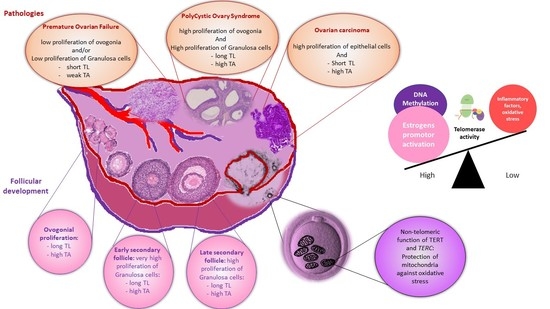
Ovarian Telomerase and Female Fertility
Abstract
:1. Introduction
2. The Telomerase
3. Human Folliculogenesis, the Importance of Proliferative Capacity
3.1. Establishment of a Pool of Primordial Follicles at the Fetal Time
3.2. Follicle Growth
3.3. Telomerase and Follicle Growth
3.4. Regulation of Telomerase Activity in Ovaries
3.4.1. Effect of Age
3.4.2. Estradiol and Telomerase Activity
4. Polycystic Ovary Syndrome (PCOS)
5. Premature Ovarian Failure
6. Discussion
7. Conclusions (Abstract Figure)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Greider, C.W.; Blackburn, E.H. Identification of a Specific Telomere Terminal Transferase Activity in Tetrahymena Extracts. Cell 1985, 43, 405–413. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Collins, K. Telomerase: An RNP Enzyme Synthesizes DNA. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, W.E.; Piatyszek, M.A.; Rainey, W.E.; Byrd, W.; Shay, J.W. Telomerase Activity in Human Germline and Embryonic Tissues and Cells. Dev. Genet. 1996, 18, 173–179. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and Telomerase in Stem Cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shay, J.W.; Wright, W.E. Role of Telomeres and Telomerase in Cancer. Semin. Cancer Biol. 2011, 21, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, N.P.; Levine, B.L.; June, C.H.; Hodes, R.J. Regulated Expression of Telomerase Activity in Human T Lymphocyte Development and Activation. J. Exp. Med. 1996, 183, 2471–2479. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomerase Activity in Human Cancer. Curr. Opin. Oncol. 1996, 8, 66–71. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Implications for Cancer and Aging. Radiat. Res. 2001, 155, 188–193. [Google Scholar] [CrossRef]
- Shay, J.W.; Bacchetti, S. A Survey of Telomerase Activity in Human Cancer. Eur. J. Cancer 1997, 33, 787–791. [Google Scholar] [CrossRef]
- Feng, J.; Funk, W.D.; Wang, S.S.; Weinrich, S.L.; Avilion, A.A.; Chiu, C.P.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J. The RNA Component of Human Telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomeres and Telomerase: Three Decades of Progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef]
- Weinrich, S.L.; Pruzan, R.; Ma, L.; Ouellette, M.; Tesmer, V.M.; Holt, S.E.; Bodnar, A.G.; Lichtsteiner, S.; Kim, N.W.; Trager, J.B.; et al. Reconstitution of Human Telomerase with the Template RNA Component HTR and the Catalytic Protein Subunit HTRT. Nat. Genet. 1997, 17, 498–502. [Google Scholar] [CrossRef]
- Wu, R.A.; Upton, H.E.; Vogan, J.M.; Collins, K. Telomerase Mechanism of Telomere Synthesis. Annu. Rev. Biochem. 2017, 86, 439–460. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, J.R.; Wood, E.; Collins, K. A Telomerase Component Is Defective in the Human Disease Dyskeratosis Congenita. Nature 1999, 402, 551–555. [Google Scholar] [CrossRef]
- Egan, E.D.; Collins, K. Biogenesis of Telomerase Ribonucleoproteins. RNA 2012, 18, 1747–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, S.E.; Aisner, D.L.; Baur, J.; Tesmer, V.M.; Dy, M.; Ouellette, M.; Trager, J.B.; Morin, G.B.; Toft, D.O.; Shay, J.W.; et al. Functional Requirement of P23 and Hsp90 in Telomerase Complexes. Genes Dev. 1999, 13, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; Abreu, E.B.; Meng, Z.; McCann, K.E.; Terns, R.M.; Veenstra, T.D.; Terns, M.P.; Artandi, S.E. A Human Telomerase Holoenzyme Protein Required for Cajal Body Localization and Telomere Synthesis. Science 2009, 323, 644–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinkle-Mulcahy, L.; Sleeman, J.E. The Cajal Body and the Nucleolus: “In a Relationship” or “It’s Complicated”? RNA Biol. 2017, 14, 739–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomlinson, R.L.; Ziegler, T.D.; Supakorndej, T.; Terns, R.M.; Terns, M.P. Cell Cycle-Regulated Trafficking of Human Telomerase to Telomeres. Mol. Biol. Cell 2006, 17, 955–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; De Lange, T. Human Telomerase Caught in the Act. Cell 2009, 138, 432–434. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Sfeir, A.J.; Zou, Y.; Buseman, C.M.; Chow, T.T.; Shay, J.W.; Wright, W.E. Telomere Extension Occurs at Most Chromosome Ends and Is Uncoupled from Fill-in in Human Cancer Cells. Cell 2009, 138, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.T.; Arneric, M.; Sperisen, P.; Lingner, J. Telomere Length Homeostasis Is Achieved via a Switch between Telomerase- Extendible and -Nonextendible States. Cell 2004, 117, 323–335. [Google Scholar] [CrossRef] [Green Version]
- Greider, C.W. Regulating Telomere Length from the inside out: The Replication Fork Model. Genes Dev. 2016, 30, 1483–1491. [Google Scholar] [CrossRef] [Green Version]
- Ségal-Bendirdjian, E.; Geli, V. Non-Canonical Roles of Telomerase: Unraveling the Imbroglio. Front. Cell Dev. Biol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, C.; Hou, M.; Komurasaki, T.; Zheng, C.; Henriksson, M.; Sedivy, J.M.; Björkholm, M.; Teh, B.T.; Nordenskjöld, M.; Xu, D. Molecular Characterization of Human Telomerase Reverse Transcriptase-Immortalized Human Fibroblasts by Gene Expression Profiling: Activation of the Epiregulin Gene. Cancer Res. 2003, 63, 1743–1747. [Google Scholar]
- Ghosh, A.; Saginc, G.; Leow, S.C.; Khattar, E.; Shin, E.M.; Yan, T.D.; Wong, M.; Zhang, Z.; Li, G.; Sung, W.-K.; et al. Telomerase Directly Regulates NF-ΚB-Dependent Transcription. Nat. Cell Biol. 2012, 14, 1270–1281. [Google Scholar] [CrossRef]
- Park, J.-I.; Venteicher, A.S.; Hong, J.Y.; Choi, J.; Jun, S.; Shkreli, M.; Chang, W.; Meng, Z.; Cheung, P.; Ji, H.; et al. Telomerase Modulates Wnt Signalling by Association with Target Gene Chromatin. Nature 2009, 460, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Li, Q.; Li, K.; Chen, L.; Li, W.; Hou, M.; Liu, T.; Yang, J.; Lindvall, C.; Björkholm, M.; et al. Telomerase Reverse Transcriptase Promotes Epithelial-Mesenchymal Transition and Stem Cell-like Traits in Cancer Cells. Oncogene 2013, 32, 4203–4213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zheng, D.; Wang, M.; Cong, Y.-S. Telomerase Reverse Transcriptase Activates the Expression of Vascular Endothelial Growth Factor Independent of Telomerase Activity. Biochem. Biophys. Res. Commun. 2009, 386, 739–743. [Google Scholar] [CrossRef]
- Liu, N.; Ding, D.; Hao, W.; Yang, F.; Wu, X.; Wang, M.; Xu, X.; Ju, Z.; Liu, J.-P.; Song, Z.; et al. HTERT Promotes Tumor Angiogenesis by Activating VEGF via Interactions with the Sp1 Transcription Factor. Nucleic Acids Res. 2016, 44, 8693–8703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yuan, X.; Sjöholm, L.; Liu, T.; Kong, F.; Ekström, T.J.; Björkholm, M.; Xu, D. Telomerase Reverse Transcriptase Regulates DNMT3B Expression/Aberrant DNA Methylation Phenotype and AKT Activation in Hepatocellular Carcinoma. Cancer Lett. 2018, 434, 33–41. [Google Scholar] [CrossRef]
- Young, J.I.; Sedivy, J.M.; Smith, J.R. Telomerase Expression in Normal Human Fibroblasts Stabilizes DNA 5-Methylcytosine Transferase, I. J. Biol. Chem. 2003, 278, 19904–19908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.H.; Meyer, J.N.; Van Houten, B. Mitochondrial Localization of Telomerase as a Determinant for Hydrogen Peroxide-Induced Mitochondrial DNA Damage and Apoptosis. Hum. Mol. Genet. 2006, 15, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase Does Not Counteract Telomere Shortening but Protects Mitochondrial Function under Oxidative Stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haendeler, J.; Dröse, S.; Büchner, N.; Jakob, S.; Altschmied, J.; Goy, C.; Spyridopoulos, I.; Zeiher, A.M.; Brandt, U.; Dimmeler, S. Mitochondrial Telomerase Reverse Transcriptase Binds to and Protects Mitochondrial DNA and Function from Damage. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 929–935. [Google Scholar] [CrossRef] [Green Version]
- Indran, I.R.; Hande, M.P.; Pervaiz, S. HTERT Overexpression Alleviates Intracellular ROS Production, Improves Mitochondrial Function, and Inhibits ROS-Mediated Apoptosis in Cancer Cells. Cancer Res. 2011, 71, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Santos, J.H.; Meyer, J.N.; Skorvaga, M.; Annab, L.A.; Houten, B.V. Mitochondrial HTERT Exacerbates Free-Radical-Mediated MtDNA Damage. Aging Cell 2004, 3, 399–411. [Google Scholar] [CrossRef]
- Shin, W.H.; Chung, K.C. Human Telomerase Reverse Transcriptase Positively Regulates Mitophagy by Inhibiting the Processing and Cytoplasmic Release of Mitochondrial PINK1. Cell Death Dis. 2020, 11, 425. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, P.; Zheng, Q.; Gao, G.; Yuan, J.; Wang, P.; Huang, J.; Xie, L.; Lu, X.; Tong, T.; et al. Mitochondrial Trafficking and Processing of Telomerase RNA TERC. Cell Rep. 2018, 24, 2589–2595. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.; Liu, P.; Gao, G.; Yuan, J.; Wang, P.; Huang, J.; Xie, L.; Lu, X.; Di, F.; Tong, T.; et al. Mitochondrion-Processed TERC Regulates Senescence without Affecting Telomerase Activities. Protein Cell 2019, 10, 631–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, T.G. A Quantitative And Cytological Study Of Germ Cells In Human Ovaries. Proc. R. Soc. Lond. B Biol. Sci. 1963, 158, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Pepling, M.E. Follicular Assembly: Mechanisms of Action. Reprod. Camb. Engl. 2012, 143, 139–149. [Google Scholar] [CrossRef] [Green Version]
- Keefe, D.L.; Marquard, K.; Liu, L. The Telomere Theory of Reproductive Senescence in Women. Curr. Opin. Obstet. Gynecol. 2006, 18, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, A. Ovarian Follicular Growth in Humans: Ovarian Ageing and Population of Growing Follicles. Maturitas 1998, 30, 137–142. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Irving-Rodgers, H.F. Morphological Classification of Bovine Ovarian Follicles. Reprod. Camb. Engl. 2010, 139, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Lavranos, T.C.; Mathis, J.M.; Latham, S.E.; Kalionis, B.; Shay, J.W.; Rodgers, R.J. Evidence for Ovarian Granulosa Stem Cells: Telomerase Activity and Localization of the Telomerase Ribonucleic Acid Component in Bovine Ovarian Follicles. Biol. Reprod. 1999, 61, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Kosebent, E.G.; Uysal, F.; Ozturk, S. Telomere Length and Telomerase Activity during Folliculogenesis in Mammals. J. Reprod. Dev. 2018, 64, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Cleément, F.; Gruet, M.A.; Monget, P.; Terqui, M.; Jolivet, E.; Monniaux, D. Growth Kinetics of the Granulosa Cell Population in Ovarian Follicles: An Approach by Mathematical Modelling. Cell Prolif. 1997, 30, 255–270. [Google Scholar] [CrossRef]
- Van Deerlin, P.G.; Cekleniak, N.; Coutifaris, C.; Boyd, J.; Strauss, J.F. Evidence for the Oligoclonal Origin of the Granulosa Cell Population of the Mature Human Follicle. J. Clin. Endocrinol. Metab. 1997, 82, 3019–3024. [Google Scholar] [CrossRef]
- Kossowska-Tomaszczuk, K.; De Geyter, C. Cells with Stem Cell Characteristics in Somatic Compartments of the Ovary. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slack, J.M.W. What Is a Stem Cell? WIREs Dev. Biol. 2018, 7, e323. [Google Scholar] [CrossRef] [PubMed]
- Lavranos, T.C. Anchorage-Independent Culture of Bovine Granulosa Cells: The Effects of Basic Fibroblast Growth Factor and Dibutyryl CAMP on Cell Division and Differentiation. Exp. Cell Res. 1994, 211, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kossowska-Tomaszczuk, K.; Geyter, C.D.; Geyter, M.D.; Martin, I.; Holzgreve, W.; Scherberich, A.; Zhang, H. The Multipotency of Luteinizing Granulosa Cells Collected from Mature Ovarian Follicles. Stem Cells 2009, 27, 210–219. [Google Scholar] [CrossRef]
- Goto, H.; Iwata, H.; Takeo, S.; Nisinosono, K.; Murakami, S.; Monji, Y.; Kuwayama, T. Effect of Bovine Age on the Proliferative Activity, Global DNA Methylation, Relative Telomere Length and Telomerase Activity of Granulosa Cells. Zygote Camb. Engl. 2013, 21, 256–264. [Google Scholar] [CrossRef]
- Russo, V.; Berardinelli, P.; Martelli, A.; Giacinto, O.D.; Nardinocchi, D.; Fantasia, D.; Barboni, B. Expression of Telomerase Reverse Transcriptase Subunit (TERT) and Telomere Sizing in Pig Ovarian Follicles. J. Histochem. Cytochem. 2006, 54, 443–455. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-P.; Li, H. Telomerase in the Ovary. Reprod. Camb. Engl. 2010, 140, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, R.J.; Irving-Rodgers, H.F.; van Wezel, I.L.; Krupa, M.; Lavranos, T.C. Dynamics of the Membrana Granulosa during Expansion of the Ovarian Follicular Antrum. Mol. Cell. Endocrinol. 2001, 171, 41–48. [Google Scholar] [CrossRef]
- Tománek, M.; Chronowska, E.; Kott, T.; Czerneková, V. Telomerase Activity in Pig Granulosa Cells Proliferating and Differentiating in Vitro. Anim. Reprod. Sci. 2008, 104, 284–298. [Google Scholar] [CrossRef]
- Butts, S.; Riethman, H.; Ratcliffe, S.; Shaunik, A.; Coutifaris, C.; Barnhart, K. Correlation of Telomere Length and Telomerase Activity with Occult Ovarian Insufficiency. J. Clin. Endocrinol. Metab. 2009, 94, 4835–4843. [Google Scholar] [CrossRef]
- Kosebent, E.G.; Uysal, F.; Ozturk, S. The Altered Expression of Telomerase Components and Telomere-Linked Proteins May Associate with Ovarian Aging in Mouse. Exp. Gerontol. 2020, 138, 110975. [Google Scholar] [CrossRef]
- Uysal, F.; Kosebent, E.G.; Toru, H.S.; Ozturk, S. Decreased Expression of TERT and Telomeric Proteins as Human Ovaries Age May Cause Telomere Shortening. J. Assist. Reprod. Genet. 2021, 38, 429–441. [Google Scholar] [CrossRef]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and Telomere Length: Systematic Review and Meta-Analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Mayer, S.; Brüderlein, S.; Perner, S.; Waibel, I.; Holdenried, A.; Ciloglu, N.; Hasel, C.; Mattfeldt, T.; Nielsen, K.V.; Möller, P. Sex-Specific Telomere Length Profiles and Age-Dependent Erosion Dynamics of Individual Chromosome Arms in Humans. Cytogenet. Genome Res. 2006, 112, 194–201. [Google Scholar] [CrossRef]
- Lulkiewicz, M.; Bajsert, J.; Kopczynski, P.; Barczak, W.; Rubis, B. Telomere Length: How the Length Makes a Difference. Mol. Biol. Rep. 2020, 47, 7181–7188. [Google Scholar] [CrossRef]
- Chen, W.; Kimura, M.; Kim, S.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Kark, J.D.; Aviv, A. Longitudinal versus Cross-Sectional Evaluations of Leukocyte Telomere Length Dynamics: Age-Dependent Telomere Shortening Is the Rule. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 312–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalgård, C.; Benetos, A.; Verhulst, S.; Labat, C.; Kark, J.D.; Christensen, K.; Kimura, M.; Kyvik, K.O.; Aviv, A. Leukocyte Telomere Length Dynamics in Women and Men: Menopause vs. Age Effects. Int. J. Epidemiol. 2015, 44, 1688–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Factor-Litvak, P.; Susser, E.; Kezios, K.; McKeague, I.; Kark, J.D.; Hoffman, M.; Kimura, M.; Wapner, R.; Aviv, A. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics 2016, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piontkewitz, Y.; Sundfeldt, K.; Hedin, L. The Expression of C-Myc during Follicular Growth and Luteal Formation in the Rat Ovary in Vivo. J. Endocrinol. 1997, 152, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.; Jones, M.E.E.; Li, H.; Liu, J.-P. Potential Roles for Estrogen Regulation of Telomerase Activity in Aging. Ann. N. Y. Acad. Sci. 2007, 1114, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borrás, C.; Gambini, J.; Sastre, J.; Pallardó, F.V. Why Females Live Longer than Males? Importance of the Upregulation of Longevity-Associated Genes by Oestrogenic Compounds. FEBS Lett. 2005, 579, 2541–2545. [Google Scholar] [CrossRef] [Green Version]
- Yamagata, Y.; Nakamura, Y.; Umayahara, K.; Harada, A.; Takayama, H.; Sugino, N.; Kato, H. Changes in Telomerase Activity in Experimentally Induced Atretic Follicles of Immature Rats. Endocr. J. 2002, 49, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Bayne, S.; Li, H.; Jones, M.E.E.; Pinto, A.R.; van Sinderen, M.; Drummond, A.; Simpson, E.R.; Liu, J.-P. Estrogen Deficiency Reversibly Induces Telomere Shortening in Mouse Granulosa Cells and Ovarian Aging in Vivo. Protein Cell 2011, 2, 333–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordechai, A.; Wasserman, M.; Abramov, M.; Ben-Menahem, D.; Har-Vardi, I.; Levitas, E.; Priel, E. Increasing Telomerase Enhanced Steroidogenic Genes Expression and Steroid Hormones Production in Rat and Human Granulosa Cells and in Mouse Ovary. J. Steroid Biochem. Mol. Biol. 2020, 197, 105551. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Xie, J.; Yin, B.; Hao, H.; Song, X.; Liu, Q.; Zhang, C.; Sun, Y. Significantly Lengthened Telomere in Granulosa Cells from Women with Polycystic Ovarian Syndrome (PCOS). J. Assist. Reprod. Genet. 2017, 34, 861–866. [Google Scholar] [CrossRef]
- Dunaif, A. Insulin Resistance and the Polycystic Ovary Syndrome: Mechanism and Implications for Pathogenesis. Endocr. Rev. 1997, 18, 774–800. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, N.; Khan, S.Z.; Shaikh, R. Polycystic Ovary Syndrome (PCOS) and Genetic Predisposition: A Review Article. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2019, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Du, J.; Feng, R.; Xu, Y.; Wang, H.; Sang, Q.; Xing, Q.; Zhao, X.; Jin, L.; He, L.; et al. A Possible New Mechanism in the Pathophysiology of Polycystic Ovary Syndrome (PCOS): The Discovery That Leukocyte Telomere Length Is Strongly Associated with PCOS. J. Clin. Endocrinol. Metab. 2014, 99, E234–E240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, M. Oxidative Stress and Polycystic Ovary Syndrome: A Brief Review. Int. J. Prev. Med. 2019, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.C.; Miranda-Furtado, C.L.; Kogure, G.S.; Meola, J.; Okuka, M.; Silva, C.; Calado, R.T.; Ferriani, R.A.; Keefe, D.L.; dos Reis, R.M. Inflammatory Biomarkers and Telomere Length in Women with Polycystic Ovary Syndrome. Fertil. Steril. 2015, 103, 542–547.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, R.; Ouyang, N.; Dai, K.; Yuan, P.; Zheng, L.; Wang, W. Investigating the Impact of Local Inflammation on Granulosa Cells and Follicular Development in Women with Ovarian Endometriosis. Fertil. Steril. 2019, 112, 882–891.e1. [Google Scholar] [CrossRef]
- Pedroso, D.C.C.; Santana, V.P.; Donaires, F.S.; Picinato, M.C.; Giorgenon, R.C.; Santana, B.A.; Pimentel, R.N.; Keefe, D.L.; Calado, R.T.; Ferriani, R.A.; et al. Telomere Length and Telomerase Activity in Immature Oocytes and Cumulus Cells of Women with Polycystic Ovary Syndrome. Reprod. Sci. Thousand Oaks Calif. 2020, 27, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.O.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.M.; Georgiadis, G.; et al. The Association of Female and Male Infertility with Telomere Length (Review). Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Yewdell, W.T.; Wilkerson, K.L.; Regal, J.A.; Kajigaya, S.; Stratakis, C.A.; Young, N.S. Sex Hormones, Acting on the TERT Gene, Increase Telomerase Activity in Human Primary Hematopoietic Cells. Blood 2009, 114, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Deng, B.; Ouyang, N.; Yuan, P.; Zheng, L.; Wang, W. Telomere Length Is Short in PCOS and Oral Contraceptive Does Not Affect the Telomerase Activity in Granulosa Cells of Patients with PCOS. J. Assist. Reprod. Genet. 2017, 34, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.J.; Stubbs, S.; Stark, J.; Trew, G.H.; Margara, R.; Hardy, K.; Franks, S. Formation and Early Development of Follicles in the Polycystic Ovary. Lancet Lond. Engl. 2003, 362, 1017–1021. [Google Scholar] [CrossRef]
- Beck-Peccoz, P.; Persani, L. Premature Ovarian Failure. Orphanet. J. Rare Dis. 2006, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI; Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E.; et al. ESHRE Guideline: Management of Women with Premature Ovarian Insufficiency. Hum. Reprod. Oxf. Engl. 2016, 31, 926–937. [Google Scholar] [CrossRef] [Green Version]
- Committee on Gynecologic Practice Committee Opinion No. 605: Primary Ovarian Insufficiency in Adolescents and Young Women. Obstet. Gynecol. 2014, 124, 193–197. [CrossRef]
- Podfigurna-Stopa, A.; Czyzyk, A.; Grymowicz, M.; Smolarczyk, R.; Katulski, K.; Czajkowski, K.; Meczekalski, B. Premature Ovarian Insufficiency: The Context of Long-Term Effects. J. Endocrinol. Investig. 2016, 39, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Rocca, M.S.; Foresta, C.; Ferlin, A. Telomere Length: Lights and Shadows on Their Role in Human Reproduction. Biol. Reprod. 2019, 100, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.; Sozen, B.; Demir, N. Telomere Length and Telomerase Activity during Oocyte Maturation and Early Embryo Development in Mammalian Species. Mol. Hum. Reprod. 2014, 20, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattet, A.-J.; Toupance, S.; Thornton, S.N.; Monnin, N.; Guéant, J.-L.; Benetos, A.; Koscinski, I. Telomere Length in Granulosa Cells and Leukocytes: A Potential Marker of Female Fertility? A Systematic Review of the Literature. J. Ovarian Res. 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Daniali, L.; Benetos, A.; Susser, E.; Kark, J.D.; Labat, C.; Kimura, M.; Desai, K.; Granick, M.; Aviv, A. Telomeres Shorten at Equivalent Rates in Somatic Tissues of Adults. Nat. Commun. 2013, 4, 1597. [Google Scholar] [CrossRef] [PubMed]
- Hanna, C.W.; Bretherick, K.L.; Gair, J.L.; Fluker, M.R.; Stephenson, M.D.; Robinson, W.P. Telomere Length and Reproductive Aging. Hum. Reprod. Oxf. Engl. 2009, 24, 1206–1211. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Furtado, C.L.; Luchiari, H.R.; Chielli Pedroso, D.C.; Kogure, G.S.; Caetano, L.C.; Santana, B.A.; Santana, V.P.; Benetti-Pinto, C.L.; Reis, F.M.; Maciel, M.A.; et al. Skewed X-Chromosome Inactivation and Shorter Telomeres Associate with Idiopathic Premature Ovarian Insufficiency. Fertil. Steril. 2018, 110, 476–485.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayban, S.; Mirfakhraie, R.; Omrani, M.D.; Ghaedi, H.; Heidary, H.; Yaghoobi, H.; Azizi, F.; Pouresmaeili, F. Idiopathic Premature Ovarian Failure and Its Association to the Abnormal Longitudinal Changes of Telomere Length in a Population of Iranian Infertile Women: A Pilot Study. Meta Gene 2018, 18, 58–61. [Google Scholar] [CrossRef]
- Kinugawa, C.; Murakami, T.; Okamura, K.; Yajima, A. Telomerase Activity in Normal Ovaries and Premature Ovarian Failure. Tohoku J. Exp. Med. 2000, 190, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Chen, X.; Zhang, X.; Liu, Y.; Wang, Z.; Wang, P.; Du, Y.; Qin, Y.; Chen, Z.-J. Impaired Telomere Length and Telomerase Activity in Peripheral Blood Leukocytes and Granulosa Cells in Patients with Biochemical Primary Ovarian Insufficiency. Hum. Reprod. Oxf. Engl. 2017, 32, 201–207. [Google Scholar] [CrossRef]
- Liu, C.C.; Ma, D.L.; Yan, T.-D.; Fan, X.; Poon, Z.; Poon, L.-F.; Goh, S.-A.; Rozen, S.G.; Hwang, W.Y.K.; Tergaonkar, V.; et al. Distinct Responses of Stem Cells to Telomere Uncapping-A Potential Strategy to Improve the Safety of Cell Therapy. Stem Cells 2016, 34, 2471–2484. [Google Scholar] [CrossRef] [Green Version]
- Portillo, A.M.; Varela, E.; García-Velasco, J.A. Mathematical Model to Study the Aging of the Human Follicle According to the Telomerase Activity. J. Theor. Biol. 2019, 462, 446–454. [Google Scholar] [CrossRef]
- Portillo, A.M.; Peláez, C. Mathematical Modelling of Ageing Acceleration of the Human Follicle Due to Oxidative Stress and Other Factors. Math. Med. Biol. J. IMA 2021. [Google Scholar] [CrossRef]
- Chen, H.; Wang, W.; Mo, Y.; Ma, Y.; Ouyang, N.; Li, R.; Mai, M.; He, Y.; Bodombossou-Djobo, M.M.A.; Yang, D. Women with High Telomerase Activity in Luteinised Granulosa Cells Have a Higher Pregnancy Rate during in Vitro Fertilisation Treatment. J. Assist. Reprod. Genet. 2011, 28, 797–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, K.; Xu, H.; Ouyang, N.; Li, Y.; Yuan, P.; Wang, L.; Zhao, X.; Wang, W. Correlation of Human Telomerase Reverse Transcriptase Single Nucleotide Polymorphisms with in Vitro Fertilisation Outcomes. J. Assist. Reprod. Genet. 2019, 36, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Sklavos, M.M.; Stratton, P.; Giri, N.; Alter, B.P.; Savage, S.A.; Pinto, L.A. Reduced Serum Levels of Anti-Müllerian Hormone in Females with Inherited Bone Marrow Failure Syndromes. J. Clin. Endocrinol. Metab. 2015, 100, E197–E203. [Google Scholar] [CrossRef] [Green Version]
- Robinson, L.G.; Pimentel, R.; Wang, F.; Kramer, Y.G.; Gonullu, D.C.; Agarwal, S.; Navarro, P.A.; McCulloh, D.; Keefe, D.L. Impaired Reproductive Function and Fertility Preservation in a Woman with a Dyskeratosis Congenita. J. Assist. Reprod. Genet. 2020, 37, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of Endometrial, Ovarian and Breast Cancer in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Turner, M.A.; Drury, J.A.; Quenby, S.; Saretzki, G.; Martin-Ruiz, C.; Von Zglinicki, T. Endometriosis Is Associated with Aberrant Endometrial Expression of Telomerase and Increased Telomere Length. Hum. Reprod. Oxf. Engl. 2008, 23, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Polonio, A.M.; Chico-Sordo, L.; Córdova-Oriz, I.; Medrano, M.; García-Velasco, J.A.; Varela, E. Impact of Ovarian Aging in Reproduction: From Telomeres and Mice Models to Ovarian Rejuvenation. Yale J. Biol. Med. 2020, 93, 561–569. [Google Scholar]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xie, L.Y.; Allan, S.; Beach, D.; Hannon, G.J. Myc Activates Telomerase. Genes Dev. 1998, 12, 1769–1774. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.-W.; Hou, P.-S.; Tseng, S.-F.; Chien, C.-L.; Wu, K.-J.; Chen, H.-F.; Ho, H.-N.; Kyo, S.; Teng, S.-C. Krüppel-like Transcription Factor 4 Contributes to Maintenance of Telomerase Activity in Stem Cells. Stem Cells 2010, 28, 1510–1517. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Vera, E.; Schneeberger, K.; Tejera, A.M.; Ayuso, E.; Bosch, F.; Blasco, M.A. Telomerase Gene Therapy in Adult and Old Mice Delays Aging and Increases Longevity without Increasing Cancer. EMBO Mol. Med. 2012, 4, 691–704. [Google Scholar] [CrossRef]
- Jaskelioff, M.; Muller, F.L.; Paik, J.-H.; Thomas, E.; Jiang, S.; Adams, A.C.; Sahin, E.; Kost-Alimova, M.; Protopopov, A.; Cadiñanos, J.; et al. Telomerase Reactivation Reverses Tissue Degeneration in Aged Telomerase-Deficient Mice. Nature 2011, 469, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Bretschneider, H.; Quade, M.; Lode, A.; Gelinsky, M.; Rammelt, S.; Zwingenberger, S.; Schaser, K.-D.; Vater, C. Characterization of Naturally Occurring Bioactive Factor Mixtures for Bone Regeneration. Int. J. Mol. Sci. 2020, 21, 1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakiroglu, Y.; Saltik, A.; Yuceturk, A.; Karaosmanoglu, O.; Kopuk, S.Y.; Scott, R.T.; Tiras, B.; Seli, E. Effects of Intraovarian Injection of Autologous Platelet Rich Plasma on Ovarian Reserve and IVF Outcome Parameters in Women with Primary Ovarian Insufficiency. Aging 2020, 12, 10211–10222. [Google Scholar] [CrossRef]
- Sills, E.S.; Wood, S.H. Autologous Activated Platelet-Rich Plasma Injection into Adult Human Ovary Tissue: Molecular Mechanism, Analysis, and Discussion of Reproductive Response. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Shingu, T.; Jaskelioff, M.; Yuan, L.; Ding, Z.; Protopopov, A.; Kost-Alimova, M.; Hu, J. Utilizing Murine Inducible Telomerase Alleles in the Studies of Tissue Degeneration/Regeneration and Cancer. J. Vis. Exp. JoVE 2015. [Google Scholar] [CrossRef] [Green Version]
- Counter, C.M.; Hirte, H.W.; Bacchetti, S.; Harley, C.B. Telomerase Activity in Human Ovarian Carcinoma. Proc. Natl. Acad. Sci. USA 1994, 91, 2900–2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baykal, A.; Thompson, J.A.; Xu, X.-C.; Hahn, W.C.; Deavers, M.T.; Malpica, A.; Gershenson, D.M.; Silva, E.G.; Liu, J. In Situ Human Telomerase Reverse Transcriptase Expression Pattern in Normal and Neoplastic Ovarian Tissues. Oncol. Rep. 2004, 11, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Narisawa-Saito, M.; Yugawa, T.; Fujita, M.; Tashiro, H.; Katabuchi, H.; Kiyono, T. Oncogenic Transformation of Human Ovarian Surface Epithelial Cells with Defined Cellular Oncogenes. Carcinogenesis 2009, 30, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Osborne, C.K.; Schiff, R. Estrogen-Receptor Biology: Continuing Progress and Therapeutic Implications. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Hu, W.; Fu, S.Q.; Li, J.D.; Liu, J.H.; Kavanagh, J.J. Aromatase Inhibitors in Ovarian Cancer: Is There a Role? Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2008, 18, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Simpson, E.R.; Liu, J.-P. Oestrogen, Telomerase, Ovarian Ageing and Cancer. Clin. Exp. Pharmacol. Physiol. 2010, 37, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Losi, L.; Botticelli, L.; Garagnani, L.; Fabbiani, L.; Panini, R.; Gallo, G.; Sabbatini, R.; Maiorana, A.; Benhattar, J. TERT Promoter Methylation and Protein Expression as Predictive Biomarkers for Recurrence Risk in Patients with Serous Borderline Ovarian Tumours. Pathology 2021, 53, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ghareghomi, S.; Ahmadian, S.; Zarghami, N.; Hemmati, S. HTERT-Molecular Targeted Therapy of Ovarian Cancer Cells via Folate-Functionalized PLGA Nanoparticles Co-Loaded with MNPs/SiRNA/Wortmannin. Life Sci. 2021, 277, 119621. [Google Scholar] [CrossRef]
- Devereux, T.R.; Horikawa, I.; Anna, C.H.; Annab, L.A.; Afshari, C.A.; Barrett, J.C. DNA Methylation Analysis of the Promoter Region of the Human Telomerase Reverse Transcriptase (HTERT) Gene. Cancer Res. 1999, 59, 6087–6090. [Google Scholar]
- Boccardi, V.; Paolisso, G.; Mecocci, P. Nutrition and Lifestyle in Healthy Aging: The Telomerase Challenge. Aging 2016, 8, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Widschwendter, A.; Müller, H.M.; Hubalek, M.M.; Wiedemair, A.; Fiegl, H.; Goebel, G.; Mueller-Holzner, E.; Marth, C.; Widschwendter, M. Methylation Status and Expression of Human Telomerase Reverse Transcriptase in Ovarian and Cervical Cancer. Gynecol. Oncol. 2004, 93, 407–416. [Google Scholar] [CrossRef]
- Rafie, N.; Golpour Hamedani, S.; Barak, F.; Safavi, S.M.; Miraghajani, M. Dietary Patterns, Food Groups and Telomere Length: A Systematic Review of Current Studies. Eur. J. Clin. Nutr. 2017, 71, 151–158. [Google Scholar] [CrossRef]
- Davinelli, S.; Trichopoulou, A.; Corbi, G.; De Vivo, I.; Scapagnini, G. The Potential Nutrigeroprotective Role of Mediterranean Diet and Its Functional Components on Telomere Length Dynamics. Ageing Res. Rev. 2019, 49, 1–10. [Google Scholar] [CrossRef]
- Pepper, G.V.; Bateson, M.; Nettle, D. Telomeres as Integrative Markers of Exposure to Stress and Adversity: A Systematic Review and Meta-Analysis. R. Soc. Open Sci. 2018, 5, 180744. [Google Scholar] [CrossRef] [Green Version]
- Canudas, S.; Becerra-Tomás, N.; Hernández-Alonso, P.; Galié, S.; Leung, C.; Crous-Bou, M.; De Vivo, I.; Gao, Y.; Gu, Y.; Meinilä, J.; et al. Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1544–1554. [Google Scholar] [CrossRef]
- Navarro-Ibarra, M.J.; Hernández, J.; Caire-Juvera, G. Diet, Physical Activity and Telomere Length in Adults. Nutr. Hosp. 2019, 36, 1403–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreerangaraja Urs, D.B.; Wu, W.-H.; Komrskova, K.; Postlerova, P.; Lin, Y.-F.; Tzeng, C.-R.; Kao, S.-H. Mitochondrial Function in Modulating Human Granulosa Cell Steroidogenesis and Female Fertility. Int. J. Mol. Sci. 2020, 21, 3592. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.-M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian Ageing: The Role of Mitochondria in Oocytes and Follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Clinic and Biologic Symptoms | Long-Term Clinical Consequences | |
|---|---|---|
| Diagnostic criteria according to Androgen Excess-PCOS Society (2006) | Biochemical and clinical hyperandrogenism (hirsutism and acne) | Hirsutism |
| Ovarian dysfunction (oligo-amenorrhea) | Infertility | |
| Polycystic ovary morphology | ||
| Insulin resistance/increased incidence of type 2 diabetes | Metabolic syndrome | |
| Increased incidence of cardiovascular disease |
| Authors | Variable Measured/Method | Population No. | Population Age (Years) | Results |
|---|---|---|---|---|
| Li et al., 2017 [85] | GTL: PCR GTA: TRAP | (n = 65) PCOS (IVF) (n = 98) Non-PCOS (IVF) | 30.3 ± 4.3 31.7 ± 3.8 | GTL: shorter in PCOS GTA: no difference Earlier infertility symptoms when shorter TL and lower TA |
| Wei et al., 2017 [75] | GTL: PCR | (n = 75) PCOS (IVF) (n = 81) Non-PCOS (IVF) | 28.36 ± 2.55 28.09 ± 2.26 | GTL: longer in PCOS |
| Pedroso et al., 2020 [82] | GTL: PCR | (n = 43) PCOS (IVF) (n = 67) Non-PCOS (IVF) | 33.7 ± 4.1 | GTL: no difference GTA: higher in PCOS Similar TA and TL in GV and MI oocytes |
| Clinic and Biologic Symptoms | Long-Term Clinical Consequences | |
|---|---|---|
| Diagnostic criteria according to ESHRE | Ovarian dysfunction (Oligo-amenorrhea for at least 4 months) | Infertility |
| Clinical estrogen deficiency (climacteric syndrome) and sex hormonal dysfunction with: elevated FSH level >25 IU/L on two occasions >4 weeks apart | Urogenital symptoms (vaginal dryness, irritation and itching, sexual disorders) Cognitive dysfunction (memory and concentration problems, increased risk of dementia) Autoimmunity (increased risk of autoimmune disease) Bone alteration (osteopenia, osteoporosis, and increased risk of fracture) Increased all-cause mortality |
| Authors | Method | Population | Population Age (Years) | Results |
|---|---|---|---|---|
| Kinugawa et al., 2000 [98] | TRAP | (n = 5) POF (n = 20) Controls | 31.4 <38 | Ovarian TA: lower level in POF |
| Butts et al., 2009 [60] | PCR TRAP | (n = 12) POF (n = 42) Controls | 30–37 23–37 | GTL: shorter in POF GTA: absence of TA is more frequent in POF |
| Xu et al., 2017 [99] | PCR TRAP | (n = 120) POF (n = 90) Controls | 32.95 ± 4.27 29.98 ± 4.28 | GTL: shorter in POF GTA: lower level in POF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toupance, S.; Fattet, A.-J.; Thornton, S.N.; Benetos, A.; Guéant, J.-L.; Koscinski, I. Ovarian Telomerase and Female Fertility. Biomedicines 2021, 9, 842. https://doi.org/10.3390/biomedicines9070842
Toupance S, Fattet A-J, Thornton SN, Benetos A, Guéant J-L, Koscinski I. Ovarian Telomerase and Female Fertility. Biomedicines. 2021; 9(7):842. https://doi.org/10.3390/biomedicines9070842
Chicago/Turabian StyleToupance, Simon, Anne-Julie Fattet, Simon N. Thornton, Athanase Benetos, Jean-Louis Guéant, and Isabelle Koscinski. 2021. "Ovarian Telomerase and Female Fertility" Biomedicines 9, no. 7: 842. https://doi.org/10.3390/biomedicines9070842
APA StyleToupance, S., Fattet, A.-J., Thornton, S. N., Benetos, A., Guéant, J.-L., & Koscinski, I. (2021). Ovarian Telomerase and Female Fertility. Biomedicines, 9(7), 842. https://doi.org/10.3390/biomedicines9070842