Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice
Abstract
1. Introduction
2. Materials and Methods
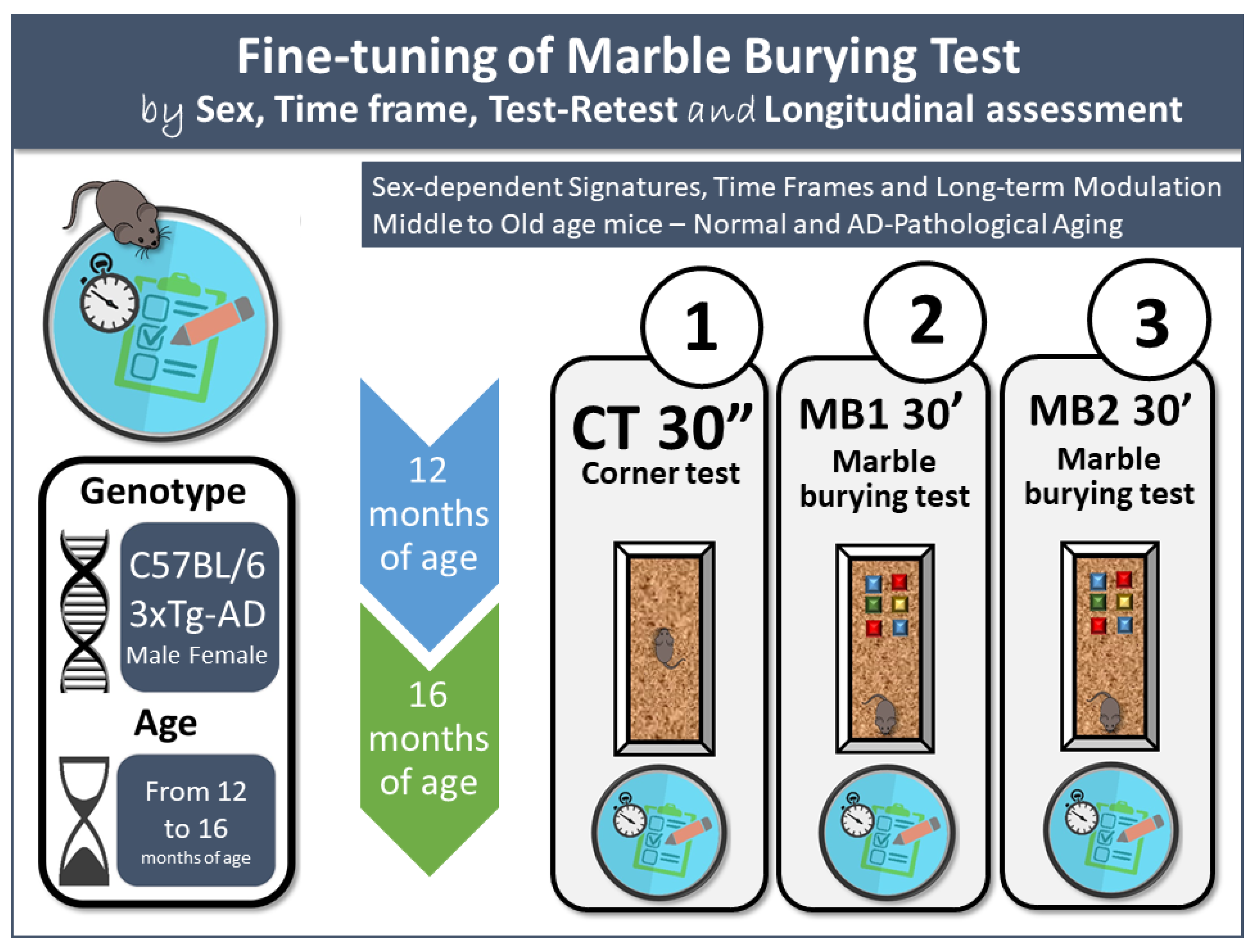
2.1. Animals
2.2. Experimental Design
2.3. Behavioral Assessments
2.4. Statistics
3. Results
3.1. Corner Test for Neophobia
3.2. Longitudinal Assessment of Marble Burying Test and Repeated Test
3.3. Corner Test and Marble Burying Test Correlations
4. Discussion
4.1. New Insight of Burying Behavior in 3xTg-AD Mice
4.2. Corner Test and Its Relationship with Marble Burying Test in 3xTg-AD Mice
4.3. Marble Test as a Model of Anxiety-Like or OCD-Like Behavior in 3xTg-AD Mice?
4.4. Integrating Our New Findings into Our Previous Knowledge of The Burying Behavior of 3xTg-AD Mice
4.5. Benefits of Implementing the Time-Course of Marble Buried
4.6. Methodological Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, C.; Corbett, A. Management of neuropsychiatric symptoms in people with dementia. CNS Drugs 2010, 24, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.S.; Carter, M.; Masterman, D.; Fairbanks, L.; Cummings, J.L. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am. J. Geriatr. Psychiatry 2005, 13, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Wong, H.B.; Allen, H. The impact of neuropsychiatric symptoms of dementia on distress in family and professional caregivers in Singapore. Int. Psychogeriatr. 2005, 17, 253–263. [Google Scholar] [CrossRef]
- Hope, T.; Keene, J.; Gedling, k.; Fairburn, C.G.; Jacoby, R. Predictors of institutionalization for people with dementia living at home with a carer. Int. J. Geriatr. Psychiatry 1998, 13, 682–690. [Google Scholar] [CrossRef]
- Mielke, M.M. Sex and Gender Differences in Alzheimer’s Disease Dementia. Psychiatr. Times 2018, 35, 14–17. [Google Scholar] [PubMed]
- Torres-Lista, V.; López-Pousa, S.; Giménez-Llort, L. Marble-burying is enhanced in 3xTg-AD mice, can be reversed by risperidone and it is modulable by handling. Behav. Process. 2015, 116, 69–74. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Giménez-Llort, L. Impairment of nesting behaviour in 3xTg-AD mice. Behav. Brain Res. 2013, 247, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Torres-Lista, V. Social Nesting, Animal Welfare, and Disease Monitoring. Animals 2021, 11, 1079. [Google Scholar] [CrossRef]
- Deacon, R.M. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Pinel, J.P.J.; Treit, D. Burying as a defensive response in rats. J. Comp. Physiol. Psychol. 1978, 92, 708–712. [Google Scholar] [CrossRef]
- Broekkamp, C.L.; Rijk, H.W.; Joly-Gelouin, D.; Lloyd, K.L. Major tranquillizers can be distinguished from minor tranquillizers on the basis of effects on marble burying and swim-induced grooming in mice. Eur. J. Pharmacol. 1986, 126, 223–229. [Google Scholar] [CrossRef]
- Njung’e, K.; Handley, S.L. Effects of 5-HT uptake inhibitors, agonists and antagonists on the burying of harmless objects by mice; a putative test for anxiolytic agents. Br. J. Pharmacol. 1991, 104, 105–112. [Google Scholar] [CrossRef] [PubMed]
- de Brouwer, G.; Fick, A.; Harvey, B.H.; Wolmarans, W. A critical inquiry into marble-burying as a preclinical screening paradigm of relevance for anxiety and obsessive-compulsive disorder: Mapping the way forward. Cogn. Affect. Behav. Neurosci. 2019, 19, 1–39. [Google Scholar] [CrossRef] [PubMed]
- de Brouwer, G.; Wolmarans, W. Back to basics: A methodological perspective on marble-burying behavior as a screening test for psychiatric illness. Behav. Process. 2018, 157, 590–600. [Google Scholar] [CrossRef]
- Lazic, S.E. Analytical strategies for the marble burying test: Avoiding impossible predictions and invalid p-values. BMC Res. Notes 2015, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Belfiore, R.; Rodin, A.; Ferreira, E.; Velazquez, R.; Branca, C.; Caccamo, A.; Oddo, S. Temporal and regional progression of Alzheimer’s disease-like pathology in 3xTg-AD mice. Aging Cell 2019, 18, e12873. [Google Scholar] [CrossRef]
- Torres-Lista, V.; López-Pousa, S.; Giménez-Llort, L. Impact of Chronic Risperidone Use on Behavior and Survival of 3xTg-AD Mice Model of Alzheimer’s Disease and Mice With Normal Aging. Front. Pharmacol. 2019, 10, 1061. [Google Scholar] [CrossRef]
- Gimenez-Llort, L.; Alveal-Mellado, D. Digging Signatures in 13-Month-Old 3xTg-AD Mice for Alzheimer’s Disease and Its Disruption by Isolation Despite Social Life Since They Were Born. Front. Behav. Neurosci. 2021, 19. [Google Scholar] [CrossRef]
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef]
- Çalışkan, H.; Şentunali, B.; Özden, F.M.; Cihan, K.H.; Uzunkulaoğlu, M.; Çakan, O.; Kankal, S.; Zaloğlu, N. Marble burying test analysis in terms of biological and non-biological factors. J. Appl. Biol. Sci. 2017, 11, 54–57. [Google Scholar]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; ohansson, B.; Oddo, S.; Tobeña, A.; LaFerla, F.M.; Fernández-Teruel, A. Modeling behavioral and neuronal symptoms of Alzheimer’s disease in mice: A role for intraneuronal amyloid. Neurosci. Biobehav. Rev. 2007, 31, 125–147. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; Giménez-Llort, L. Bizarre behaviors and risk assessment in 3xTg-AD mice at early stages of the disease. Behav. Brain Res. 2014, 258, 97–105. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 24. [Google Scholar] [CrossRef]
- Schneider, T.; Popik, P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology 2007, 32, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Llaneza, D.C.; Frye, C.A. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol. Biochem. Behav. 2009, 93, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Bastos, C.P.; Chesworth, S.; Frye, C.; Bult-Ito, A. Strain and sex based characterization of behavioral expressions in non-induced compulsive-like mice. Physiol. Behav. 2017, 168, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.T.; Lerch, S.; Chourbaji, S. Marble burying as compulsive behaviors in male and female mice. Acta Neurobiol. Exp. (Wars) 2017, 77, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Sanathara, N.M.; Garau, C.; Alachkar, A.; Wang, L.; Wang, Z.; Nishimori, K.; Xu, X.; Civelli, O. Melanin concentrating hormone modulates oxytocin-mediated marble burying. Neuropharmacology 2018, 128, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Savy, C.Y.; Fitchett, A.E.; McQuade, R.; Gartside, S.E.; Morris, C.M.; Blain, P.G.; Judge, S.J. Low-level repeated exposure to diazinon and chlorpyrifos decrease anxiety-like behaviour in adult male rats as assessed by marble burying behaviour. Neurotoxicology 2015, 50, 149–156. [Google Scholar] [CrossRef]
- Thomas, A.; Burant, A.; Bui, N.; Graham, D.; Yuva-Paylor, L.A.; Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 2009, 204, 361–373. [Google Scholar] [CrossRef]
- Njung’e, K.; Handley, S.L. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav. 1991, 38, 63–67. [Google Scholar] [CrossRef]
- Kaehler, S.T.; Singewald, N.; Sinner, C.; Philippu, A. Nitric oxide modulates the release of serotonin in the rat hypothalamus. Brain Res. 1999, 835, 346–349. [Google Scholar] [CrossRef]
- Nicolas, L.B.; Kolb, Y.; Prinssen, E.P. A combined marble burying-locomotor activity test in mice: A practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol. 2006, 547, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Bouwer, C. A neuro-evolutionary approach to the anxiety disorders. J. Anxiety Disord. 1997, 11, 409–429. [Google Scholar] [CrossRef]
- Steimer, T. Animal models of anxiety disorders in rats and mice: Some conceptual issues. Dialogues Clin. Neurosci. 2011, 13, 495–506. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Everts, H.; de Ruiter, A.J.; de Boer, S.F.; Bohus, B. Coping with stress in rats and mice: Differential peptidergic modulation of the amygdala-lateral septum complex. Prog. Brain Res. 1998, 119, 437–448. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; de Boer, S.F.; Buwalda, B.; van Reenen, K. Individual variation in coping with stress: A multidimensional approach of ultimate and proximate mechanisms. Brain Behav. Evol. 2007, 70, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Bruins Slot, L.A.; Bardin, L.; Auclair, A.L.; Depoortere, R.; Newman-Tancredi, A. Effects of antipsychotics and reference monoaminergic ligands on marble burying behavior in mice. Behav. Pharmacol. 2008, 19, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Coppens, C.M.; de Boer, S.F.; Koolhaas, J.M. Coping styles and behavioural flexibility: Towards underlying mechanisms. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 4021–4028. [Google Scholar] [CrossRef]
- Kinsey, S.G.; O’Neal, S.T.; Long, J.Z.; Cravatt, B.F.; Lichtman, A.H. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol. Biochem. Behav. 2011, 98, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Masino, S.A.; Diao, L.; Fernández-Teruel, A.; Tobeña, A.; Halldner, L.; Fredholm, B.B. Mice lacking the adenosine A1 receptor have normal spatial learning and plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse 2005, 57, 8–16. [Google Scholar] [CrossRef]
- Gyertyán, I. Analysis of the marble burying response: Marbles serve to measure digging rather than evoke burying. Behav. Pharmacol. 1995, 6, 24–31. [Google Scholar]
- Poling, A.; Cleary, J.; Monaghan, M. Burying by rats in response to aversive and nonaversive stimuli. J. Exp. Anal. Behav. 1981, 35, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wolmarans de, W.; Stein, D.J.; Harvey, B.H. Of mice and marbles: Novel perspectives on burying behavior as a screening test for psychiatric illness. Cogn. Affect. Behav. Neurosci. 2016, 16, 551–560. [Google Scholar] [CrossRef]
- de Boer, S.F.; Koolhaas, J.M. Defensive burying in rodents: Ethology, neurobiology and psychopharmacology. Eur. J. Pharmacol. 2003, 463, 145–161. [Google Scholar] [CrossRef]
- Mrabet Khiari, H.; Achouri, A.; Ben Ali, N.; Cherif, A.; Batti, H.; Messaoud, T.; Mrabet, A. Obsessive-compulsive disorder: A new risk factor for Alzheimer disease? Neurol Sci. 2011, 32, 959–962. [Google Scholar] [CrossRef]
- Dondu, A.; Sevincoka, L.; Akyol, A.; Tataroglu, C. Is obsessive-compulsive symptomatology a risk factor for Alzheimer-type dementia? Psychiatry Res. 2015, 225, 381–386. [Google Scholar] [CrossRef]
- Pekkala, S.; Albert, M.L.; Spiro, A., 3rd; Erkinjuntti, T. Perseveration in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 109–114. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Grossberg, G.T. Behavioral and psychological symptoms in Alzheimer’s dementia and vascular dementia. Handb. Clin. Neurol. 2019, 165, 5–32. [Google Scholar] [CrossRef]
- Cipriani, G.; Vedovello, M.; Ulivi, M.; Nuti, A.; Lucetti, C. Repetitive and stereotypic phenomena and dementia. Am. J. Alzheimers Dis Other Demen. 2013, 28, 223–227. [Google Scholar] [CrossRef]
- Torres-Lista, V.; Giménez-Llort, L. Persistence of behaviours in the Forced Swim Test in 3xTg-AD mice at advanced stages of disease. Behav. Processes 2014, 106, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Romberg, C.; Mattson, M.P.; Mughal, M.R.; Bussey, T.J.; Saksida, L.M. Impaired attention in the 3xTgAD mouse model of Alzheimer’s disease: Rescue by donepezil (Aricept). J. Neurosci. 2011, 31, 3500–3507. [Google Scholar] [CrossRef]
- Layne, J.N.; Ehrhart, L.M. Digging behavior of four species of deer mice (Peromyscus). In American Museum Novitates; American Museum of Natural History: Washington, DC, USA, 1970; p. 2429. [Google Scholar]
- Boix, F.; Fernandez-Terula, A.; Tobeña, A. The anxiolytic action of benzodiazepines is not present in handling-habituated rats. Pharmacol. Biochem. Behav. 1988, 31, 541–546. [Google Scholar] [CrossRef]
- Cañete, T.; Blázquez, G.; Tobeña, A.; Giménez-Llort, L.; Fernández-Teruel, A. Cognitive and emotional alterations in young Alzheimer’s disease (3xTgAD) mice: Effects of neonatal handling stimulation and sexual dimorphism. Behav. Brain Res. 2015, 281, 156–171. [Google Scholar] [CrossRef]
- Gouveia, K.; Hurst, J.L. Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Sci. Rep. 2019, 9, 20305. [Google Scholar] [CrossRef] [PubMed]
- Barnum, C.J.; Pace, T.W.; Hu, F.; Neigh, G.N.; Tansey, M.G. Psychological stress in adolescent and adult mice increases neuroinflammation and attenuates the response to LPS challenge. J. Neuroinflamm. 2012, 9, 9. [Google Scholar] [CrossRef]
- Kedia, S.; Chattarji, S. Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J. Neurosci Methods. 2014, 233, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Yohn, N.L.; Blendy, J.A. Adolescent Chronic Unpredictable Stress Exposure Is a Sensitive Window for Long-Term Changes in Adult Behavior in Mice. Neuropsychopharmacology 2017, 42, 1670–1678. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Tagawa, N.; Kobayashi, Y.; Hotta, Y.; Yamada, J. Effects of the serotonin and noradrenaline reuptake inhibitor (SNRI) milnacipran on marble burying behavior in mice. Biol. Pharm. Bull. 2007, 30, 2399–2401. [Google Scholar] [CrossRef][Green Version]


| Post-Hoc Analysis CORNER TEST | Genotype (vs. NTg Mice) | Sex (vs. Female Mice) | Aging (vs. 12-Month-Old Mice) | |||
|---|---|---|---|---|---|---|
| Behavioral variable | 12 mo | 16 mo | 12 mo | 16 mo | Each group | |
| CTc | All n.s. | Males p = 0.040 | All n.s. | All n.s. | fNTg f3xTg-AD mNTg m3xTg-AD | p < 0.001 p = 0.003 p < 0.001 p < 0.001 |
| CTlatR | All n.s. | All n.s. | All n.s. | NTg p = 0.024 | fNTg f3xTg-AD mNTg m3xTg-AD | p < 0.001 n.s. n.s. p = 0.048 |
| CTr | All n.s. | All n.s. | All n.s. | All n.s. | fNTg f3xTg-AD mNTg m3xTg-AD | p = 0.001 n.s. n.s. p = 0.035 |
| CTratio | All n.s. | Females p = 0.043 | All n.s. | All n.s. | fNTg f3xTg-AD mNTg m3xTgAD | p = 0.001 n.s. p = 0.001 p = 0.021 |
| Post-Hoc Analysis MARBLE TEST | Genotype (vs. NTg Mice) | Sex (vs. Female Mice) | Aging (vs. 12 mo) | Repeated Test(vs. Day 1) | ||
|---|---|---|---|---|---|---|
| Behavioral variable | 12 mo | 16 mo | 12 mo | 16 mo | Each group | Each group |
| Day 1 (MB1) | ||||||
| MB1.5 | Females p = 0.010 | All n.s. | 3xTg-AD p = 0.036 | NTg p = 0.025 | All n.s. | All n.s. |
| MB1.10 | Females p = 0.013 | Males p = 0.016 | All n.s. | NTg p = 0.043 | All n.s. | All n.s. |
| MB1.15 | Females p = 0.009 | Females p = 0.046 Males p = 0.000 | All n.s. | NTg p = 0.002 3xTg-AD p = 0.011 | All n.s. | All n.s. |
| MB1.20 | Females p = 0.014 | Females p = 0.040 Males p = 0.000 | All n.s. | NTg p = 0.003 3xTg-AD p = 0.003 | f3xTg-AD p = 0.043 | All n.s. |
| MB1.25 | All n.s. | Females p = 0.011 Males p = 0.001 | All n.s. | NTg p = 0.004 3xTg-AD p = 0.003 | f3xTg-AD p = 0.010 | All n.s. |
| MB1.30 | All n.s. | Females p = 0.002 | All n.s. | NTg p = 0.012 3xTg-AD p = 0.007 | f3xTg-AD p = 0.010 | All n.s. |
| Day 2 (MB2) | ||||||
| MB2.5 | Males p = 0.005 | Males p = 0.021 | 3xTg-AD p = 0.009 | All n.s. | All n.s. | All n.s. |
| MB2.10 | All n.s. | Males p = 0.024 | 3xTg-AD p = 0.045 | All n.s. | All n.s. | All n.s. |
| MB2.15 | Males p = 0.006 | Males p = 0.008 | All n.s. | All n.s. | All n.s. | All n.s. |
| MB2.20 | Males p = 0.007 | Males p = 0.004 | All n.s. | 3xTg-AD p = 0.012 | All n.s. | All n.s. |
| MB2.25 | Males p = 0.024 | Males p = 0.020 | Alln.s. | 3xTg-AD p = 0.039 | All n.s. | All n.s. |
| MB2.30 | All n.s. | Males p = 0.038 | All n.s. | 3xTg-AD p = 0.025 | All n.s. | fNTg p = 0.005 |
| CTc | CTlatR | CTr | CTratio | ||
|---|---|---|---|---|---|
| fNTg (n = 13) at 12 moa | |||||
| MB1.5 | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | 0.716 ** 0.006 | n.s. |
| MB1.10 | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | 0.617 * 0.025 | n.s. |
| MB1.15 | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | 0.697 ** 0.008 | n.s. |
| MB1.20 | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | 0.631 * 0.021 | n.s. |
| MB1.25 | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | n.s. | n.s. |
| MB1.30 | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | 0.620 * 0.024 | n.s. |
| fNTg at 16 moa | |||||
| MB1.25 | Spearman correlation Sig. (2-tailed) | 0.568 * 0.043 | n.s. | n.s. | n.s. |
| f3xTg-AD (n = 8) at 12 moa | |||||
| MB1.5 | Spearman correlation Sig. (2-tailed) | n.s. | −0.845 ** 0.008 | 0.0835 ** 0.010 | n.s. |
| All the other groups | |||||
| MB1.all | Spearman correlation Sig. (2-tailed) | n.s. | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-Santana, M.; Bayascas, J.-R.; Giménez-Llort, L. Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice. Biomedicines 2021, 9, 994. https://doi.org/10.3390/biomedicines9080994
Santana-Santana M, Bayascas J-R, Giménez-Llort L. Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice. Biomedicines. 2021; 9(8):994. https://doi.org/10.3390/biomedicines9080994
Chicago/Turabian StyleSantana-Santana, Mikel, José-Ramón Bayascas, and Lydia Giménez-Llort. 2021. "Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice" Biomedicines 9, no. 8: 994. https://doi.org/10.3390/biomedicines9080994
APA StyleSantana-Santana, M., Bayascas, J.-R., & Giménez-Llort, L. (2021). Sex-Dependent Signatures, Time Frames and Longitudinal Fine-Tuning of the Marble Burying Test in Normal and AD-Pathological Aging Mice. Biomedicines, 9(8), 994. https://doi.org/10.3390/biomedicines9080994








