Standardized Method for the Assessment of Behavioral Responses of Zebrafish Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Animals and Husbandry
2.2. Experimental Protocol
2.3. Statistics
3. Results
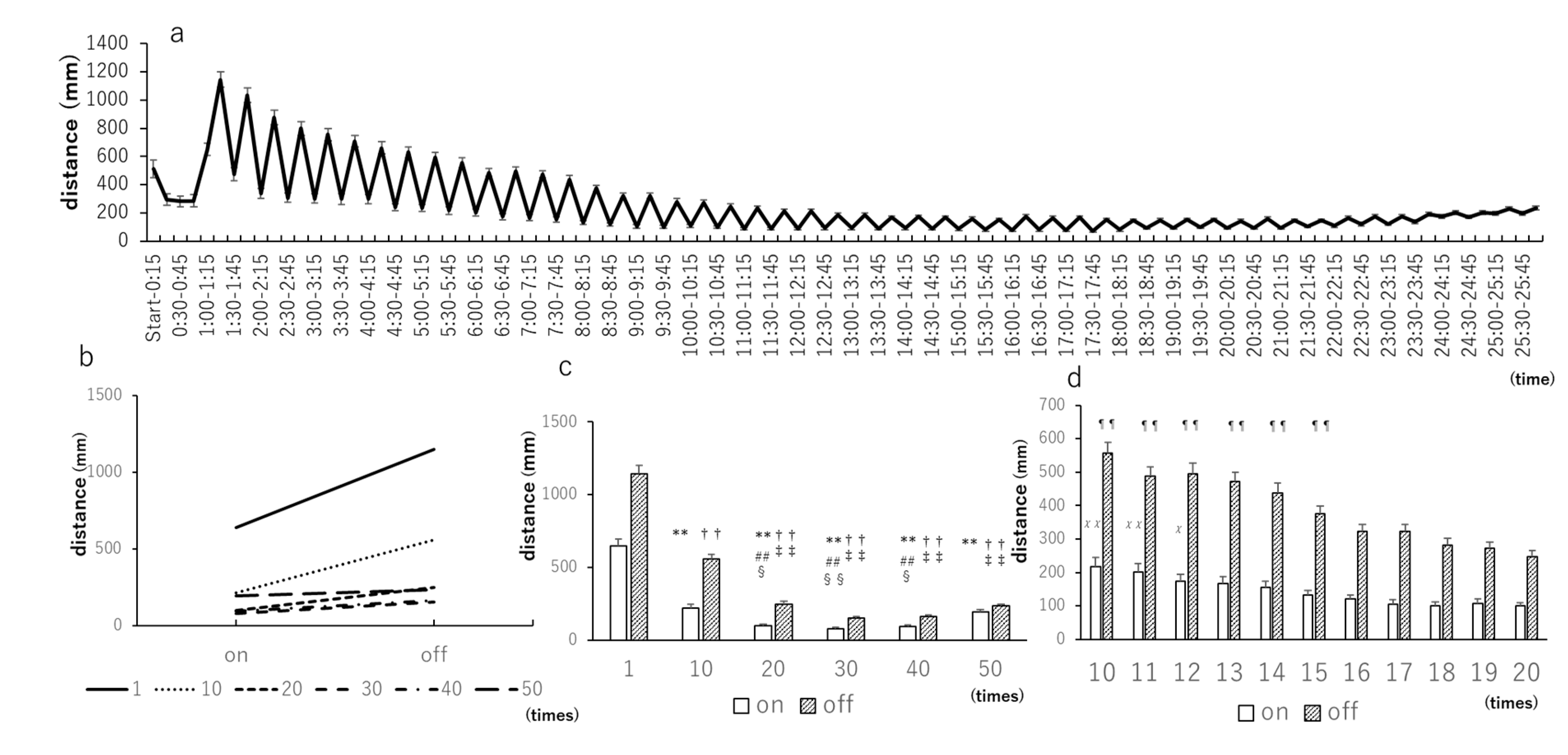
3.1. Light–Dark Tests with 15-Min Intervals
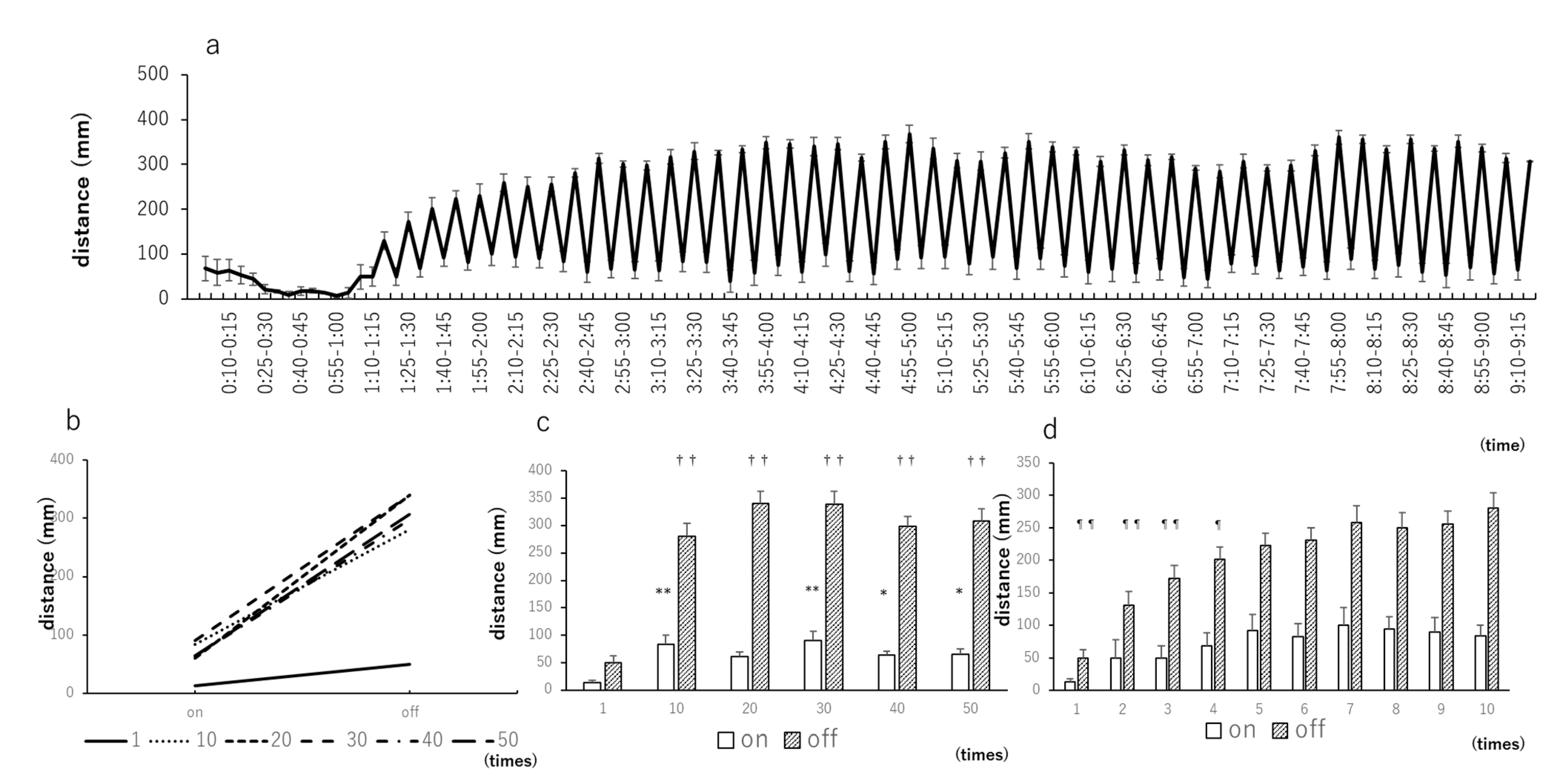
3.2. Light–Dark Tests with 5-Min Intervals
4. Discussion
4.1. Fear and Anxiety in Fish
4.2. Light–Dark Stimulation Test of Zebrafish Larvae
4.3. Number of Light and Dark Stimuli Repetitions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Maximino, C.; Lima, M.G.; Olivera, K.R.; Picanço, D.L.; Herculano, A.M. Adenosine A1, but not A2, receptor blockade in-creases anxiety and arousal in Zebrafish. Basic Clin. Pharmacol. Toxicol. 2011, 109, 203–207. [Google Scholar] [CrossRef]
- Stewart, A.M.; Kalueff, A.V. The developing utility of zebrafish models for cognitive enhancers research. Curr. Neuropharmacol. 2012, 10, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Irons, T.; MacPhail, R.; Hunter, D.; Padilla, S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol. Teratol. 2010, 32, 84–90. [Google Scholar] [CrossRef]
- Maximino, C.; de Brito, T.M.; Dias, C.A.G.D.M.; Jr, A.G.; Morato, S. Scototaxis as anxiety-like behavior in fish. Nat. Protoc. 2010, 5, 209–216. [Google Scholar] [CrossRef]
- Maeda, H.; Hasumi, A.; Yoshida, K.-I. Caffeine-induced bradycardia, death, and anxiety-like behavior in zebrafish larvae. Forensic Toxicol. 2021, 39, 427–436. [Google Scholar] [CrossRef]
- Hasumi, A.; Maeda, H.; Yoshida, K.-I. Analyzing cannabinoid-induced abnormal behavior in a zebrafish model. PLoS ONE 2020, 15, e0236606. [Google Scholar] [CrossRef]
- Jesuthasan, S. Fear, anxiety, and control in the zebrafish. Dev. Neurobiol. 2012, 72, 395–403. [Google Scholar] [CrossRef]
- Blanchard, R.J.; Yudko, E.B.; Rodgers, R.; Blanchard, D. Defense system psychopharmacology: An ethological approach to the pharmacology of fear and anxiety. Behav. Brain Res. 1993, 58, 155–165. [Google Scholar] [CrossRef]
- Rodgers, R.; Cao, B.-J.; Dalvi, A.; Holmes, A. Animal models of anxiety: An ethological perspective. Braz. J. Med. Biol. Res. 1997, 30, 289–304. [Google Scholar] [CrossRef] [Green Version]
- Champagne, D.L.; Hoefnagels, C.C.; de Kloet, R.; Richardson, M.K. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- Bourin., M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Cachat, J.; Stewart, A.; Grossman, L.; Gaikwad, S.; Kadri, F.; Chung, K.M.; Wu, N.; Wong, K.; Roy, S.; Suciu, C.; et al. Measuring behavioral and endocrine responses to novelty stress in adult zebrafish. Nat. Protoc. 2010, 5, 1786–1799. [Google Scholar] [CrossRef]
- Davis, M.; Walker, D.L.; Miles, L.; Grillon, C. Phasic vs Sustained Fear in Rats and Humans: Role of the Extended Amygdala in Fear vs Anxiety. Neuropsychopharmacology 2009, 35, 105–135. [Google Scholar] [CrossRef] [Green Version]
- Orger, M.B.; Polavieja, G.G. Zebrafish Behavior: Opportunities and Challenges. Annu. Rev. Neurosci. 2017, 40, 125–147. [Google Scholar] [CrossRef] [Green Version]
- Eddins, D.; Cerutti, D.; Williams, P.; Linney, E.; Levin, E.D. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: Comparison with nicotine and pilocarpine effects and relationship to dopa-mine deficits. Neurotoxicol. Teratol. 2010, 32, 99–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Carmer, R.; Zhang, G.; Venkatraman, P.; Brown, S.A.; Pang, C.-P.; Zhang, M.; Ma, P.; Leung, Y.F. Statistical Analysis of Zebrafish Locomotor Response. PLoS ONE 2015, 10, e0139521. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ma, P.; Cassidy, P.A.; Carmer, R.; Zhang, G.; Venkatraman, P.; Brown, S.A.; Pang, C.-P.; Zhong, W.; Zhang, M.; et al. Statistical Analysis of Zebrafish Locomotor Behaviour by Generalized Linear Mixed Models. Sci. Rep. 2017, 7, 2937. [Google Scholar] [CrossRef]
- Facciol, A.; Tran, S.; Gerlai, R. Re-examining the factors affecting choice in the light-dark preference test in zebrafish. Behav. Brain Res. 2017, 327, 21–28. [Google Scholar] [CrossRef]
- Singh, J. The national centre for the replacement, refinement, and reduction of animals in research. J. Pharmacol. Pharmacother. 2012, 3, 87–89. [Google Scholar]
- Graham, M.L.; Prescott, M.J. The multifactorial role of the 3Rs in shifting the harm-benefit analysis in animal models of disease. Eur. J. Pharmacol. 2015, 759, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenzetti, S.; Altieri, I.; Arabi, S.; Balduzzi, D.; Bechi, N.; Cordelli, E.; Galli, C.; Ietta, F.; Modina, S.C.; Narciso, L.; et al. Innovative non-animal testing strategies for reproductive toxicology: The contribution of Italian partners within the EU project ReProTect. Ann. Ist. Super. Sanita 2011, 47, 429–444. [Google Scholar] [PubMed]
- Best, J.D.; Berghmans, S.; Hunt, J.J.F.G.; Clarke, S.C.; Fleming, A.; Goldsmith, P.; Roach, A.G. Non-associative learning in larval zebrafish. Neuropsychopharmacology 2008, 33, 1206–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, R.F.; Spencer, W.A. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychol. Rev. 1966, 73, 16–43. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maeda, H.; Fukushima, N.; Hasumi, A. Standardized Method for the Assessment of Behavioral Responses of Zebrafish Larvae. Biomedicines 2021, 9, 884. https://doi.org/10.3390/biomedicines9080884
Maeda H, Fukushima N, Hasumi A. Standardized Method for the Assessment of Behavioral Responses of Zebrafish Larvae. Biomedicines. 2021; 9(8):884. https://doi.org/10.3390/biomedicines9080884
Chicago/Turabian StyleMaeda, Hideyuki, Noritoshi Fukushima, and Akihiro Hasumi. 2021. "Standardized Method for the Assessment of Behavioral Responses of Zebrafish Larvae" Biomedicines 9, no. 8: 884. https://doi.org/10.3390/biomedicines9080884





