Clinical Implication of Metformin in Relation to Diabetes Mellitus and Ovarian Cancer
Abstract
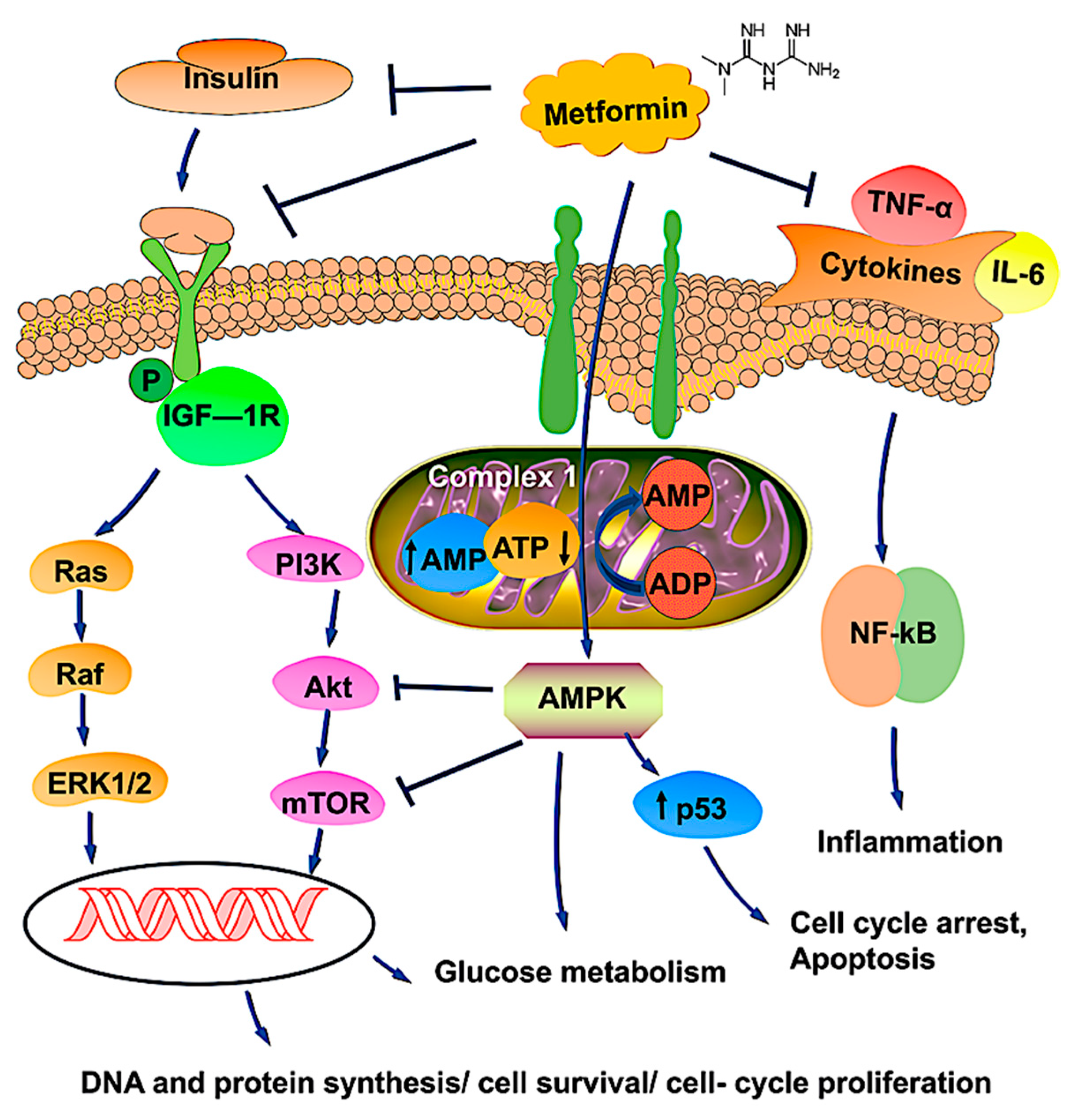
:1. Introduction
2. DM and Cancer Development
2.1. Hyperinsulinemia Due to Insulin Resistance as Seen in T2DM
2.2. Hyperglycemia as Seen in T1DM and T2DM and Its Effect on Cancer Development
2.3. Chronic Inflammation as Seen in DM and Its Relationship with the Development of OvCa
3. Theories Regarding the Biological Link between DM and OvCa
4. Antineoplastic Mechanisms of Metformin on OvCa Cells
5. The Future of Metformin as an Adjunct for OvCa Therapy
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [Green Version]
- Gajjar, K.; Ogden, G.; Mujahid, M.I.; Razvi, K. Symptoms and risk factors of ovarian cancer: A survey in primary care. ISRN Obstet. Gynecol. 2012, 2012, 754197. [Google Scholar] [CrossRef] [Green Version]
- Shlomai, G.; Neel, B.; LeRoith, D.; Gallagher, E.J. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, A.; Qiu, M.; Zhou, H.; Wang, T.; Guo, W. PTEN, Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3667–3676. [Google Scholar] [CrossRef]
- Zhang, D.; Li, N.; Xi, Y.; Zhao, Y.; Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 2017, 130, 43–52. [Google Scholar] [CrossRef]
- Romero, I.L.; McCormick, A.; McEwen, K.A.; Park, S.; Karrison, T.; Yamada, S.D.; Pannain, S.; Lengyel, E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012, 119, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rattan, R.; Giri, S.; Hartmann, L.C.; Shridhar, V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J. Cell Mol. Med. 2011, 15, 166–178. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zheng, Y.; Zhang, H.; Sun, H. Targeting cancer cell metabolism: The combination of metformin and 2-Deoxyglucose regulates apoptosis in ovarian cancer cells via p38 MAPK/JNK signaling pathway. Am. J. Transl. Res. 2016, 8, 4812–4821. [Google Scholar]
- Wang, S.B.; Lei, K.J.; Liu, J.P.; Jia, Y.M. Continuous use of metformin can improve survival in type 2 diabetic patients with ovarian cancer: A retrospective study. Medicine 2017, 96, e7605. [Google Scholar] [CrossRef]
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naïve Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dąbrowski, M.; Szymańska-Garbacz, E.; Miszczyszyn, Z.; Dereziński, T.; Czupryniak, L. Risk factors for cancer development in type 2 diabetes: A retrospective case-control study. BMC Cancer 2016, 16, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujimoto, T.; Kajio, H.; Sugiyama, T. Association between hyperinsulinemia and increased risk of cancer death in nonobese and obese people: A population-based observational study. Int. J. Cancer 2017, 141, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.E.; Koo, J.; Madarnas, Y.; Hartwick, W.; Hoffman, B.; Hood, N. Fasting insulin and outcome in early-stage breast cancer: Results of a prospective cohort study. J. Clin. Oncol. 2002, 20, 42–51. [Google Scholar] [CrossRef]
- Arcidiacono, B.; Iiritano, S.; Nocera, A.; Possidente, K.; Nevolo, M.T.; Ventura, V.; Foti, D.; Chiefari, E.; Brunetti, A. Insulin resistance and cancer risk: An overview of the pathogenetic mechanisms. Exp. Diabetes Res. 2012, 2012, 789174. [Google Scholar] [CrossRef] [Green Version]
- Denduluri, S.K.; Idowu, O.; Wang, Z.; Liao, Z.; Yan, Z.; Mohammed, M.K.; Ye, J.; Wei, Q.; Wang, J.; Zhao, L.; et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015, 2, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Zha, J.; Lackner, M.R. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin. Cancer Res. 2010, 16, 2512–2517. [Google Scholar] [CrossRef] [Green Version]
- Pollak, M. Insulin and insulin-like growth factor signalling in neoplasia. Nat. Rev. Cancer 2008, 8, 915–928. [Google Scholar] [CrossRef]
- Osborne, C.K.; Bolan, G.; Monaco, M.E.; Lippman, M.E. Hormone responsive human breast cancer in long-term tissue culture: Effect of insulin. Proc. Natl. Acad. Sci. USA 1976, 73, 4536–4540. [Google Scholar] [CrossRef] [Green Version]
- Heuson, J.C.; Legros, N. Influence of insulin deprivation on growth of the 7,12-dimethylbenz(a)anthracene-induced mammary carcinoma in rats subjected to alloxan diabetes and food restriction. Cancer Res. 1972, 32, 226–232. [Google Scholar] [PubMed]
- Kellenberger, L.D.; Petrik, J. Hyperglycemia promotes insulin-independent ovarian tumor growth. Gynecol. Oncol. 2018, 149, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Wahdan-Alaswad, R.; Fan, Z.; Edgerton, S.M.; Liu, B.; Deng, X.S.; Arnadottir, S.S.; Richer, J.K.; Anderson, S.M.; Thor, A.D. Glucose promotes breast cancer aggression and reduces metformin efficacy. Cell Cycle 2013, 12, 3759–3769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, W.; Shen, X.; Lei, J.; Xu, Q.; Yu, Y.; Li, R.; Wu, E.; Ma, Q. Hyperglycemia, a neglected factor during cancer progression. Biomed Res. Int. 2014, 2014, 461917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negre-Salvayre, A.; Salvayre, R.; Augé, N.; Pamplona, R.; Portero-Otín, M. Hyperglycemia and glycation in diabetic complications. Antioxid. Redox Signal. 2009, 11, 3071–3109. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bali, A.; Singh, N.; Jaggi, A.S. Advanced glycation end products and diabetic complications. Korean J. Physiol. Pharmacol. 2014, 18, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Sharaf, H.; Matou-Nasri, S.; Wang, Q.; Rabhan, Z.; Al-Eidi, H.; Al Abdulrahman, A.; Ahmed, N. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochim. Biophys. Acta 2015, 1852, 429–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shikata, K.; Ninomiya, T.; Kiyohara, Y. Diabetes mellitus and cancer risk: Review of the epidemiological evidence. Cancer Sci. 2013, 104, 9–14. [Google Scholar] [CrossRef]
- Andreasen, A.S.; Kelly, M.; Berg, R.M.; Møller, K.; Pedersen, B.K. Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PLoS ONE 2011, 6, e23999. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Onco. Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018, 10, 6685–6693. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, P.C.; Behrmann, I.; Müller-Newen, G.; Schaper, F.; Graeve, L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998, 334 Pt 2, 297–314. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Mu, W.; Kahn, A.; Jing, N.; Li, J.H.; Lan, H.Y.; Nakagawa, T.; Ohashi, R.; Johnson, R.J. Role of JAK/STAT pathway in IL-6-induced activation of vascular smooth muscle cells. Am. J. Nephrol. 2004, 24, 387–392. [Google Scholar] [CrossRef]
- Beauchamp, M.C.; Yasmeen, A.; Knafo, A.; Gotlieb, W.H. Targeting insulin and insulin-like growth factor pathways in epithelial ovarian cancer. J. Oncol. 2010, 2010, 257058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kagan, P.; Sultan, M.; Tachlytski, I.; Safran, M.; Ben-Ari, Z. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation. PLoS ONE 2017, 12, e0176173. [Google Scholar] [CrossRef]
- Colomiere, M.; Ward, A.C.; Riley, C.; Trenerry, M.K.; Cameron-Smith, D.; Findlay, J.; Ackland, L.; Ahmed, N. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br. J. Cancer 2009, 100, 134–144. [Google Scholar] [CrossRef]
- Adler, A.I.; Weiss, N.S.; Kamb, M.L.; Lyon, J.L. Is diabetes mellitus a risk factor for ovarian cancer? A case-control study in Utah and Washington (United States). Cancer Causes Control 1996, 7, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Gapstur, S.M.; Patel, A.V.; Diver, W.R.; Hildebrand, J.S.; Gaudet, M.M.; Jacobs, E.J.; Campbell, P.T. Type II diabetes mellitus and the incidence of epithelial ovarian cancer in the cancer prevention study-II nutrition cohort. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2000–2005. [Google Scholar] [CrossRef] [Green Version]
- La Vecchia, C.; Negri, E.; Franceschi, S.; D’Avanzo, B.; Boyle, P. A case-control study of diabetes mellitus and cancer risk. Br. J. Cancer 1994, 70, 950–953. [Google Scholar] [CrossRef]
- Inoue, M.; Iwasaki, M.; Otani, T.; Sasazuki, S.; Noda, M.; Tsugane, S. Diabetes mellitus and the risk of cancer: Results from a large-scale population-based cohort study in Japan. Arch. Intern. Med. 2006, 166, 1871–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Jeon, I.; Kim, J.W.; Song, Y.S.; Yoon, J.M.; Park, S.M. Diabetes mellitus and ovarian cancer risk: A systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer 2013, 23, 402–412. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, J.; Wang, B.; Liu, H. Association between diabetes mellitus and subsequent ovarian cancer in women: A systematic review and meta-analysis of cohort studies. Medicine 2017, 96, e6396. [Google Scholar] [CrossRef]
- Lauf, P.K.; Adragna, N.C. K-Cl cotransport: Properties and molecular mechanism. Cell Physiol. Biochem. 2000, 10, 341–354. [Google Scholar] [CrossRef]
- Shen, M.R.; Lin, A.C.; Hsu, Y.M.; Chang, T.J.; Tang, M.J.; Alper, S.L.; Ellory, J.C.; Chou, C.Y. Insulin-like growth factor 1 stimulates KCl cotransport, which is necessary for invasion and proliferation of cervical cancer and ovarian cancer cells. J. Biol. Chem. 2004, 279, 40017–40025. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.R.; Chou, C.Y.; Hsu, K.F.; Hsu, Y.M.; Chiu, W.T.; Tang, M.J.; Alper, S.L.; Ellory, J.C. KCl cotransport is an important modulator of human cervical cancer growth and invasion. J. Biol. Chem. 2003, 278, 39941–39950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.F.; Chou, C.Y.; Wilkins, R.J.; Ellory, J.C.; Mount, D.B.; Shen, M.R. Motor protein-dependent membrane trafficking of KCl cotransporter-4 is important for cancer cell invasion. Cancer Res. 2009, 69, 8585–8593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef] [Green Version]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikhlas, S.; Ahmad, M. Metformin: Insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 2017, 185, 53–62. [Google Scholar] [CrossRef]
- Li, H.; Zeng, J.; Shen, K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet. 2014, 290, 1067–1078. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Bardhi, E.; Ruscito, I.; Papadia, A.; Farooqi, A.A.; Marchetti, C.; Bogani, G.; Ceccacci, I.; Mueller, M.D.; Benedetti Panici, P. PI3K/AKT/mTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd 2017, 77, 1095–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Chen, H.; Du, J.; Wang, B.; Yang, L. Anticancer Activity of Metformin, an Antidiabetic Drug, Against Ovarian Cancer Cells Involves Inhibition of Cysteine-Rich 61 (Cyr61)/Akt/Mammalian Target of Rapamycin (mTOR) Signaling Pathway. Med. Sci. Monit. 2018, 24, 6093–6101. [Google Scholar] [CrossRef]
- Lee, K.B.; Byun, H.J.; Park, S.H.; Park, C.Y.; Lee, S.H.; Rho, S.B. CYR61 controls p53 and NF-κB expression through PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells. Cancer Lett. 2012, 315, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.L.; Zhang, Q.H.; Wang, X.W.; He, H. Antidiabetic drug metformin mitigates ovarian cancer SKOV3 cell growth by triggering G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1169–1175. [Google Scholar] [PubMed]
- Xu, S.; Yang, Z.; Jin, P.; Yang, X.; Li, X.; Wei, X.; Wang, Y.; Long, S.; Zhang, T.; Chen, G.; et al. Metformin Suppresses Tumor Progression by Inactivating Stromal Fibroblasts in Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 1291–1302. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zhu, J.; Zhang, H.; Liu, Y.; Sun, H. Metformin inhibits ovarian cancer growth and migration in vitro and in vivo by enhancing cisplatin cytotoxicity. Am. J. Transl. Res. 2018, 10, 3086–3098. [Google Scholar]
- Lengyel, E.; Litchfield, L.M.; Mitra, A.K.; Nieman, K.M.; Mukherjee, A.; Zhang, Y.; Johnson, A.; Bradaric, M.; Lee, W.; Romero, I.L. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am. J. Obstet. Gynecol. 2015, 212, 479.e1–479.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mert, I.; Chhina, J.; Allo, G.; Dai, J.; Seward, S.; Carey, M.S.; Llaurado, M.; Giri, S.; Rattan, R.; Munkarah, A.R. Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol. Oncol. 2017, 146, 319–326. [Google Scholar] [CrossRef]
- Hamedi, B.; Khalili, A.; Roozmeh, S.; Namazi, G.; Saraf, Z. Combination of Metformin and Chemotherapy Decreases the Recurrence Rates of Epithelial Ovarian Cancers: A Randomized Clinical Trial. Int. J. Cancer Manag. 2018, 11, e11621. [Google Scholar] [CrossRef]
- Facchinetti, F.; Orru, B.; Grandi, G.; Unfer, V. Short-term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2019, 35, 198–206. [Google Scholar] [CrossRef]
- Hameed, M.; Khan, K.; Salman, S.; Mehmood, N. Dose Comparison and Side Effect Profile of Metformin Extended Release Versus Metformin Immediate Release. J. Ayub Med. Coll. Abbottabad 2017, 29, 225–229. [Google Scholar]
- Haikala, H.M.; Anttila, J.M.; Marques, E.; Raatikainen, T.; Ilander, M.; Hakanen, H.; Ala-Hongisto, H.; Savelius, M.; Balboa, D.; Von Eyss, B.; et al. Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 3724. [Google Scholar] [CrossRef]
- Xue, J.; Li, L.; Li, N.; Li, F.; Qin, X.; Li, T.; Liu, M. Metformin suppresses cancer cell growth in endometrial carcinoma by inhibiting PD-L1. Eur. J. Pharmacol. 2019, 859, 172541. [Google Scholar] [CrossRef] [PubMed]
| Disease Condition | Treatment | Summary | Trial Phase Status | Clinical Trial Number |
|---|---|---|---|---|
| Advanced stage OvCa | Metformin with carboplatin/paclitaxel | mTOR pathway inhibition, p53-induced apoptosis. | Phase1 | NCT02312661 |
| Advanced stage OvCa | Paclitaxel, carboplatin, and oral metformin | Increased synergy without compromising patient tolerability. | Phase 2 | NCT02437812 |
| Advanced epithelial OvCa in Stages IIIa–-IV | Metformin, acetylsalicylic acid, olaparib, and letrozole | Women with advanced (stage IIIa-IV) OvCa of the histologic subtype high-grade serous carcinoma (HGSOC) are going through a diagnostic laparoscopy. They will receive treatment with a study agent for 10–14 days before surgery. The study is randomized and unblinded. | Early Phase 1 | NCT03378297 |
| Complex endometrial hyperplasia with atypia grade 1 endometrial endometrioid adenocarcinoma | Levonorgestrel and metformin | Metformin is an effective treatment for early-stage endometrial cancer and endometrial hyperplasia with atypia. | Phase 2 | NCT01686126 |
| Ovarian, fallopian tube, and primary peritoneal cancer | Metformin | To determine if metformin administered in combination with chemotherapy to women with advanced ovarian, primary peritoneal, or fallopian tube cancer will improve recurrence-free survival at 18 months compared to controls. | Phase 2 | NCT01579812 |
| Cancer | Metformin, atorvastatin, doxycycline, and mebendazole | To determine the effectiveness of a regimen of selected metabolic treatments for patients with cancer in a real-world setting and to conduct exploratory analysis on the relationship between the degree of response and changes in biochemical markers (such as glucose and lipid levels). | Phase 3 | NCT02201381 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, S.K.; Apata, T.; Singh, S.; McFadden, M.; Singh, R. Clinical Implication of Metformin in Relation to Diabetes Mellitus and Ovarian Cancer. Biomedicines 2021, 9, 1020. https://doi.org/10.3390/biomedicines9081020
Singh SK, Apata T, Singh S, McFadden M, Singh R. Clinical Implication of Metformin in Relation to Diabetes Mellitus and Ovarian Cancer. Biomedicines. 2021; 9(8):1020. https://doi.org/10.3390/biomedicines9081020
Chicago/Turabian StyleSingh, Santosh Kumar, Tejumola Apata, Shriti Singh, Melayshia McFadden, and Rajesh Singh. 2021. "Clinical Implication of Metformin in Relation to Diabetes Mellitus and Ovarian Cancer" Biomedicines 9, no. 8: 1020. https://doi.org/10.3390/biomedicines9081020
APA StyleSingh, S. K., Apata, T., Singh, S., McFadden, M., & Singh, R. (2021). Clinical Implication of Metformin in Relation to Diabetes Mellitus and Ovarian Cancer. Biomedicines, 9(8), 1020. https://doi.org/10.3390/biomedicines9081020








