Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans
Abstract
:1. Introduction
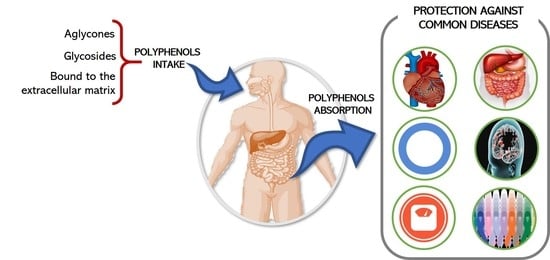
2. Absorption and Metabolism
2.1. Stomach
2.2. Intestine
2.3. Colon
3. Bioavailability
Experimental Studies
4. In Vitro Studies
4.1. Cardiovascular Disease
4.2. Diabetes
4.3. Obesity
4.4. Digestive Diseases
4.5. Neurodegenerative Disease
4.6. Cancer
5. Animal Models
5.1. Cardiovascular Disease
5.2. Diabetes Mellitus
5.3. Obesity
5.4. Neurodegenerative Diseases
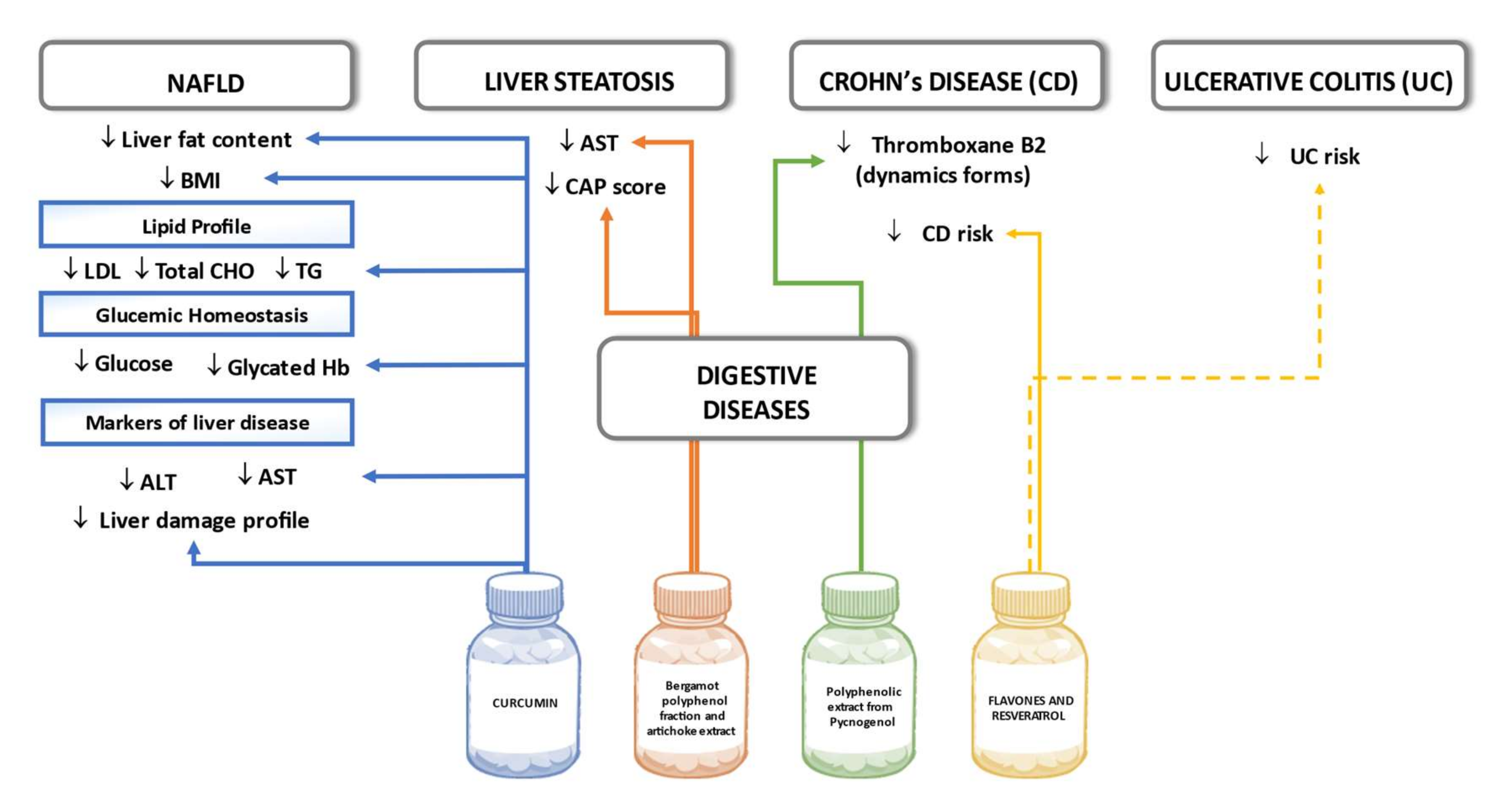
5.5. Digestive Diseases
5.6. Cancer
6. Human Studies
6.1. Cardiovascular Diseases
6.2. Diabetes Mellitus
6.3. Obesity
6.4. Neurodegenerative Diseases
6.5. Digestive Disease
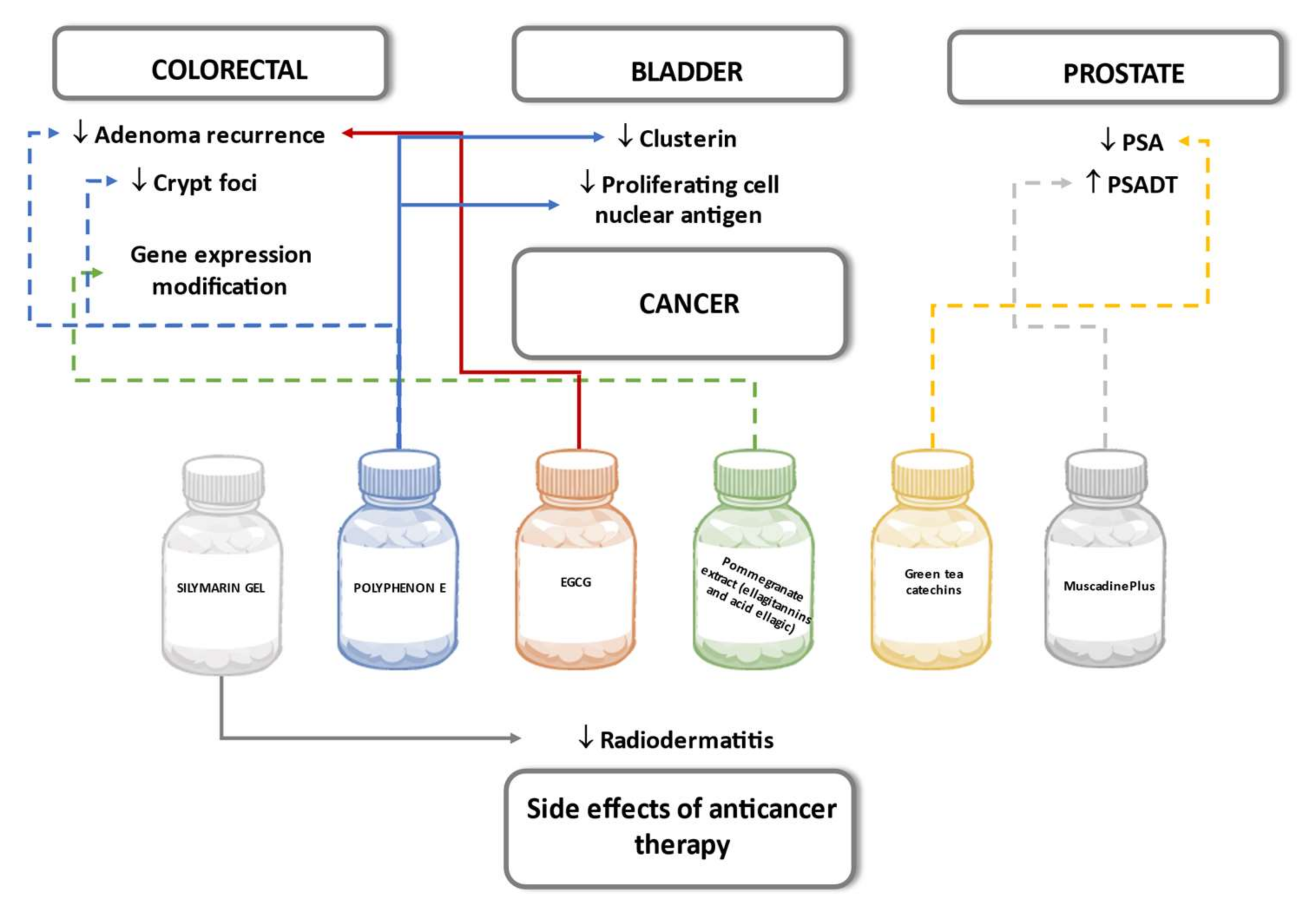
6.6. Cancer
6.6.1. Colorectal
6.6.2. Prostate
6.6.3. Bladder
6.6.4. Side Effects of Anticancer Therapy
7. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| AFAB2 | adipocyte fatty acid binding protein 2 |
| AKT | protein kinase B |
| BB | Davallia formosana |
| BP | blood pressure |
| BMI | body mass index |
| BRC | biochemically recurrent |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| EGCG | epigallocatechin-3-gallate |
| FOXO1 | forkhead box protein O 1 |
| IR | insulin resistance |
| LDL-C | low-density lipoprotein cholesterol |
| NAFLD | nonalcoholic fatty liver diseases |
| OGTT | oral glucose tolerance test |
| PI3K | phosphatidylinositol 3-kinase |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PSA | prostate-specific antigen |
| PSADT | PSA doubling time |
| SREBP1c | sterol regulatory element-binding protein 1 |
| T2DM | type 2 diabetes mellitus |
| TAS | total antioxidant status |
| TC | total cholesterol |
References
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Dell’istituto Super. Sanità 2007, 43, 348–361. [Google Scholar]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Mas-Bargues, C.; Gambini, J.; Gimeno-Mallench, L. Protective Effects of Polyphenols Present in Mediterranean Diet on Endothelial Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 2097096. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Bargues, C.M.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol:In VitroandIn VivoStudies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [Green Version]
- Gambini, J.; Gimeno-Mallench, L.; Olaso-Gonzalez, G.; Mastaloudis, A.; Traber, M.; Monleón, D.; Borrás, C.; Viña, J. Moderate Red Wine Consumption Increases the Expression of Longevity-associated Genes in Controlled Human Populations and Extends Lifespan in Drosophila melanogaster. Antioxidants 2021, 10, 301. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Iskandar, M.M.; Sleno, L.; Sabally, K.; Khairallah, J.; Prakash, S.; Kubow, S. Absorption and Metabolism of Phenolics from Digests of Polyphenol-Rich Potato Extracts Using the Caco-2/HepG2 Co-Culture System. Foods 2018, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Dhital, S.; Wu, P.; Chen, X.-D.; Gidley, M.J. In Vitro Digestion of Apple Tissue Using a Dynamic Stomach Model: Grinding and Crushing Effects on Polyphenol Bioaccessibility. J. Agric. Food Chem. 2019, 68, 574–583. [Google Scholar] [CrossRef]
- Ariza, M.T.; Reboredo-Rodríguez, P.; Cervantes, L.; Soria, C.; Martínez-Ferri, E.; González-Barreiro, C.; Cancho-Grande, B.; Battino, M.; Simal-Gándara, J. Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem. 2018, 248, 155–165. [Google Scholar] [CrossRef]
- Silberberg, M.; Morand, C.; Mathevon, T.; Besson, C.; Manach, C.; Scalbert, A.; Remesy, C. The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur. J. Nutr. 2006, 45, 88–96. [Google Scholar] [CrossRef]
- Ayrton, A.; Morgan, P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 2001, 31, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, R.P.; Mills, C.E.; Istas, G.; Heiss, C.; Rodriguez-Mateos, A. Absorption, Metabolism and Excretion of Cranberry (Poly)phenols in Humans: A Dose Response Study and Assessment of Inter-Individual Variability. Nutrients 2017, 9, 268. [Google Scholar] [CrossRef] [PubMed]
- Bokkenheuser, V.D.; Shackleton, C.H.; Winter, J. Hydrolysis of dietary flavonoid glycosides by strains of intestinal Bacteroides from humans. Biochem. J. 1987, 248, 953–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, H.; Simmering, R.; Hartmann, L.; Pforte, H.; Blaut, M. Degradation of quercetin-3-glucoside in gnotobiotic rats associated with human intestinal bacteria. J. Appl. Microbiol. 2000, 89, 1027–1037. [Google Scholar] [CrossRef]
- Déprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [CrossRef] [Green Version]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Williamson, G. Solubilisation of Ferulic Acid from Plant Cell Wall Materials in a Model Human Gut System. Biochem. Soc. Trans. 1996, 24, 384S. [Google Scholar] [CrossRef] [Green Version]
- Aziz, A.A.; Edwards, C.A.; Lean, M.E.; Crozier, A. Absorption and excretion of conjugated flavonols, including quercetin-4′-O-β-glucoside and isorhamnetin-4′-O-β-glucoside by human volunteers after the consumption of onions. Free Radic. Res. 1998, 29, 257–269. [Google Scholar] [CrossRef]
- Hollman, P.C.; Van Trijp, J.; Mengelers, M.J.; de Vries, J.H.; Katan, M.B. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Lett. 1997, 114, 139–140. [Google Scholar] [CrossRef]
- Nesbitt, P.D.; Lam, Y.; Thompson, L.U. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am. J. Clin. Nutr. 1999, 69, 549–555. [Google Scholar] [CrossRef]
- Nitsa, A.; Toutouza, M.; Machairas, N.; Mariolis, A.; Philippou, A.; Koutsilieris, M. Vitamin D in Cardiovascular Disease. Vivo 2018, 32, 977–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Huang, Y.; Zheng, W.; Yan, J.; Cheng, M.; Zhao, R.; Chen, L.; Hu, C.; Jia, W. Resveratrol reduces intracellular reactive oxygen species levels by inducing autophagy through the AMPK-mTOR pathway. Front. Med. 2018, 12, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Cruz, E.; Cerezo, A.B.; Cantos-Villar, E.; Richard, T.; Troncoso, A.M.; Parrilla, M.C.G. Inhibition of VEGFR-2 Phosphorylation and Effects on Downstream Signaling Pathways in Cultivated Human Endothelial Cells by Stilbenes from Vitis Spp. J. Agric. Food Chem. 2019, 67, 3909–3918. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, P.; Xu, S.; Koroleva, M.; Zhang, S.; Si, S.; Jin, Z.G. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci. Rep. 2017, 7, 6686. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Chen, Y.; Wang, J.; Qiu, J.; Chang, C.; Bi, F.; Wu, X.; Liu, W. EGCG protects vascular endothelial cells from oxidative stress-induced damage by targeting the autophagy-dependent PI3K-AKT-mTOR pathway. Ann. Transl. Med. 2020, 8, 200. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, S.S.; He, Y.; Bi, X.Y.; Gao, S.; Yan, T.D.; Zheng, G.D.; Chen, T.T.; Ye, J.T.; Liu, P.Q. EGCG inhibits pressure overload-induced cardiac hypertrophy via the PSMB5/Nmnat2/SIRT6-dependent signalling pathways. Acta Physiol. 2021, 231, e13602. [Google Scholar] [CrossRef]
- Checkouri, E.; Ramin-Mangata, S.; Diotel, N.; Viranaicken, W.; Marodon, C.; Reignier, F.; Silva, C.; Meilhac, O. Protective Effects of Medicinal Plant Decoctions on Macrophages in the Context of Atherosclerosis. Nutrients 2021, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Yan, Z.; Xu, T.T.; Wu, X.; Pi, A.; Liu, Q.; Chai, H.; Li, S.; Dou, X. Inhibition of TLR4/MAPKs Pathway Contributes to the Protection of Salvianolic Acid A Against Lipotoxicity-Induced Myocardial Damage in Cardiomyocytes and Obese Mice. Front. Pharmacol. 2021, 12, 627123. [Google Scholar] [CrossRef] [PubMed]
- Pham-Hua, D.; Padgett, L.E.; Xue, B.; Anderson, B.; Zeiger, M.; Barra, J.M.; Bethea, M.; Hunter, C.S.; Kozlovskaya, V.; Kharlampieva, E.; et al. Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking. Biomaterials 2017, 128, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Venkateswaran, M.; Jayabal, S.; Hemaiswarya, S.; Murugesan, S.; Enkateswara, S.; Doble, M.; Periyasamy, S. Polyphenol-rich Indian ginger cultivars ameliorate GLUT4 activity in C2C12 cells, inhibit diabetes-related enzymes and LPS-induced inflammation: An in vitro study. J. Food Biochem. 2021, 45, e13600. [Google Scholar] [CrossRef]
- Gomes, J.H.D.S.; Mbiakop, U.C.; Oliveira, R.L.; Stehmann, J.R.; de Pádua, R.M.; Cortes, S.F.; Braga, F.C. Polyphenol-rich extract and fractions of Terminalia phaeocarpa Eichler possess hypoglycemic effect, reduce the release of cytokines, and inhibit lipase, α-glucosidase, and α-amilase enzymes. J. Ethnopharmacol. 2021, 271, 113847. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Naimi, M.; Murphy, B.; Hudlicky, T.; Tsiani, E. Rosmarinic Acid, a Rosemary Extract Polyphenol, Increases Skeletal Muscle Cell Glucose Uptake and Activates AMPK. Molecules 2017, 22, 1669. [Google Scholar] [CrossRef] [Green Version]
- Les, F.; Arbones-Mainar, J.M.; Valero, M.S.; López, V. Pomegranate polyphenols and urolithin A inhibit α-glucosidase, dipeptidyl peptidase-4, lipase, triglyceride accumulation and adipogenesis related genes in 3T3-L1 adipocyte-like cells. J. Ethnopharmacol. 2018, 220, 67–74. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1ɑ Signaling Pathway by Ginger ( Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Park, M.; Sharma, A.; Kim, K.; Lee, H.-J. Black Ginseng and Ginsenoside Rb1 Promote Browning by Inducing UCP1 Expression in 3T3-L1 and Primary White Adipocytes. Nutrients 2019, 11, 2747. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.; Rehman, A.U.; Luckett, D.J.; Blanchard, C.L.; Obied, H.K.; Strappe, P. Phenolic Compounds with Antioxidant Properties from Canola Meal Extracts Inhibit Adipogenesis. Int. J. Mol. Sci. 2019, 21, 1. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Hochstetter, D.; Yao, L.; Zhao, Y.; Zhou, J.; Wang, Y.; Xu, P. Green Tea Polyphenol (-)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 5081. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.; Song, Z.; Shao, W.; Du, W.W.; Zhao, L.R.; Zeng, K.; Yang, B.B.; Jin, T. Curcumin represses mouse 3T3-L1 cell adipogenic differentiation via inhibiting miR-17-5p and stimulating the Wnt signalling pathway effector Tcf7l2. Cell Death Dis. 2018, 8, e2559. [Google Scholar] [CrossRef] [Green Version]
- Carpéné, C.; Pejenaute, H.; del Moral, R.; Boulet, N.; Hijona, E.; Andrade, F.; Villanueva-Millán, M.J.; Aguirre, L.; Arbones-Mainar, J.M. The Dietary Antioxidant Piceatannol Inhibits Adipogenesis of Human Adipose Mesenchymal Stem Cells and Limits Glucose Transport and Lipogenic Activities in Adipocytes. Int. J. Mol. Sci. 2018, 19, 2081. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; He, S.; Yin, P.; Li, D.; Mei, C.; Yu, X.; Shi, Y.; Jiang, L.; Liu, F. Punicalagin induces Nrf2 translocation and HO-1 expression via PI3K/Akt, protecting rat intestinal epithelial cells from oxidative stress. Int. J. Hyperth. 2016, 32, 465–473. [Google Scholar] [CrossRef]
- Guo, R.; Zhao, B.; Wang, Y.; Wu, D.; Wang, Y.; Yu, Y.; Yan, Y.; Zhang, W.; Liu, Z.; Liu, X. Cichoric Acid Prevents Free-Fatty-Acid-Induced Lipid Metabolism Disorders via Regulating Bmal1 in HepG2 Cells. J. Agric. Food Chem. 2018, 66, 9667–9678. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; An, J.M.; Han, Y.M.; Surh, Y.J.; Hwang, S.J.; Kim, S.J.; Hahm, K.B. Walnut polyphenol extracts inhibit Helicobacter pylori-induced STAT3Tyr705 phosphorylation through activation of PPAR-γ and SOCS1 induction. J. Clin. Biochem. Nutr. 2020, 67, 20–89. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.J.; Cho, J.Y.; Kim, D. PDK1 in NF-?B signaling is a target of Xanthium strumarium methanolic extract-mediated anti-inflammatory activities. J. Ethnopharmacol. 2016, 190, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; de Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [Green Version]
- Dias, R.J.C.; Brás, N.F.; Gregorio, M.R.P.; Fernandes, I.; Mateus, N.; Freitas, V. A multi-spectroscopic study on the interaction of food polyphenols with a bioactive gluten peptide: From chemistry to biological implications. Food Chem. 2019, 299, 125051. [Google Scholar] [CrossRef]
- Kim, H.; Baek, S.; Sok, D.-E.; Lee, K.; Kim, Y.-J.; Kim, M. Neuroprotective Activity of Polyphenol-Rich Ribes diacanthum Pall against Oxidative Stress in Glutamate-Stimulated HT-22 Cells and a Scopolamine-Induced Amnesia Animal Model. Antioxidants 2020, 9, 895. [Google Scholar] [CrossRef]
- Hou, Y.; Peng, S.; Li, X.; Yao, J.; Xu, J.; Fang, J. Honokiol Alleviates Oxidative Stress-Induced Neurotoxicity via Activation of Nrf2. ACS Chem. Neurosci. 2018, 9, 3108–3116. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective Effects of Bioavailable Polyphenol-Derived Metabolites against Oxidative Stress-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Wang, S.; Ma, F.; Zhang, Y.; Peng, Y.; Xing, C.; Feng, Y.; Wang, X.; Peng, Y. From stroke to neurodegenerative diseases: The multi-target neuroprotective effects of 3-n-butylphthalide and its derivatives. Pharmacol. Res. 2018, 135, 201–211. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Liu, T.; Ma, Y.; Huang, S.; Lei, L.; Wen, A.; Ding, Y. Resveratrol: Multi-Targets Mechanism on Neurodegenerative Diseases Based on Network Pharmacology. Front. Pharmacol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Arús, B.; Souza, D.G.; Bellaver, B.; Souza, D.; Gonçalves, C.-A.; Quincozes-Santos, A.; Bobermin, L.D. Resveratrol modulates GSH system in C6 astroglial cells through heme oxygenase 1 pathway. Mol. Cell. Biochem. 2017, 428, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Hu, J.; Ma, Q.; Xu, Y.; Zhang, Y.; Zhang, F.; Liu, Y. Tea polyphenols induce S phase arrest and apoptosis in gallbladder cancer cells. Braz. J. Med Biol. Res. 2018, 51, e6891. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Yan, F.; Zhao, Y.; Chen, X.; Sun, S.; Wang, Y.; Ying, L. Green Tea Polyphenol EGCG Attenuates MDSCs-mediated Immunosuppression through Canonical and Non-Canonical Pathways in a 4T1 Murine Breast Cancer Model. Nutrients 2020, 12, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyza, J.R.; Arora, S.; Zhang, H.; Conner, K.L.; Lei, W.; Floyd, A.M.; Deshmukh, R.R.; Sarver, J.; Trabbic, C.J.; Erhardt, P.; et al. Targeting the DNA Repair Endonuclease ERCC1-XPF with Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCG) and Its Prodrug to Enhance Cisplatin Efficacy in Human Cancer Cells. Nutrients 2018, 10, 1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.; Correia-Branco, A.; Andrade, N.; Ferreira, A.C.; Soares, M.L.; Sonveaux, P.; Stephenne, J.; Martel, F. Selective pro-apoptotic and antimigratory effects of polyphenol complex catechin:lysine 1:2 in breast, pancreatic and colorectal cancer cell lines. Eur. J. Pharmacol. 2019, 859, 172533. [Google Scholar] [CrossRef]
- D’Angelo, S.; Martino, E.; Ilisso, C.P.; Bagarolo, M.L.; Porcelli, M.; Cacciapuoti, G. Pro-oxidant and pro-apoptotic activity of polyphenol extract from Annurca apple and its underlying mechanisms in human breast cancer cells. Int. J. Oncol. 2017, 51, 939–948. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Cheng, M.; Zhang, W.; He, R.; Yang, H. New Insights into the Mechanisms of Polyphenol from Plum Fruit Inducing Apoptosis in Human Lung Cancer A549 Cells via PI3K/AKT/FOXO1 Pathway. Plant Foods Hum. Nutr. 2021, 76, 125–132. [Google Scholar] [CrossRef]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; La Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [Green Version]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018, 12, 1753465817750524. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Sun, J.; Mohammadtursun, N.; Wu, J.; Dong, J.; Li, L. Curcumin inhibits cigarette smoke-induced inflammation via modulating the PPARγ-NF-κB signaling pathway. Food Funct. 2019, 10, 7983–7994. [Google Scholar] [CrossRef]
- Lv, Y.-L.; Jia, Y.; Wan, Z.; An, Z.-L.; Yang, S.; Han, F.-F.; Gong, L.-L.; Xuan, L.-L.; Ren, L.-L.; Zhang, W.; et al. Curcumin inhibits the formation of atherosclerosis in ApoE mice by suppressing cytomegalovirus activity in endothelial cells. Life Sci. 2020, 257, 117658. [Google Scholar] [CrossRef]
- Masodsai, K.; Lin, Y.-Y.; Chaunchaiyakul, R.; Su, C.-T.; Lee, S.-D.; Yang, A.-L. Twelve-Week Protocatechuic Acid Administration Improves Insulin-Induced and Insulin-Like Growth Factor-1-Induced Vasorelaxation and Antioxidant Activities in Aging Spontaneously Hypertensive Rats. Nutrients 2019, 11, 699. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Zhang, D.-L.; Jia, M.; Hao, W.-H.; Li, Y.-J. Mangiferin inhibits high-fat diet induced vascular injury via regulation of PTEN/AKT/eNOS pathway. J. Pharmacol. Sci. 2018, 137, 265–273. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Zhang, S.; Yang, C.; Mai, Z.; Hu, X.; Gao, Z.; Deng, H. Anti-myocardial Ischemia Effect and Components of Litchi Pericarp Extracts. Phytother. Res. 2017, 31, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Wu, J.-B.; Jian, J.-Y.; Shih, C.-C. (−)-Epicatechin-3-O-β-D-allopyranoside from Davallia formosana prevents diabetes and dyslipidemia in streptozotocin-induced diabetic mice. PLoS ONE 2017, 12, e0173984. [Google Scholar] [CrossRef]
- Iftikhar, A.; Aslam, B.; Iftikhar, M.; Majeed, W.; Batool, M.; Zahoor, B.; Amna, N.; Gohar, H.; Latif, I. Effect of Caesalpinia bonduc Polyphenol Extract on Alloxan-Induced Diabetic Rats in Attenuating Hyperglycemia by Upregulating Insulin Secretion and Inhibiting JNK Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 9020219. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Cheng, H.-Y.; Chang, M.-L.; Huang, S.-S.; Liao, J.-W.; Cheng, Y.-C.; Peng, W.-H.; Pao, L.-H. Gallic Acid Ameliorated Impaired Lipid Homeostasis in a Mouse Model of High-Fat Diet—and Streptozotocin-Induced NAFLD and Diabetes through Improvement of β-oxidation and Ketogenesis. Front. Pharmacol. 2021, 11, 606759. [Google Scholar] [CrossRef] [PubMed]
- Polce, S.A.; Burke, C.; França, L.M.; Kramer, B.; Paes, A.M.D.A.; Carrillo-Sepulveda, M.A. Ellagic Acid Alleviates Hepatic Oxidative Stress and Insulin Resistance in Diabetic Female Rats. Nutrients 2018, 10, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-W.; Chen, Y.-J.; Chang, Y.-C.; Chang, S.-J. Oligonol, a Low-Molecular Weight Polyphenol Derived from Lychee, Alleviates Muscle Loss in Diabetes by Suppressing Atrogin-1 and MuRF1. Nutrients 2017, 9, 1040. [Google Scholar] [CrossRef]
- Gimeno-Mallench, L.; Bargues, C.M.; Inglés, M.; Olaso, G.; Borras, C.; Gambini, J.; Vina, J. Resveratrol shifts energy metabolism to increase lipid oxidation in healthy old mice. Biomed. Pharmacother. 2019, 118, 109130. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.-Y.; Wei, Y.-L.; Hao, J.-Y.; Lei, Y.-Q.; Zhao, W.-B.; Xiao, Y.-H.; Sun, A.-D. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. 2020, 17, 54. [Google Scholar] [CrossRef]
- Tuzcu, Z.; Orhan, C.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Inhibits Hyperlipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1583098. [Google Scholar] [CrossRef]
- Kim, Y.; Rouse, M.; González-Mariscal, I.; Egan, J.M.; O’Connell, J.F. Dietary curcumin enhances insulin clearance in diet-induced obese mice via regulation of hepatic PI3K-AKT axis and IDE, and preservation of islet integrity. Nutr. Metab. 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Lee, H.G.; Seo, K.-H.; Yokoyama, W.; Kim, H. Antiobesity Effect of Prebiotic Polyphenol-Rich Grape Seed Flour Supplemented with Probiotic Kefir-Derived Lactic Acid Bacteria. J. Agric. Food Chem. 2018, 66, 12498–12511. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J.; Kang, H.; Yun, S.P.; Lee, Y.-S.; Lee, Y. Activation of the Akt1-CREB pathway promotes RNF146 expression to inhibit PARP1-mediated neuronal death. Sci. Signal. 2020, 13, eaax7119. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Misawa, K.; Nishimura, H.; Hirata, T.; Yamamoto, M.; Ota, N. 5-Caffeoylquinic Acid Ameliorates Cognitive Decline and Reduces Aβ Deposition by Modulating Aβ Clearance Pathways in APP/PS2 Transgenic Mice. Nutrients 2020, 12, 494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Liu, W.; Zhou, Y.; Mo, Y.; Liu, Y.; Chen, X.; Pan, H.; Yuan, D.; Wang, Q.; Chen, T. Enhancement of oral bioavailability and anti-Parkinsonian efficacy of resveratrol through a nanocrystal formulation. Asian J. Pharm. Sci. 2020, 15, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lv, R.; Wang, L.; Hou, H.; Liu, H.; Shao, S. Resveratrol, an antioxidant, protects spinal cord injury in rats by suppressing MAPK pathway. Saudi J. Biol. Sci. 2018, 25, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.-S.; Li, S.-R.; Li, K.; Li, X.; Yin, X.; Pang, Z. Ellagic acid improves endogenous neural stem cells proliferation and neurorestoration through Wnt/β-catenin signaling in vivo and in vitro. Mol. Nutr. Food Res. 2017, 61, 1600587. [Google Scholar] [CrossRef] [PubMed]
- Badi, R.M.; Mostafa, D.G.; Khaleel, E.F.; Satti, H.H. Resveratrol protects against hepatic insulin resistance in a rat’s model of non-alcoholic fatty liver disease by down-regulation of GPAT -1 and DGAT 2 expression and inhibition of PKC membranous translocation. Clin. Exp. Pharmacol. Physiol. 2019, 46, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, L.; Li, W.; Dai, T.; Nie, L.; Xie, J.; Ai, Y.; Li, L.; Tian, Y.; Sheng, J. Polyphenol Extract of Moringa Oleifera Leaves Alleviates Colonic Inflammation in Dextran Sulfate Sodium-Treated Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 6295402. [Google Scholar] [CrossRef]
- Duan, J.; Zhan, J.-C.; Wang, G.-Z.; Zhao, X.-C.; Huang, W.-D.; Zhou, G.-B. The red wine component ellagic acid induces autophagy and exhibits anti-lung cancer activity in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ren, F.; Li, B.; Song, Z.; Chen, P.; Ouyang, L. Ellagic acid exerts antitumor effects via the PI3K signaling pathway in endometrial cancer. J. Cancer 2019, 10, 3303–3314. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, J.; Gan, R.; Wu, Z.; Luo, H.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Synergistic inhibition of lung cancer cells by EGCG and NF-κB inhibitor BAY11-7082. J. Cancer 2019, 10, 6543–6556. [Google Scholar] [CrossRef]
- Nemec, M.J.; Kim, H.; Marciante, A.B.; Barnes, R.C.; Hendrick, E.D.; Bisson, W.H.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenolics from mango (Mangifera indica L.) suppress breast cancer ductal carcinoma in situ proliferation through activation of AMPK pathway and suppression of mTOR in athymic nude mice. J. Nutr. Biochem. 2017, 41, 12–19. [Google Scholar] [CrossRef]
- Zeriouh, W.; Nani, A.; Belarbi, M.; Dumont, A.; De Rosny, C.; Aboura, I.; Ghanemi, F.Z.; Murtaza, B.; Patoli, D.; Thomas, C.; et al. Phenolic extract from oleaster (Olea europaea var. Sylvestris) leaves reduces colon cancer growth and induces caspase-dependent apoptosis in colon cancer cells via the mitochondrial apoptotic pathway. PLoS ONE 2017, 12, e0170823. [Google Scholar] [CrossRef]
- Pan, Z.; Zhuang, J.; Ji, C.; Cai, Z.; Liao, W. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol. Lett. 2018, 15, 4821–4826. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, S.; Michaels, H.A.; Quadros, A.S.; Qian, Y.; Rabovsky, A.B.; Badger, R.S.; Jalili, T. A combination of isolated phytochemicals and botanical extracts lowers diastolic blood pressure in a randomized controlled trial of hypertensive subjects. Eur. J. Clin. Nutr. 2015, 70, 10–16. [Google Scholar] [CrossRef]
- Loo, B.-M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of chokeberry ( Aronia mitschurinii ) products modestly lowered blood pressure and reduced low-grade inflammation in patients with mildly elevated blood pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef]
- Valls, R.M.; Llauradó, E.; Fernández-Castillejo, S.; Puiggrós, F.; Solà, R.; Arola, L.; Pedret, A. Effects of low molecular weight procyanidin rich extract from french maritime pine bark on cardiovascular disease risk factors in stage-1 hypertensive subjects: Randomized, double-blind, crossover, placebo-controlled intervention trial. Phytomedicine 2016, 23, 1451–1461. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macarro, M.S.; Rodríguez, J.P.M.; Morell, E.B.; Pérez-Piñero, S.; Victoria-Montesinos, D.; García-Muñoz, A.M.; García, F.C.; Sánchez, J.C.; López-Román, F.J. Effect of a Combination of Citrus Flavones and Flavanones and Olive Polyphenols for the Reduction of Cardiovascular Disease Risk: An Exploratory Randomized, Double-Blind, Placebo-Controlled Study in Healthy Subjects. Nutrients 2020, 12, 1475. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.; Mathison, B.; Kimble, L.; McKay, D.; Kaspar, K.; Khoo, C.; Chen, C.-Y.O.; Blumberg, J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 1223–1235. [Google Scholar] [CrossRef]
- Tenore, G.C.; D’Avino, M.; Caruso, D.; Buonomo, G.; Acampora, C.; Caruso, G.; Simone, C.; Ciampaglia, R.; Novellino, E. Effect of Annurca Apple Polyphenols on Intermittent Claudication in Patients With Peripheral Artery Disease. Am. J. Cardiol. 2018, 123, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ponzo, V.; Evangelista, A.; Ciccone, G.; Goitre, I.; Saba, F.; Procopio, M.; Cassader, M.; Gambino, R. Effects of 6 months of resveratrol versus placebo on pentraxin 3 in patients with type 2 diabetes mellitus: A double-blind randomized controlled trial. Acta Diabetol. 2017, 54, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The effects of curcumin supplementation on high-sensitivity C-reactive protein, serum adiponectin, and lipid profile in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Shoji, T.; Yamada, M.; Miura, T.; Nagashima, K.; Ogura, K.; Inagaki, N.; Maeda-Yamamoto, M. Chronic administration of apple polyphenols ameliorates hyperglycaemia in high-normal and borderline subjects: A randomised, placebo-controlled trial. Diabetes Res. Clin. Pract. 2017, 129, 43–51. [Google Scholar] [CrossRef]
- Paquette, M.; Larqué, A.S.M.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and cranberry polyphenols improve insulin sensitivity in insulin-resistant, non-diabetic adults: A parallel, double-blind, controlled and randomised clinical trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [Green Version]
- Herranz-López, M.; Olivares-Vicente, M.; Boix-Castejón, M.; Caturla, N.; Roche, E.; Micol, V. Differential effects of a combination of Hibiscus sabdariffa and Lippia citriodora polyphenols in overweight/obese subjects: A randomized controlled trial. Sci. Rep. 2019, 9, 2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boix-Castejón, M.; Herranz-López, M.; Gago, A.P.; Olivares-Vicente, M.; Caturla, N.; Roche, E.; Micol, V. Hibiscus and lemon verbena polyphenols modulate appetite-related biomarkers in overweight subjects: A randomized controlled trial. Food Funct. 2018, 9, 3173–3184. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-J.; Sharma, A.; Bae, M.H.; Sung, H.C.; Kim, N.K.; Sung, E.; Lee, H.-J. Efficacy and Safety of Sinetrol-XPur on Weight and Body Fat Reduction in Overweight or Obese Adults: A 12-Week, Randomized, Double-Blind, Parallel, Placebo-Controlled Trial. J. Med. Food 2020, 23, 335–342. [Google Scholar] [CrossRef]
- Ashigai, H.; Taniguchi, Y.; Suzuki, M.; Ikeshima, E.; Kanaya, T.; Zembutsu, K.; Tomita, S.; Miyake, M.; Fukuhara, I. Fecal lipid excretion after consumption of a black tea polyphenol containing beverage. Biol. Pharm. Bull. 2016, 39, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Capomolla, A.S.; Janda, E.; Paone, S.; Parafati, M.; Sawicki, T.; Mollace, R.; Ragusa, S.; Mollace, V. Atherogenic Index Reduction and Weight Loss in Metabolic Syndrome Patients Treated with A Novel Pectin-Enriched Formulation of Bergamot Polyphenols. Nutrients 2019, 11, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala-Vila, A.; Valls-Pedret, C.; Rajaram, S.; Coll-Padrós, N.; Cofán, M.; Serra-Mir, M.; Pérez-Heras, A.M.; Roth, I.; Freitas-Simoes, T.M.; Doménech, M.; et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: A randomized controlled trial. Am. J. Clin. Nutr. 2020, 111, 590–600. [Google Scholar] [CrossRef]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Maaß, S.; Schuberth, M.; Giese, A.; Oertel, W.H.; Poewe, W.; Trenkwalder, C.; Wenning, G.K.; Mansmann, U.; Südmeyer, M.; et al. Safety and efficacy of epigallocatechin gallate in multiple system atrophy (PROMESA): A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2019, 18, 724–735. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Nezhad, M.A.; Gh, B.F.N.M. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Stud. Biomark. New Targets Aging Res. Iran 2021, 1308, 25–35. [Google Scholar] [CrossRef]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Kolacek, M.; Paduchova, Z.; Dvorakova, M.; Zitnanova, I.; Cierna, I.; Durackova, Z.; Muchova, J. Effect of natural polyphenols on thromboxane levels in children with Crohn’s disease. Bratisl. Med. J. 2019, 120, 924–928. [Google Scholar] [CrossRef]
- Lu, Y.; Zamora-Ros, R.; Chan, S.S.M.; Cross, A.J.; Ward, H.; Jakszyn, P.; Luben, R.; Opstelten, J.L.; Oldenburg, B.; Hallmans, G.; et al. Dietary Polyphenols in the Aetiology of Crohnʼs Disease and Ulcerative Colitis—A Multicenter European Prospective Cohort Study (EPIC). Inflamm. Bowel Dis. 2017, 23, 2072–2082. [Google Scholar] [CrossRef] [Green Version]
- Filippini, T.; Malavolti, M.; Borrelli, F.; Izzo, A.A.; Fairweather-Tait, S.J.; Horneber, M.; Vinceti, M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst. Rev. 2020, 2020, CD005004. [Google Scholar] [CrossRef] [Green Version]
- Sinicrope, F.A.; Viggiano, T.R.; Buttar, N.S.; Song, L.M.W.K.; Schroeder, K.W.; Kraichely, R.E.; Larson, M.V.; Sedlack, R.E.; Kisiel, J.B.; Gostout, C.J.; et al. Randomized Phase II Trial of Polyphenon E versus Placebo in Patients at High Risk of Recurrent Colonic Neoplasia. Cancer Prev. Res. 2021, 14, 573–580. [Google Scholar] [CrossRef]
- Seufferlein, T.; Ettrich, T.; Menzler, S.; Messmann, H.; Kleber, G.; Zipprich, A.; Frank-Gleich, S.; Algül, H.; Metter, K.; Odemar, F.; et al. MIRACLE: Green tea extract versus placebo for the prevention of colorectal adenomas: A randomized, controlled trial. Ann. Oncol. 2019, 30, v869. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; González-Sarrías, A.; Villalba, R.G.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; et al. Gene expression changes in colon tissues from colorectal cancer patients following the intake of an ellagitannin-containing pomegranate extract: A randomized clinical trial. J. Nutr. Biochem. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Lane, J.A.; Er, V.; Avery, K.N.; Horwood, J.; Cantwell, M.; Caro, G.P.; Crozier, A.; Smith, G.D.; Donovan, J.L.; Down, L.; et al. ProDiet: A Phase II Randomized Placebo-controlled Trial of Green Tea Catechins and Lycopene in Men at Increased Risk of Prostate Cancer. Cancer Prev. Res. 2018, 11, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Paller, C.J.; Zhou, X.C.; Heath, E.I.; Taplin, M.-E.; Mayer, T.; Stein, M.N.; Bubley, G.J.; Pili, R.; Hudson, T.S.; Kakarla, R.; et al. Muscadine Grape Skin Extract (MPX) in Men with Biochemically Recurrent Prostate Cancer: A Randomized, Multicenter, Placebo-Controlled Clinical Trial. Clin. Cancer Res. 2018, 24, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gee, J.R.; Saltzstein, D.R.; Kim, K.; Kolesar, J.; Huang, W.; Havighurst, T.C.; Wollmer, B.W.; Stublaski, J.; Downs, T.; Mukhtar, H.; et al. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev. Res. 2017, 10, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karbasforooshan, H.; Hosseini, S.; Elyasi, S.; Pakdel, A.F.; Karimi, G. Topical silymarin administration for prevention of acute radiodermatitis in breast cancer patients: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Elyasi, S.; Shojaee, F.S.R.; Allahyari, A.; Karimi, G. Topical Silymarin Administration for Prevention of Capecitabine-Induced Hand-Foot Syndrome: A Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Phytother. Res. 2017, 31, 1323–1329. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stromsnes, K.; Lagzdina, R.; Olaso-Gonzalez, G.; Gimeno-Mallench, L.; Gambini, J. Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans. Biomedicines 2021, 9, 1074. https://doi.org/10.3390/biomedicines9081074
Stromsnes K, Lagzdina R, Olaso-Gonzalez G, Gimeno-Mallench L, Gambini J. Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans. Biomedicines. 2021; 9(8):1074. https://doi.org/10.3390/biomedicines9081074
Chicago/Turabian StyleStromsnes, Kristine, Rudite Lagzdina, Gloria Olaso-Gonzalez, Lucia Gimeno-Mallench, and Juan Gambini. 2021. "Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans" Biomedicines 9, no. 8: 1074. https://doi.org/10.3390/biomedicines9081074
APA StyleStromsnes, K., Lagzdina, R., Olaso-Gonzalez, G., Gimeno-Mallench, L., & Gambini, J. (2021). Pharmacological Properties of Polyphenols: Bioavailability, Mechanisms of Action, and Biological Effects in In Vitro Studies, Animal Models, and Humans. Biomedicines, 9(8), 1074. https://doi.org/10.3390/biomedicines9081074