Differential Diagnosis and Treatment of Itching in Children and Adolescents
Abstract
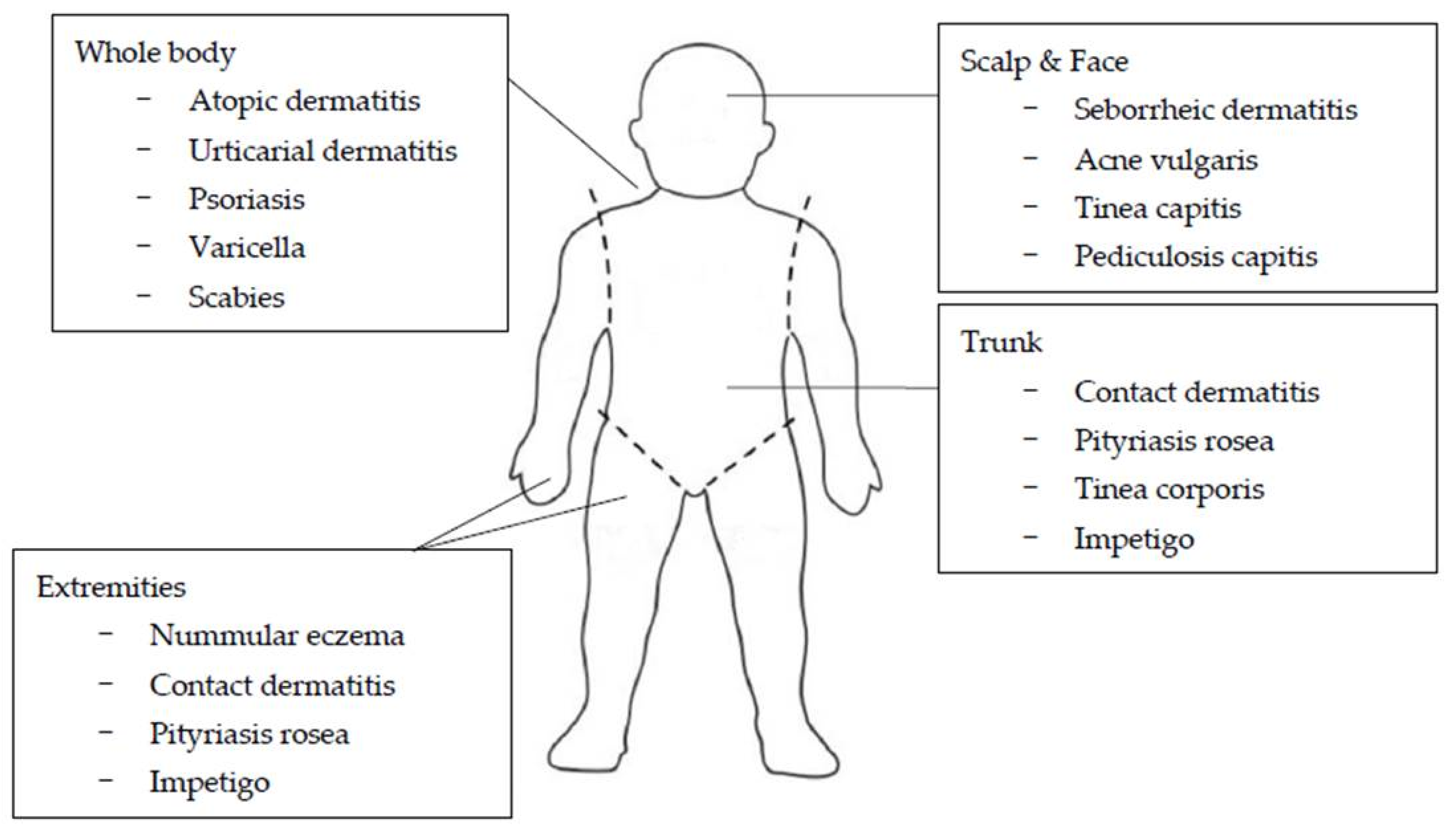
:1. Introduction
2. Common Disorders in Children and Adolescents That Cause Itching
2.1. Atopic Dermatitis
2.2. Nummular Eczema
2.3. Urticaria
2.4. Mastocytosis
2.5. Seborrheic Dermatitis
2.6. Psoriasis
2.7. Infectious Diseases
2.7.1. Varicella (Chickenpox)
2.7.2. Pityriasis Rosea (Paraviral Exanthema)
2.7.3. Fungal Infections
2.7.4. Scabies
2.7.5. Pediculosis Capitis
2.8. Hereditary Disorders
2.9. Dermatologic Disorders Due to Systemic Diseases or Drugs
2.10. Neuropathic Pruritus and Postburn Pruritus
3. Treatments of Itch in Children and Adolescence
3.1. Topical Treatments
3.1.1. Emollient
3.1.2. Topical Corticosteroids
3.1.3. Topical Calcineurin Inhibitors
3.1.4. Small Molecules Drug
3.2. Systemic Treatments
3.2.1. H1-Antihistamines
3.2.2. Immunomodulators
3.2.3. Biologics and Small Molecules
3.2.4. Ultraviolet Phototherapy
3.3. Treatment for Itch Due to Systemic Diseases and Drugs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Atopic dermatitis |
| EASI | Eczema Area and Severity Index |
| IGA | Investigator global assessment |
| IL | interleukin |
| NB-UVB | Narrowband ultraviolet B |
| PsA | psoriatic arthritis |
| PR | Pityriasis rosea |
| TCIs | Topical calcineurin inhibitors |
| TRPV1 | Transient receptor potential vanilloid 1 |
| PDE-4 | Phosphodiesterase-4 |
References
- Blume-Peytavi, U.; Metz, M. Atopic dermatitis in children: Management of pruritus. J. Eur. Acad. Dermatol. Venereol. 2012, 26 (Suppl. S6), 2–8. [Google Scholar] [CrossRef]
- Fölster-Holst, R. Itch Management in Childhood. Curr. Probl. Dermatol. 2016, 50, 173–191. [Google Scholar]
- El Hachem, M.; Di Mauro, G.; Rotunno, R.; Giancristoforo, S.; De Ranieri, C.; Carlevaris, C.M.; Verga, M.C.; Iacono, I.D. Pruritus in pediatric patients with atopic dermatitis: A multidisciplinary approach—Summary document from an Italian expert group. Ital. J. Pediatr. 2020, 46, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caffarelli, C.; Paravati, F.; El Hachem, M.; Duse, M.; Bergamini, M.; Simeone, G.; Barbagallo, M.; Bernardini, R.; Bottau, P.; Bugliaro, F.; et al. Management of chronic urticaria in children: A clinical guideline. Ital. J. Pediatr. 2019, 45, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oranges, T.; Dini, V.; Romanelli, M. Skin Physiology of the Neonate and Infant: Clinical Implications. Adv. Wound Care 2015, 4, 587–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, V.; Murphy, L.M.; Rosenberg, M.; Rosenberg, L.; Holzer, C.E., III; Meyer, W.J., III. Itch assessment scale for the pediatric burn survivor. J. Burn Care Res. 2012, 33, 419–424. [Google Scholar] [CrossRef]
- Elman, S.; Hynan, L.S.; Gabriel, V.; Mayo, M.J. The 5-D itch scale: A new measure of pruritus. Br. J. Dermatol. 2010, 162, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Özçelik, S.; Kulaç, İ.; Yazıcı, M.; Öcal, E. Distribution of childhood skin diseases according to age and gender, a single institution experience. Turk. Pediatri. Ars. 2018, 53, 105–112. [Google Scholar] [CrossRef]
- Wenk, C.; Itin, P.H. Epidemiology of pediatric dermatology and allergology in the region of Aargau, Switzerland. Pediatr. Dermatol. 2003, 20, 482–487. [Google Scholar] [CrossRef]
- Furue, M.; Yamazaki, S.; Jimbow, K.; Tsuchida, T.; Amagai, M.; Tanaka, T.; Matsunaga, K.; Muto, M.; Morita, E.; Akiyama, M.; et al. Prevalence of dermatological disorders in Japan: A nationwide, cross-sectional, seasonal, multicenter, hospital-based study. J. Dermatol. 2011, 38, 310–320. [Google Scholar] [CrossRef]
- Tamer, E.; Ilhan, M.N.; Polat, M.; Lenk, N.; Alli, N. Prevalence of skin diseases among pediatric patients in Turkey. J. Dermatol. 2008, 35, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Polat, A.K.; Yesilova, Y.; Alatas, E.T.; Belli, A.A.; Dogan, G.; Picakciefe, M. Prevalence of skin diseases of the pediatric population in the Southeastern Anatolia, Turkey. Med. Sci. 2018, 7, 664–667. [Google Scholar] [CrossRef]
- Ravindaranath, M.; Swaroop, M.R.; Ghosh, A.; Devaraj, Y.; Shale, K.S.M. A clinico-epidemiological study on various patterns of facial dermatoses in adolescents attending a rural tertiary centre: A cross-sectional study. IP Indian J. Clin. Exp. Dermatol. 2020, 6, 126–135. [Google Scholar] [CrossRef]
- Chou, J.C.; Horswell, B.B. Facial dermatologic lesions in children. Oral Maxillofac. Surg. Clin. N. Am. 2012, 24, 497–509. [Google Scholar] [CrossRef]
- Conti, R.; Colucci, R.; Arunachalam, M.; Berti, S.; Fabroni, C.; De Martino, M.; Dragoni, F.; Lazzeri, L.; Pisaneschi, L.; Moretti, S. Hair and Scalp Disorders in a Tuscan Pediatric Dermatological Outpatient Clinic: A Clinical and Epidemiological Evaluation. Med. Princ. Pract. 2016, 25, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Macini, A.J. Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence; Elsevier Health Sciences: Amsterdam, The Netheralands, 2020. [Google Scholar]
- Reich, A.; Bożek, A.; Janiszewska, K.; Szepietowski, J.C. 12-Item Pruritus Severity Scale: Development and Validation of New Itch Severity Questionnaire. Biomed. Res. Int. 2017, 2017, 3896423. [Google Scholar] [CrossRef] [Green Version]
- Corcimaru, A.; Morrell, D.S.; Burkhart, C.N. The Internet for patient education on atopic dermatitis: Friend or foe? J. Am. Acad. Dermatol. 2017, 76, 1197–1198. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.P.; Ly, K.; Thibodeaux, Q.; Weerasinghe, T.; Wu, J.J.; Yosipovitch, G.; Bhutani, T.; Liao, W. Emerging Methods to Objectively Assess Pruritus in Atopic Dermatitis. Dermatol. Ther. 2019, 9, 407–420. [Google Scholar] [CrossRef] [Green Version]
- de Waard-van der Spek, F.B.; Andersen, K.E.; Darsow, U.; Mortz, C.G.; Orton, D.; Worm, M.; Muraro, A.; Schmid-Grendelmeier, P.; Grimalt, R.; Spiewak, R.; et al. Allergic contact dermatitis in children: Which factors are relevant? (review of the literature). Pediatr. Allergy Immunol. 2013, 24, 321–329. [Google Scholar] [CrossRef]
- Lyons, J.J.; Milner, J.D.; Stone, K.D. Atopic dermatitis in children: Clinical features, pathophysiology, and treatment. Immunol. Allergy Clin. N. Am. 2015, 35, 161–183. [Google Scholar] [CrossRef] [Green Version]
- Draelos, Z.D.; Stein Gold, L.F.; Murrell, D.F.; Hughes, M.H.; Zane, L.T. Post Hoc Analyses of the Effect of Crisaborole Topical Ointment, 2% on Atopic Dermatitis: Associated Pruritus from Phase 1 and 2 Clinical Studies. J. Drugs Dermatol. 2016, 15, 172–176. [Google Scholar]
- Montes-Torres, A.; Llamas-Velasco, M.; Pérez-Plaza, A.; Solano-López, G.; Sánchez-Pérez, J. Biological Treatments in Atopic Dermatitis. J. Clin. Med. 2015, 4, 593–613. [Google Scholar] [CrossRef]
- Leung, A.; Lam, J.M.; Leong, K.F.; Leung, A.; Wong, A. Nummular Eczema: An Updated Review. Recent Pat. Inflamm. Allergy Drug Discov. 2020, 14, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Perlman, H.H.; Gropen, J.; Westfall, P. Nummular eczema in children; report of six cases. J. Pediatr. 1951, 39, 565–574. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, M.K.; Weller, K.; Stock, P.; Maurer, M. Chronic spontaneous urticaria in children: Itching for insight. Pediatr. Allergy Immunol. 2011, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Knöpfel, N.; Noguera-Morel, L.; Hernández-Martín, A.; Torrelo, A. Methotrexate for severe nummular eczema in children: Efficacy and tolerability in a retrospective study of 28 patients. Pediatr. Dermatol. 2018, 35, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Sekerel, B.E.; Orhan, F.; Kocabas, C.N.; Tuncer, A.; Adalioglu, G. The etiology of different forms of urticaria in childhood. Pediatr. Dermatol. 2004, 21, 102–108. [Google Scholar] [CrossRef]
- Hide, M.; Francis, D.M.; Grattan, C.; Hakimi, J.; Kochan, J.P.; Greaves, M.W. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 1993, 328, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Niimi, N.; Francis, D.M.; Kermani, F.; O’Donnell, B.F.; Hide, M.; Kobza-Black, A.; Winkelmann, R.K.; Greaves, M.W.; Barr, R.M. Dermal mast cell activation by autoantibodies against the high affinity IgE receptor in chronic urticaria. J. Investig. Dermatol. 1996, 106, 1001–1006. [Google Scholar] [CrossRef] [Green Version]
- Heide, R.; Van Doorn, K.; Mulder, P.G.; Van Toorenenbergen, A.W.; Beishuizen, A.; De Groot, H.; Tank, B.; Oranje, A.P. Serum tryptase and SCORMA (SCORing MAstocytosis) Index as disease severity parameters in childhood and adult cutaneous mastocytosis. Clin. Exp. Dermatol. 2009, 34, 462–468. [Google Scholar] [CrossRef]
- Carter, M.C.; Clayton, S.T.; Komarow, H.D.; Brittain, E.H.; Scott, L.M.; Cantave, D.; Gaskins, D.M.; Maric, I.; Metcalfe, D.D. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J. Allergy Clin. Immunol. 2015, 136, 1673–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borda, L.J.; Wikramanayake, T.C. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J. Clin. Investig. Dermatol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Relvas, M.; Torres, T. Pediatric Psoriasis. Am. J. Clin. Dermatol. 2017, 18, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Bonigen, J.; Phan, A.; Hadj-Rabia, S.; Boralevi, F.; Bursztejn, A.C.; Bodemer, C.; Ferneiny, M.; Souillet, A.L.; Chiavérini, C.; Bourrat, E.; et al. Impact de l’âge et du sexe sur les aspects cliniques et épidémiologiques du psoriasis de l’enfant. Données d’une étude transversale multicentrique française [Impact of sex and age on the clinical and epidemiological aspects of childhood psoriasis: Data from a French cross-sectional multicentre study]. Ann. Dermatol. Venereol. 2016, 143, 354–363. [Google Scholar]
- Shah, K.N. Diagnosis and treatment of pediatric psoriasis: Current and future. Am. J. Clin. Dermatol. 2013, 14, 195–213. [Google Scholar] [CrossRef]
- O’Neill, J.L.; Feldman, S.R. Vitamine D analogue-based therapies for psoriasis. Drugs Today 2010, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- de Jager, M.E.; de Jong, E.M.; van de Kerkhof, P.C.; Seyger, M.M. Efficacy and safety of treatments for childhood psoriasis: A systematic literature review. J. Am. Acad. Dermatol. 2010, 62, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Marqueling, A.L.; Cordoro, K.M. Systemic treatments for severe pediatric psoriasis: A practical approach. Dermatol. Clin. 2013, 31, 267–288. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Gordon, K.B.; Fakharzadeh, S.; Yeilding, N.; Szapary, P.O.; Schenkel, B.; Guzzo, C.; Li, S.; Papp, K.A. Long-term efficacy of ustekinumab in patients with moderate-to-severe psoriasis: Results from the PHOENIX 1 trial through up to 3 years. Br. J. Dermatol. 2012, 166, 861–872. [Google Scholar] [CrossRef]
- Kimball, A.B.; Gordon, K.B.; Langley, R.G.; Menter, A.; Chartash, E.K.; Valdes, J.; ABT-874 Psoriasis Study Investigators. Safety and efficacy of ABT-874, a fully human interleukin 12/23 monoclonal antibody, in the treatment of moderate to severe chronic plaque psoriasis: Results of a randomized, placebo-controlled, phase 2 trial. Arch. Dermatol. 2008, 144, 200–207. [Google Scholar] [CrossRef]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers 2015, 1, 15016. [Google Scholar] [CrossRef] [Green Version]
- Paller, A.S.; Mancini, A.J. Exanthematous diseases of childhood. In Hurwitz Clinical Pediatric Dermatology: A Textbook of Skin Disorders of Childhood and Adolescence; Paller, A.S., Mancini, A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 348–369. [Google Scholar]
- González, L.M.; Allen, R.; Janniger, C.K.; Schwartz, R.A. Pityriasis rosea: An important papulosquamous disorder. Int. J. Dermatol. 2005, 44, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Drago, F.; Broccolo, F.; Ciccarese, G.; Rebora, A.; Parodi, A. Persistent pityriasis rosea: An unusual form of pityriasis rosea with persistent active HHV-6 and HHV-7 infection. Dermatology 2015, 230, 23–26. [Google Scholar] [CrossRef]
- Fölster-Holst, R.; Kreth, H.W. Viral exanthems in childhood. Part 3: Parainfectious exanthems and those associated with virus-drug interactions. J. Dtsch. Dermatol. Ges. 2009, 7, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Rassai, S.; Feily, A.; Sina, N.; Abtahian, S. Low dose of acyclovir may be an effective treatment against pityriasis rosea: A random investigator-blind clinical trial on 64 patients. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 24–26. [Google Scholar] [CrossRef]
- Chuh, A.A.T. Pediatric viral exanthems. In Yearbook of Dermatology and Dermatologic Surgery; Thiers, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 16–43. [Google Scholar]
- Verma, S.; Vasani, R.; Reszke, R.; Matusiak, Ł.; Szepietowski, J.C. Prevalence and clinical characteristics of itch in epidemic-like scenario of dermatophytoses in India: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, A.; Pinto, H.P.; Bhat, R.M.; Sukumar, D.; Srinath, M.K. A study of the prevalence and precipitating factors of pruritus in pityriasis versicolor. Indian Derm. Online J. 2014, 5, 223–224. [Google Scholar]
- Jacobsen, I.D. Fungal infection strategies. Virulence 2019, 10, 835–838. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Friedlander, S.F. Pediatric Onychomycosis: The Emerging Role of Topical Therapy. J. Drugs Dermatol. 2017, 16, 105–109. [Google Scholar] [PubMed]
- Thomas, C.; Coates, S.J.; Engelman, D.; Chosidow, O.; Chang, A.Y. Ectoparasites: Scabies. J. Am. Acad. Dermatol. 2020, 82, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.A. Scabies and pediculosis pubis: An update of treatment regimens and general review. Clin. Infect. Dis. 2007, 44 (Suppl. S3), S153–S159. [Google Scholar] [CrossRef] [Green Version]
- Panzer, R.; Fölster-Holst, R. Ekzematöse Hautveränderungen bei einem Säugling [Eczematous skin lesions of a suckling]. J. Dtsch. Dermatol. Ges. 2009, 7, 913–914. [Google Scholar] [CrossRef] [PubMed]
- Bialek, R.; Zelck, U.E.; Fölster-Holst, R. Permethrin treatment of head lice with knockdown resistance-like gene. N. Engl. J. Med. 2011, 364, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.E.; Mettang, T. Pruritus bei systemischen Erkrankungen: Häufiges und Seltenes [Pruritus in systemic diseases: Common and rare etiologies]. Hautarzt 2016, 67, 606–614. [Google Scholar] [CrossRef]
- Park, J.S.; Suh, D.I. Drug Allergy in Children: What Should We Know? Clin. Exp. Pediatr. 2020, 63, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Estabrook, R.W.; Bouxsein, P.; Pitluck, S.; Davis, J.R.; Yaffe, S. Institute of Medicine (US) Roundtable on Research and Development of Drugs, Biologics, and Medical Devices, Similarities and Dissimilarities in Physiology, Metabolism, and Disease States and Responses to Therapy in Children and Adults. In Rational Therapeutics for Infants and Children: Workshop Summary; Yaffe, S., Ed.; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Ahuja, R.B.; Gupta, R.; Gupta, G.; Shrivastava, P. A comparative analysis of cetirizine, gabapentin and their combination in the relief of post-burn pruritus. Burns 2011, 37, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Fields-Blache, C.; Luterman, A. Severe itching in the patient with burns. J. Burn Care Rehabil. 1991, 12, 330–333. [Google Scholar] [CrossRef]
- Van Loey, N.E.; Bremer, M.; Faber, A.W.; Middelkoop, E.; Nieuwenhuis, M.K. Itching following burns: Epidemiology and predictors. Br. J. Dermatol. 2008, 158, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Nagamine, M.; Carstens, M.I.; Carstens, E. Behavioral model of itch, alloknesis, pain and allodynia in the lower hindlimb and correlative responses of lumbar dorsal horn neurons in the mouse. Neuroscience 2014, 266, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Mendham, J.E. Gabapentin for the treatment of itching produced by burns and wound healing in children: A pilot study. Burns 2004, 30, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Kaul, I.; Amin, A.; Rosenberg, M.; Rosenberg, L.; Meyer, W.J. Use of gabapentin and pregabalin for pruritus and neuropathic pain associated with major burn injury: A retrospective chart review. Burns 2018, 44, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Misery, L.; Proksch, E.; Metz, M.; Ständer, S.; Schmelz, M. Skin Barrier Damage and Itch: Review of Mechanisms, Topical Management and Future Directions. Acta Derm. Venereol. 2019, 99, 1201–1209. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.Y.; Um, J.Y.; Kim, J.C.; Kang, S.Y.; Park, C.W.; Kim, H.O. Pathophysiology and Treatment of Pruritus in Elderly. Int. J. Mol. Sci. 2020, 22, 174. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Mueller, K.; Lor, J.; Smith, P.; Paller, A.S.; Kaat, A. Systematic Review and Meta-analysis Comparing Topical Corticosteroids With Vehicle/Moisturizer in Childhood Atopic Dermatitis. J. Pediatr. Nurs. 2019, 47, 36–43. [Google Scholar] [CrossRef]
- Papier, A.; Strowd, L.C. Atopic dermatitis: A review of topical nonsteroid therapy. Drugs Context 2018, 7, 212521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, C.Y. Treatments for Childhood Atopic Dermatitis: An Update on Emerging Therapies. Clin. Rev. Allergy Immunol. 2020, in press. [Google Scholar] [CrossRef]
- Ishii, N.; Shirato, M.; Wakita, H.; Miyazaki, K.; Takase, Y.; Asano, O.; Kusano, K.; Yamamoto, E.; Inoue, C.; Hishinuma, I. Antipruritic effect of the topical phosphodiesterase 4 inhibitor E6005 ameliorates skin lesions in a mouse atopic dermatitis model. J. Pharmacol. Exp. Ther. 2013, 346, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Bäumer, W.; Hoppmann, J.; Rundfeldt, C.; Kietzmann, M. Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm. Allergy Drug Targets 2007, 6, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, J.L.; Punzón, C.; Navarro, J.; Muñoz-Fernández, M.A.; Fresno, M. Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J. Pharmacol. Exp. Ther. 2001, 299, 753–759. [Google Scholar]
- Schlessinger, J.; Shepard, J.S.; Gower, R.; Su, J.C.; Lynde, C.; Cha, A.; Ports, W.C.; Purohit, V.; Takiya, L.; Werth, J.L.; et al. Safety, Effectiveness, and Pharmacokinetics of Crisaborole in Infants Aged 3 to <24 Months with Mild-to-Moderate Atopic Dermatitis: A Phase IV Open-Label Study (CrisADe CARE 1). Am. J. Clin. Dermatol. 2020, 21, 275–284. [Google Scholar] [PubMed] [Green Version]
- Fitzsimons, R.; van der Poel, L.A.; Thornhill, W.; du Toit, G.; Shah, N.; Brough, H.A. Antihistamine use in children. Arch. Dis. Child. Educ. Pract. Ed. 2015, 100, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Baek, H.S. Approaches to the diagnosis and management of chronic urticaria in children. Korean J. Pediatr. 2015, 58, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, G.F.; Licari, A.; Papale, M.; Manti, S.; Salpietro, C.; Marseglia, G.L.; Leonardi, S. Antihistamines: ABC for the pediatricians. Pediatr. Allergy Immunol. 2020, 31 (Suppl. S24), 34–36. [Google Scholar] [CrossRef]
- Leslie, T.A.; Greaves, M.W.; Yosipovitch, G. Current topical and systemic therapies for itch. Handb. Exp. Pharmacol. 2015, 226, 337–356. [Google Scholar]
- Hernández-Martín, A.; Noguera-Morel, L.; Bernardino-Cuesta, B.; Torrelo, A.; Pérez-Martin, M.A.; Aparicio-López, C.; de Lucas-Collantes, C. Cyclosporine A for severe atopic dermatitis in children. Efficacy and safety in a retrospective study of 63 patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.E.; Hoffmann, R.; Peterson, E.; Soter, N.A. Use of Dapsone in the Treatment of Chronic Idiopathic and Autoimmune Urticaria. JAMA Dermatol. 2019, 155, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.M. Dupilumab: Human monoclonal antibody against IL-4Rα for moderate to severe atopic dermatitis. Drugs Today 2017, 53, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Hong, H.C.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Igelman, S.; Kurta, A.O.; Sheikh, U.; McWilliams, A.; Armbrecht, E.; Cullison, S.R.J.; Kress, D.W.; Smith, A.; Castelo-Soccio, L.; Treat, J.; et al. Off-label use of dupilumab for pediatric patients with atopic dermatitis: A multicenter retrospective review. J. Am. Acad. Dermatol. 2020, 82, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Siegfried, E.C.; Thaçi, D.; Wollenberg, A.; Cork, M.J.; Arkwright, P.D.; Gooderham, M.; Beck, L.A.; Boguniewicz, M.; Sher, L.; et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: A randomized, double-blinded, placebo-controlled phase 3 trial. J. Am. Acad. Dermatol. 2020, 83, 1282–1293. [Google Scholar] [CrossRef]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Heron, C.E.; Ghamrawi, R.I.; Strowd, L.C.; Feldman, S.R. Emerging Role of Janus Kinase Inhibitors for the Treatment of Atopic Dermatitis. Immunotargets Ther. 2020, 9, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.A.; Torres, T. JAK/STAT inhibitors for the treatment of atopic dermatitis. J. Dermatolog. Treat. 2020, 31, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sideris, N.; Vakirlis, E.; Tsentemeidou, A.; Kourouklidou, A.; Ioannides, D.; Sotiriou, E. Under Development JAK Inhibitors for Dermatologic Diseases. Mediterr. J. Rheumatol. 2020, 11, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Guttman-Yassky, E.; Torres, T. Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: Focus on Upadacitinib and Abrocitinib. Am. J. Clin. Dermatol. 2020, 21, 783–798. [Google Scholar] [CrossRef]
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Newsom, M.; Bashyam, A.M.; Balogh, E.A.; Feldman, S.R.; Strowd, L.C. New and Emerging Systemic Treatments for Atopic Dermatitis. Drugs 2020, 80, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Matsumura, T.; Komazaki, H.; Kawashima, M.; Nemolizumab-JP01 Study Group. Trial of Nemolizumab and Topical Agents for Atopic Dermatitis with Pruritus. N. Engl. J. Med. 2020, 383, 141–150. [Google Scholar] [CrossRef]
- Chan, S.; Cornelius, V.; Cro, S.; Harper, J.I.; Lack, G. Treatment Effect of Omalizumab on Severe Pediatric Atopic Dermatitis: The ADAPT Randomized Clinical Trial. JAMA Pediatr. 2020, 174, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zuo, J.; Tang, W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front. Pharmacol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legat, F.J. Stellenwert der Phototherapie in der Behandlung des chronischen Pruritus [Importance of phototherapy in the treatment of chronic pruritus]. Hautarzt 2018, 69, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darné, S.; Leech, S.N.; Taylor, A.E. Narrowband ultraviolet B phototherapy in children with moderate-to-severe eczema: A comparative cohort study. Br. J. Dermatol. 2014, 170, 150–156. [Google Scholar] [CrossRef] [PubMed]


| Age | Diagnosis | Frequency |
|---|---|---|
| Infancy (0–2) | Atopic dermatitis | 25–50% |
| Diaper dermatitis | 12.2% | |
| Impetigo | 6.6–6.9% | |
| Seborrheic dermatitis | 3.6% | |
| Insect bites | 2.4% | |
| Psoriasis | 2.4% | |
| Papular urticaria | 2.1% | |
| Viral exanthem | 2.1% | |
| Preschool (3–5) | Atopic dermatitis | 24–35% |
| Impetigo | 2.3–7.1% | |
| Contact dermatitis | 4.5% | |
| Insect bites | 4% | |
| Urticaria | 3.4% | |
| Seborrheic dermatitis | 2.8% | |
| Papular urticaria | 2.2% | |
| Psoriasis | 2.2% | |
| School (6–11) | Contact dermatitis | 15.1% |
| Atopic dermatitis | 10–23% | |
| Acne vulgaris | 13.0% | |
| Urticaria/Angioedema | 2.9–4.9% | |
| Impetigo | 4.7% | |
| Seborrheic dermatitis | 4.1% | |
| Psoriasis | 2.6–3.0% | |
| Xerosis | 3.4% | |
| Adolescence (12–16) | Acne vulgaris | 8.9–25.2% |
| Atopic dermatitis | 4.4–27.4% | |
| Contact dermatitis | 11.7% | |
| Seborrhic dermatitis | 4.9% | |
| Pruritus | 3.5% | |
| Psoriasis | 3.0–3.1% | |
| Urticaria/Angioedema | 3.0–5.4% | |
| Impetigo | 2.6% |
| Classifications | Diagnosis | Clinical Manifestation of Pruritus |
|---|---|---|
| Metabolic disorders | Chronic renal failure (dialysis) | Accompanied by xerosis or prurigo Generalized or localized |
| Liver diseases with or without cholestasis | Can be mechanically induced Not diminished by scratching Usually generalized pruritus | |
| Endocrinal disorders | Thyroid diseases | Hyperthyroidism/hypothyroidism Associated with urticaria |
| Diabetes mellitus | Secondary to diabetic neuropathy, Metabolic derangements associated with renal failure and xerosis | |
| Infectious diseases | HIV | Itchy, red or purple, or painful |
| Hepatitis C virus | Widespread, itchy, red rash | |
| Hematologic disease | Iron deficiency | Generalized pruritus Skin lesion or irritation provokes scratching |
| Polycythemia vera | After contact with water Accompanied by stinging sensation and prurigo Generalized pruritus | |
| Paraneoplastic Diseases | Lymphomas and solid organ tumors | Premonitory onset Area of affected lymph nodes such as mediastinal sites |
| Hereditary dermotoses | Ichthyosis, Darier disease, pityriasis rubra pilaris (juvenile familial type), hereditary angioedema | - |
| Neuropathic pruritus | Postburn pruritus | |
| Drug-induced pruritus | Drug-induced pruritus Drug eruption | With or without skin rash Can occur after several months (lichenoid type) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Differential Diagnosis and Treatment of Itching in Children and Adolescents. Biomedicines 2021, 9, 919. https://doi.org/10.3390/biomedicines9080919
Kang S-Y, Um J-Y, Chung B-Y, Kim J-C, Park C-W, Kim H-O. Differential Diagnosis and Treatment of Itching in Children and Adolescents. Biomedicines. 2021; 9(8):919. https://doi.org/10.3390/biomedicines9080919
Chicago/Turabian StyleKang, Seok-Young, Ji-Young Um, Bo-Young Chung, Jin-Cheol Kim, Chun-Wook Park, and Hye-One Kim. 2021. "Differential Diagnosis and Treatment of Itching in Children and Adolescents" Biomedicines 9, no. 8: 919. https://doi.org/10.3390/biomedicines9080919
APA StyleKang, S.-Y., Um, J.-Y., Chung, B.-Y., Kim, J.-C., Park, C.-W., & Kim, H.-O. (2021). Differential Diagnosis and Treatment of Itching in Children and Adolescents. Biomedicines, 9(8), 919. https://doi.org/10.3390/biomedicines9080919






