Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry
Abstract
1. Introduction
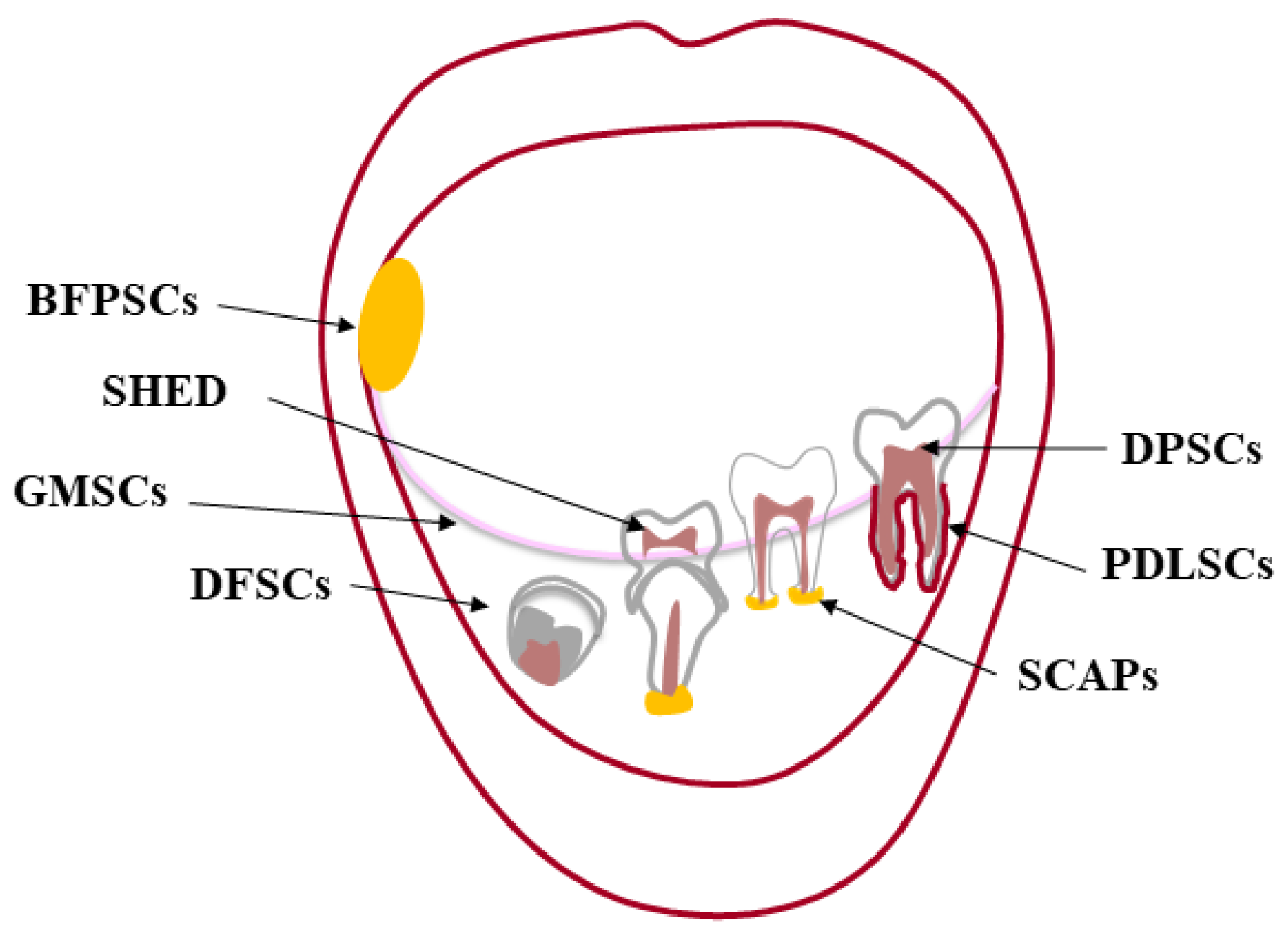
2. Oral MSCs
2.1. Dental Pulp Stem Cells (DPSCs)
2.2. Stem Cells from Exfoliated Deciduous Teeth (SHED)
2.3. Periodontal Ligament Stem Cells (PDLSCs)
2.4. Stem Cells from the Apical Papilla (SCAPs)
2.5. Dental Follicle Stem Cells (DFSCs)
2.6. Gingival Mesenchymal Stem Cells (GMSCs)
2.7. Buccal Fat Pad Stem Cells (BFPSCs)
3. MSCs-Based Therapeutic Approaches
3.1. Periodontal Diseases
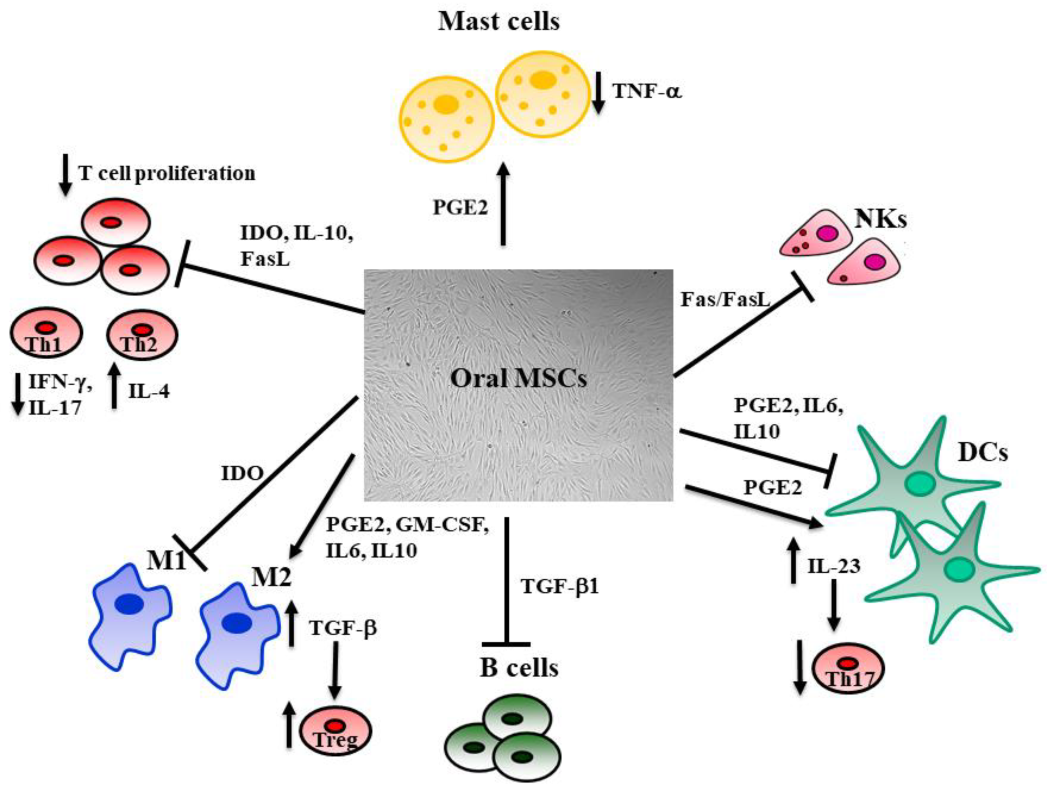
3.1.1. Exploiting the Immunomodulatory Potential of MSCs
3.1.2. Regenerating the Periodontium
3.2. Dental Pulp Restoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Kuci, S.; Kuci, Z.; Latifi-Pupovci, H.; Niethammer, D.; Handgretinger, R.; Schumm, M.; Bruchelt, G.; Bader, P.; Klingebiel, T. Adult Stem Cells as an Alternative Source of Multipotential (Pluripotential) Cells in Regenerative Medicine. Curr. Stem. Cell Res. Ther. 2009, 4, 107–117. [Google Scholar] [CrossRef]
- Tuan, R.S.; Boland, G.; Tuli, R. Adult Mesenchymal Stem Cells and Cell-Based Tissue Engineering. Arthritis Res. Ther. 2003, 5, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Wagers, A.J.; Christensen, J.L.; Weissman, I.L. Cell Fate Determination from Stem Cells. Gene Ther. 2002, 9, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Kanno, M.; Miharada, K.; Ogawa, S.; Hiroyama, T.; Saijo, K.; Nakamura, Y. Mesenchymal Progenitors Able to Differentiate into Osteogenic, Chondrogenic, and/or Adipogenic Cells in Vitro Are Present in Most Primary Fibroblast-like Cell Populations. Stem Cells 2007, 25, 1610–1617. [Google Scholar] [CrossRef]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.Y. The Meaning, the Sense and the Significance: Translating the Science of Mesenchymal Stem Cells into Medicine. Nat. Med. 2013, 19, 35–42. [Google Scholar] [CrossRef]
- Sanz, A.R.; Carrion, F.S.; Chaparro, A.P. Mesenchymal Stem Cells from the Oral Cavity and Their Potential Value in Tissue Engineering. Periodontol. 2000 2015, 67, 251–267. [Google Scholar] [CrossRef]
- Bassir, S.H.; Wisitrasameewong, W.; Raanan, J.; Ghaffarigarakani, S.; Chung, J.; Freire, M.; Andrada, L.C.; Intini, G. Potential for Stem Cell-Based Periodontal Therapy. J. Cell. Physiol. 2016, 231, 50–61. [Google Scholar] [CrossRef]
- Gronthos, S.; Akintoye, S.O.; Wang, C.Y.; Shi, S. Bone Marrow Stromal Stem Cells for Tissue Engineering. Periodontol. 2000 2006, 41, 188–195. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal Human Dental Pulp Stem Cells (DPSCs) in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.Y.; Wang, S.; et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [PubMed]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef]
- Morsczeck, C.; Gotz, W.; Schierholz, J.; Zeilhofer, F.; Kuhn, U.; Mohl, C.; Sippel, C.; Hoffmann, K.H. Isolation of Precursor Cells (PCs) from Human Dental Follicle of Wisdom Teeth. Matrix Biol. 2005, 24, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Mitrano, T.I.; Grob, M.S.; Carrion, F.; Nova-Lamperti, E.; Luz, P.A.; Fierro, F.S.; Quintero, A.; Chaparro, A.; Sanz, A. Culture and Characterization of Mesenchymal Stem Cells from Human Gingival Tissue. J. Periodontol. 2010, 81, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Farre-Guasch, E.; Marti-Page, C.; Hernadez-Alfaro, F.; Klein-Nulend, J.; Casals, N. Buccal Fat Pad, an Oral Access Source of Human Adipose Stem Cells with Potential for Osteochondral Tissue Engineering: An in Vitro Study. Tissue Eng. Part C Methods 2010, 16, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Smith, A.J. Cells and Extracellular Matrices of Dentin and Pulp: A Biological Basis for Repair and Tissue Engineering. Crit. Rev. Oral Biol. Med. 2004, 15, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Liu, Y.; Cui, D.; Pan, Y.; Zheng, L.; Wan, M. Dental Tissue-Derived Human Mesenchymal Stem Cells and Their Potential in Therapeutic Application. Stem. Cells Int. 2020, 2020, 8864572. [Google Scholar] [CrossRef]
- Noth, U.; Osyczka, A.M.; Tuli, R.; Hickok, N.J.; Danielson, K.G.; Tuan, R.S. Multilineage Mesenchymal Differentiation Potential of Human Trabecular Bone-Derived Cells. J. Orthop. Res. 2002, 20, 1060–1069. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal Stromal Cells. Biology of Adult Mesenchymal Stem Cells: Regulation of Niche, Self-Renewal and Differentiation. Arthritis Res. Ther. 2007, 9, 204. [Google Scholar] [CrossRef][Green Version]
- Menicanin, D.; Mrozik, K.M.; Wada, N.; Marino, V.; Shi, S.; Bartold, P.M.; Gronthos, S. Periodontal-Ligament-Derived Stem Cells Exhibit the Capacity for Long-Term Survival, Self-Renewal, and Regeneration of Multiple Tissue Types in Vivo. Stem. Cells Dev. 2014, 23, 1001–1011. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Achilleos, A.; Trainor, P.A. Neural Crest Stem Cells: Discovery, Properties and Potential for Therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef]
- Shi, S.; Gronthos, S. Perivascular Niche of Postnatal Mesenchymal Stem Cells in Human Bone Marrow and Dental Pulp. J. Bone. Min. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef]
- Feng, J.; Mantesso, A.; De Bari, C.; Nishiyama, A.; Sharpe, P.T. Dual Origin of Mesenchymal Stem Cells Contributing to Organ Growth and Repair. Proc. Natl. Acad. Sci. USA 2011, 108, 6503–6508. [Google Scholar] [CrossRef] [PubMed]
- Iohara, K.; Zheng, L.; Ito, M.; Ishizaka, R.; Nakamura, H.; Into, T.; Matsushita, K.; Nakashima, M. Regeneration of Dental Pulp after Pulpotomy by Transplantation of CD31-/CD146-Side Population Cells from a Canine Tooth. Regen. Med. 2009, 4, 377–385. [Google Scholar] [CrossRef]
- Huang, G.T.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/Progenitor Cell-Mediated de Novo Regeneration of Dental Pulp with Newly Deposited Continuous Layer of Dentin in an in Vivo Model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp Regeneration by Transplantation of Dental Pulp Stem Cells in Pulpitis: A Pilot Clinical Study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous Autologous Tooth Stem Cells Regenerate Dental Pulp after Implantation into Injured Teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef]
- Smith, A.J.; Tobias, R.S.; Cassidy, N.; Begue-Kirn, C.; Ruch, J.V.; Lesot, H. Influence of Substrate Nature and Immobilization of Implanted Dentin Matrix Components during Induction of Reparative Dentinogenesis. Connect. Tissue Res. 1995, 32, 291–296. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem Cell Properties of Human Dental Pulp Stem Cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Alge, D.L.; Zhou, D.; Adams, L.L.; Wyss, B.K.; Shadday, M.D.; Woods, E.J.; Gabriel Chu, T.M.; Goebel, W.S. Donor-Matched Comparison of Dental Pulp Stem Cells and Bone Marrow-Derived Mesenchymal Stem Cells in a Rat Model. J. Tissue Eng. Regen. Med. 2010, 4, 73–81. [Google Scholar] [CrossRef]
- Shi, S.; Bartold, P.M.; Miura, M.; Seo, B.M.; Robey, P.G.; Gronthos, S. The Efficacy of Mesenchymal Stem Cells to Regenerate and Repair Dental Structures. Orthod. Craniofac. Res. 2005, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Otaki, S.; Ueshima, S.; Shiraishi, K.; Sugiyama, K.; Hamada, S.; Yorimoto, M.; Matsuo, O. Mesenchymal Progenitor Cells in Adult Human Dental Pulp and Their Ability to Form Bone When Transplanted into Immunocompromised Mice. Cell. Biol. Int. 2007, 31, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, Y.; Deng, Z.; Tang, L.; Li, Y.; Shi, J.; Jin, Y. Odontogenic Capability: Bone Marrow Stromal Stem Cells versus Dental Pulp Stem Cells. Biol. Cell 2007, 99, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Laino, G.; d’Aquino, R.; Graziano, A.; Lanza, V.; Carinci, F.; Naro, F.; Pirozzi, G.; Papaccio, G. A New Population of Human Adult Dental Pulp Stem Cells: A Useful Source of Living Autologous Fibrous Bone Tissue (LAB). J. Bone Min. Res. 2005, 20, 1394–1402. [Google Scholar] [CrossRef]
- Laino, G.; Carinci, F.; Graziano, A.; d’Aquino, R.; Lanza, V.; De Rosa, A.; Gombos, F.; Caruso, F.; Guida, L.; Rullo, R.; et al. In Vitro Bone Production Using Stem Cells Derived from Human Dental Pulp. J. Craniofac. Surg. 2006, 17, 511–515. [Google Scholar] [CrossRef]
- D’Aquino, R.; Graziano, A.; Sampaolesi, M.; Laino, G.; Pirozzi, G.; De Rosa, A.; Papaccio, G. Human Postnatal Dental Pulp Cells Co-Differentiate into Osteoblasts and Endotheliocytes: A Pivotal Synergy Leading to Adult Bone Tissue Formation. Cell Death Differ. 2007, 14, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; d’Aquino, R.; Laino, G.; Papaccio, G. Dental Pulp Stem Cells: A Promising Tool for Bone Regeneration. Stem Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tang, L.; Jin, F.; Liu, X.H.; Yu, J.H.; Wu, J.J.; Yang, Z.H.; Wang, Y.X.; Duan, Y.Z.; Jin, Y. The Apical Region of Developing Tooth Root Constitutes a Complex and Maintains the Ability to Generate Root and Periodontium-like Tissues. J. Periodontal Res. 2009, 44, 275–282. [Google Scholar] [CrossRef]
- Lorusso, F.; Inchingolo, F.; Dipalma, G.; Postiglione, F.; Fulle, S.; Scarano, A. Synthetic Scaffold/Dental Pulp Stem Cell (DPSC) Tissue Engineering Constructs for Bone Defect Treatment: An Animal Studies Literature Review. Int. J. Mol. Sci. 2020, 21, 9765. [Google Scholar] [CrossRef] [PubMed]
- Kerkis, I.; Caplan, A.I. Stem Cells in Dental Pulp of Deciduous Teeth. Tissue Eng. Part B Rev. 2012, 18, 129–138. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, Y.; Katagiri, W.; Sugito, T.; Ito, K.; Ueda, M. Stem Cell Proliferation Pathways Comparison between Human Exfoliated Deciduous Teeth and Dental Pulp Stem Cells by Gene Expression Profile from Promising Dental Pulp. J. Endod. 2009, 35, 1536–1542. [Google Scholar] [CrossRef]
- Rosa, V.; Zhang, Z.; Grande, R.H.; Nor, J.E. Dental Pulp Tissue Engineering in Full-Length Human Root Canals. J. Dent. Res. 2013, 92, 970–975. [Google Scholar] [CrossRef]
- Sakai, V.T.; Zhang, Z.; Dong, Z.; Neiva, K.G.; Machado, M.A.; Shi, S.; Santos, C.F.; Nor, J.E. SHED Differentiate into Functional Odontoblasts and Endothelium. J. Dent. Res. 2010, 89, 791–796. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.H.; Kim, J.W.; Pyeon, H.J.; Lee, J.C.; Lee, G.; Nam, H. In Vivo Angiogenic Capacity of Stem Cells from Human Exfoliated Deciduous Teeth with Human Umbilical Vein Endothelial Cells. Mol. Cells 2016, 39, 790–796. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Corsalini, M.; Schierano, G.; Pettini, F.; Di Venere, D.; Carossa, S. Cytokine, Chemokine, and Growth Factor Profile Characterization of Undifferentiated and Osteoinduced Human Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 6202783. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Tsunekawa, S.; Nakamura, N.; Miura-Yura, E.; Yamada, Y.; Hayashi, Y.; Nakai-Shimoda, H.; Asano, S.; Hayami, T.; Motegi, M.; et al. Secreted Factors from Stem Cells of Human Exfoliated Deciduous Teeth Directly Activate Endothelial Cells to Promote All Processes of Angiogenesis. Cells 2020, 9, 2385. [Google Scholar] [CrossRef]
- Seo, B.M.; Sonoyama, W.; Yamaza, T.; Coppe, C.; Kikuiri, T.; Akiyama, K.; Lee, J.S.; Shi, S. SHED Repair Critical-Size Calvarial Defects in Mice. Oral. Dis. 2008, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.Y.; Su, W.T.; Chen, P.H. Comparing the Effects of Chitosan Scaffolds Containing Various Divalent Metal Phosphates on Osteogenic Differentiation of Stem Cells from Human Exfoliated Deciduous Teeth. Biol. Trace Elem. Res. 2018, 185, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Kapila, Y.; Lotz, J.; Kapila, S. Multiple Differentiation Capacity of STRO-1+/CD146+ PDL Mesenchymal Progenitor Cells. Stem Cells Dev. 2009, 18, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Su, W.T.; Chen, P.H. Magnesium and Zinc Borate Enhance Osteoblastic Differentiation of Stem Cells from Human Exfoliated Deciduous Teeth in Vitro. J. Biomater. Appl. 2018, 32, 765–774. [Google Scholar] [CrossRef]
- Gay, I.C.; Chen, S.; MacDougall, M. Isolation and Characterization of Multipotent Human Periodontal Ligament Stem Cells. Orthod. Craniofac. Res. 2007, 10, 149–160. [Google Scholar] [CrossRef]
- Lindroos, B.; Maenpaa, K.; Ylikomi, T.; Oja, H.; Suuronen, R.; Miettinen, S. Characterisation of Human Dental Stem Cells and Buccal Mucosa Fibroblasts. Biochem. Biophys. Res. Commun. 2008, 368, 329–335. [Google Scholar] [CrossRef]
- Lim, J.C.; Bae, S.H.; Lee, G.; Ryu, C.J.; Jang, Y.J. Activation of Beta-Catenin by TGF-Beta1 Promotes Ligament-Fibroblastic Differentiation and Inhibits Cementoblastic Differentiation of Human Periodontal Ligament Cells. Stem Cells 2020. [Google Scholar] [CrossRef]
- Aghamohamadi, Z.; Kadkhodazadeh, M.; Torshabi, M.; Tabatabaei, F. A Compound of Concentrated Growth Factor and Periodontal Ligament Stem Cell-Derived Conditioned Medium. Tissue Cell 2020, 65, 101373. [Google Scholar] [CrossRef]
- Huang, G.T.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The Hidden Treasure in Apical Papilla: The Potential Role in Pulp/Dentin Regeneration and Bioroot Engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, H.; Huang, Y.; Liu, C. Comparative Analysis of in Vitro Periodontal Characteristics of Stem Cells from Apical Papilla (SCAP) and Periodontal Ligament Stem Cells (PDLSCs). Arch. Oral Biol. 2013, 58, 997–1006. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, X.; Yu, S.; Yang, N.; Zeng, J.; Liu, X.; Chen, X. Exosomes Derived from Stem Cells from Apical Papilla Promote Craniofacial Soft Tissue Regeneration by Enhancing Cdc42-Mediated Vascularization. Stem Cell Res. Ther. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bai, Y.; Matsuzaka, K.; Hashimoto, S.; Fukuyama, T.; Wu, L.; Miwa, T.; Liu, X.; Wang, X.; Inoue, T. Cementum- and Periodontal Ligament-like Tissue Formation by Dental Follicle Cell Sheets Co-Cultured with Hertwig’s Epithelial Root Sheath Cells. Bone 2011, 48, 1417–1426. [Google Scholar] [CrossRef]
- Han, C.; Yang, Z.; Zhou, W.; Jin, F.; Song, Y.; Wang, Y.; Huo, N.; Chen, L.; Qian, H.; Hou, R.; et al. Periapical Follicle Stem Cell: A Promising Candidate for Cementum/Periodontal Ligament Regeneration and Bio-Root Engineering. Stem Cells Dev. 2010, 19, 1405–1415. [Google Scholar] [CrossRef]
- Yildirim, S.; Zibandeh, N.; Genc, D.; Ozcan, E.M.; Goker, K.; Akkoc, T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016, 2016, 4682875. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Bai, D.; Guo, W.; Li, J.; Zeng, J.; Yang, L.; Jiang, Z.; Feng, L.; Yu, M.; Tian, W. Comparison of Human Dental Follicle Cells and Human Periodontal Ligament Cells for Dentin Tissue Regeneration. Regen. Med. 2015, 10, 461–479. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Mrozik, K.M.; Menicanin, D.; Gronthos, S.; Bartold, P.M. Isolation and Characterization of Mesenchymal Stem Cell-like Cells from Healthy and Inflamed Gingival Tissue: Potential Use for Clinical Therapy. Regen. Med. 2012, 7, 819–832. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-Alpha-Treated Human Gingiva-Derived MSCs Enhance M2 Macrophage Polarization and Inhibit Periodontal Bone Loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Diomede, F.; Gugliandolo, A.; Cardelli, P.; Merciaro, I.; Ettorre, V.; Traini, T.; Bedini, R.; Scionti, D.; Bramanti, A.; Nanci, A.; et al. Three-Dimensional Printed PLA Scaffold and Human Gingival Stem Cell-Derived Extracellular Vesicles: A New Tool for Bone Defect Repair. Stem Cell Res. Ther. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, S.Q.; Zhang, K.; Zhang, W.J.; Liu, H.L.; Xu, Z.; Li, H.; Lou, J.N.; Ge, L.H.; Xu, B.H. Treatment of Gingival Defects with Gingival Mesenchymal Stem Cells Derived from Human Fetal Gingival Tissue in a Rat Model. Stem Cell Res. Ther. 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Meshram, M.; Anchlia, S.; Shah, H.; Vyas, S.; Dhuvad, J.; Sagarka, L. Buccal Fat Pad-Derived Stem Cells for Repair of Maxillofacial Bony Defects. J. Maxillofac. Oral Surg. 2019, 18, 112–123. [Google Scholar] [CrossRef]
- Khojasteh, A.; Hosseinpour, S.; Rezai Rad, M.; Alikhasi, M.; Zadeh, H.H. Buccal Fat Pad-Derived Stem Cells with Anorganic Bovine Bone Mineral Scaffold for Augmentation of Atrophic Posterior Mandible: An Exploratory Prospective Clinical Study. Clin. Implant. Dent. Relat. Res. 2019, 21, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Tasinato, F.; Petrillo, S.; Ruffinatti, F.A.; Mela, L.; Carossa, M.; Munaron, L.; Roato, I.; Mussano, F.; Cavagnetto, D. Isolation and Characterization of Buccal Fat Pad and Dental Pulp MSCs from the Same Donor. Biomedicines 2021, 9, 265. [Google Scholar] [CrossRef]
- Doonquah, L.; Holmes, P.J.; Ranganathan, L.K.; Robertson, H. Bone Grafting for Implant Surgery. Oral Maxillofac. Surg. Clin. 2021, 33, 211–229. [Google Scholar] [CrossRef]
- Iwata, T.; Yamato, M.; Zhang, Z.; Mukobata, S.; Washio, K.; Ando, T.; Feijen, J.; Okano, T.; Ishikawa, I. Validation of Human Periodontal Ligament-Derived Cells as a Reliable Source for Cytotherapeutic Use. J. Clin. Periodontol. 2010, 37, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Darveau, R.P. Periodontitis: A Polymicrobial Disruption of Host Homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef]
- Lourenco, T.G.; Heller, D.; Silva-Boghossian, C.M.; Cotton, S.L.; Paster, B.J.; Colombo, A.P. Microbial Signature Profiles of Periodontally Healthy and Diseased Patients. J. Clin. Periodontol. 2014, 41, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Teles, F.; Teles, R.; Figueiredo, L.C.; Faveri, M. The Subgingival Periodontal Microbiota of the Aging Mouth. Periodontol. 2000 2016, 72, 30–53. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal Diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T.; Lambris, J.D. Current Understanding of Periodontal Disease Pathogenesis and Targets for Host-Modulation Therapy. Periodontol. 2000 2020, 84, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and Immune Pathways in the Pathogenesis of Periodontal Disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Kononen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of Matrix Metalloproteinases, Especially MMP-8, in Gingival Creviclular Fluid, Mouthrinse and Saliva for Monitoring Periodontal Diseases. Periodontol. 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- Gemmell, E.; Seymour, G.J. Immunoregulatory Control of Th1/Th2 Cytokine Profiles in Periodontal Disease. Periodontol. 2000 2004, 35, 21–41. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 Cells and the IL-23/IL-17 Axis in the Pathogenesis of Periodontitis and Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Periodontal Microbial Ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are Reactive Oxygen Species Always Detrimental to Pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef]
- Zhou, L.L.; Liu, W.; Wu, Y.M.; Sun, W.L.; Dorfer, C.E.; Fawzy El-Sayed, K.M. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020, 2020, 1327405. [Google Scholar] [CrossRef] [PubMed]
- Su, W.R.; Zhang, Q.Z.; Shi, S.H.; Nguyen, A.L.; Le, A.D. Human Gingiva-Derived Mesenchymal Stromal Cells Attenuate Contact Hypersensitivity via Prostaglandin E2-Dependent Mechanisms. Stem Cells 2011, 29, 1849–1860. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, Q.Z.; Karabucak, B.; Le, A.D. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-Alpha/IDO Axis. J. Dent. Res. 2016, 95, 1274–1281. [Google Scholar] [CrossRef]
- Lan, Q.; Fan, H.; Quesniaux, V.; Ryffel, B.; Liu, Z.; Zheng, S.G. Induced Foxp3(+) Regulatory T Cells: A Potential New Weapon to Treat Autoimmune and Inflammatory Diseases? J. Mol. Cell Biol. 2012, 4, 22–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Jin, Y.; Shi, S. Fas Ligand Regulates the Immunomodulatory Properties of Dental Pulp Stem Cells. J. Dent. Res. 2012, 91, 948–954. [Google Scholar] [CrossRef]
- Ding, Q.; Yoshimitsu, M.; Kuwahata, T.; Maeda, K.; Hayashi, T.; Obara, T.; Miyazaki, Y.; Matsubara, S.; Natsugoe, S.; Takao, S. Establishment of a Highly Migratory Subclone Reveals That CD133 Contributes to Migration and Invasion through Epithelial-Mesenchymal Transition in Pancreatic Cancer. Hum. Cell 2012, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Glenn, J.D.; Whartenby, K.A. Mesenchymal Stem Cells: Emerging Mechanisms of Immunomodulation and Therapy. World J. Stem Cells 2014, 6, 526–539. [Google Scholar] [CrossRef]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous HMSCs Improve Myocardial Infarction in Mice Because Cells Embolized in Lung Are Activated to Secrete the Anti-Inflammatory Protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Shen, Z.; Guan, M.; Huang, Q.; Chen, L.; Qin, W.; Ge, X.; Chen, H.; Xiao, Y.; Lin, Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng. Part. A 2018, 24, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Roddy, G.W.; Oh, J.Y.; Lee, R.H.; Bartosh, T.J.; Ylostalo, J.; Coble, K.; Rosa, R.H., Jr.; Prockop, D.J. Action at a Distance: Systemically Administered Adult Stem/Progenitor Cells (MSCs) Reduce Inflammatory Damage to the Cornea without Engraftment and Primarily by Secretion of TNF-Alpha Stimulated Gene/Protein 6. Stem Cells 2011, 29, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. The Exciting Prospects of New Therapies with Mesenchymal Stromal Cells. Cytotherapy 2017, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, C.M.; An, S.; Cheng, Q.; Huang, Y.F.; Wang, Y.T.; Gou, Y.C.; Xiao, L.; Yu, W.J.; Wang, J. Immunomodulatory Properties of Dental Tissue-Derived Mesenchymal Stem Cells. Oral Dis. 2014, 20, 25–34. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of Mesenchymal Stem Cells in Immunomodulation: Pathological and Therapeutic Implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, D.; Zhang, Y.; Li, M. Inflammation, Mesenchymal Stem Cells and Bone Regeneration. Histochem. Cell Biol. 2018, 149, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X.; Cao, K.; Chen, Q.; Shou, P.; et al. Mesenchymal Stem Cells: A Double-Edged Sword in Regulating Immune Responses. Cell Death Differ. 2012, 19, 1505–1513. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, J.Y.; Zhou, G. Emerging Functions and Clinical Applications of Exosomes in Human Oral Diseases. Cell Biosci. 2020, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, C.; Yang, J.; Jin, Y.; Zheng, W.; Zhou, Q.; Liang, Y.; Bao, L.; Feng, G.; Ji, J.; et al. Exosomal MicroRNA-155-5p from PDLSCs Regulated Th17/Treg Balance by Targeting Sirtuin-1 in Chronic Periodontitis. J. Cell. Physiol. 2019, 234, 20662–20674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Dai, W.; Wang, H.; Xue, C.; Feng, J.; He, Y.; Wang, P.; Li, S.; Bai, D.; Shu, R. Periodontal Ligament Fibroblasts Regulate Osteoblasts by Exosome Secretion Induced by Inflammatory Stimuli. Arch. Oral Biol. 2019, 105, 27–34. [Google Scholar] [CrossRef]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic Stretch Force Induces Periodontal Ligament Cells to Secrete Exosomes That Suppress IL-1beta Production Through the Inhibition of the NF-KappaB Signaling Pathway in Macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Ye, Y.; He, S.; Song, J. SHED-Derived Conditioned Exosomes Enhance the Osteogenic Differentiation of PDLSCs via Wnt and BMP Signaling in Vitro. Differentiation 2020, 111, 1–11. [Google Scholar] [CrossRef]
- Wakayama, H.; Hashimoto, N.; Matsushita, Y.; Matsubara, K.; Yamamoto, N.; Hasegawa, Y.; Ueda, M.; Yamamoto, A. Factors Secreted from Dental Pulp Stem Cells Show Multifaceted Benefits for Treating Acute Lung Injury in Mice. Cytotherapy 2015, 17, 1119–1129. [Google Scholar] [CrossRef]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 Complex Regulates IL-1RA Secretion in Mesenchymal Stem Cells to Accelerate Wound Healing. Sci. Transl. Med. 2018, 10, eaai8524. [Google Scholar] [CrossRef]
- Shi, H.Z.; Zeng, J.C.; Shi, S.H.; Giannakopoulos, H.; Zhang, Q.Z.; Le, A.D. Extracellular Vesicles of GMSCs Alleviate Aging-Related Cell Senescence. J. Dent. Res. 2021, 100, 283–292. [Google Scholar] [CrossRef]
- Shi, Q.; Qian, Z.; Liu, D.; Sun, J.; Wang, X.; Liu, H.; Xu, J.; Guo, X. GMSC-Derived Exosomes Combined with a Chitosan/Silk Hydrogel Sponge Accelerates Wound Healing in a Diabetic Rat Skin Defect Model. Front. Physiol. 2017, 8, 904. [Google Scholar] [CrossRef] [PubMed]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shan, Z.; Ma, P.; Wang, S.; Fan, Z. Allogeneic Bone Marrow Mesenchymal Stem Cell Transplantation for Periodontal Regeneration. J. Dent. Res. 2014, 93, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.; Stavropoulos, A. Biomaterials for Promoting Periodontal Regeneration in Human Intrabony Defects: A Systematic Review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Bartold, P.M.; Gronthos, S.; Ivanovski, S.; Fisher, A.; Hutmacher, D.W. Tissue Engineered Periodontal Products. J. Periodontal Res. 2016, 51, 1–15. [Google Scholar] [CrossRef]
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-Printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94 (Suppl. S9), 153S–157S. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Sculean, A. Does Periodontal Tissue Regeneration Really Work? Periodontol. 2000 2009, 51, 208–219. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hirachi, A.; Hasegawa, N.; Iwata, T.; Hamaguchi, H.; Shiba, H.; Takata, T.; Kato, Y.; Kurihara, H. Enhancement of Periodontal Tissue Regeneration by Transplantation of Bone Marrow Mesenchymal Stem Cells. J. Periodontol. 2004, 75, 1281–1287. [Google Scholar] [CrossRef]
- Shi, H.; Zong, W.; Xu, X.; Chen, J. Improved Biphasic Calcium Phosphate Combined with Periodontal Ligament Stem Cells May Serve as a Promising Method for Periodontal Regeneration. Am. J. Transl. Res. 2018, 10, 4030–4041. [Google Scholar]
- Mohammed, E.; Khalil, E.; Sabry, D. Effect of Adipose-Derived Stem Cells and Their Exo as Adjunctive Therapy to Nonsurgical Periodontal Treatment: A Histologic and Histomorphometric Study in Rats. Biomolecules 2018, 8, 167. [Google Scholar] [CrossRef]
- Duan, X.; Tu, Q.; Zhang, J.; Ye, J.; Sommer, C.; Mostoslavsky, G.; Kaplan, D.; Yang, P.; Chen, J. Application of Induced Pluripotent Stem (IPS) Cells in Periodontal Tissue Regeneration. J. Cell. Physiol. 2011, 226, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Yamada, N.; Sakai, H.; Sakurai, Y. A Novel Recovery System for Cultured Cells Using Plasma-Treated Polystyrene Dishes Grafted with Poly(N-Isopropylacrylamide). J. Biomed. Mater. Res. 1993, 27, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yamato, M.; Kikuchi, A.; Okano, T.; Ishikawa, I. Human Periodontal Ligament Cell Sheets Can Regenerate Periodontal Ligament Tissue in an Athymic Rat Model. Tissue Eng. 2005, 11, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.G.; Hasegawa, M.; Yamato, M.; Takagi, R.; Okano, T.; Ishikawa, I. Cementum-Periodontal Ligament Complex Regeneration Using the Cell Sheet Technique. J. Periodontal Res. 2008, 43, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.G.; Yashiro, R.; Washio, K.; Yamato, M.; Okano, T.; Ishikawa, I. Periodontal Ligament Cell Sheet Promotes Periodontal Regeneration in Athymic Rats. J. Clin. Periodontol. 2008, 35, 1066–1072. [Google Scholar] [CrossRef]
- Tassi, S.A.; Sergio, N.Z.; Misawa, M.Y.O.; Villar, C.C. Efficacy of Stem Cells on Periodontal Regeneration: Systematic Review of Pre-Clinical Studies. J. Periodontal Res. 2017, 52, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Novello, S.; Debouche, A.; Philippe, M.; Naudet, F.; Jeanne, S. Clinical Application of Mesenchymal Stem Cells in Periodontal Regeneration: A Systematic Review and Meta-Analysis. J. Periodontal Res. 2020, 55, 1–12. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of Periodontal Intrabony Defects Using Autologous Periodontal Ligament Stem Cells: A Randomized Clinical Trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human Intrabony Defect Regeneration with Micrografts Containing Dental Pulp Stem Cells: A Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological Perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Hiller, K.A.; Nunez, L.J.; Stoll, J.; Weis, K. Permeability Characteristics of Bovine and Human Dentin under Different Pretreatment Conditions. J. Endod. 2001, 27, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Loghin, S.; Lin, L.M.; Spangberg, L.S.; Tay, F.R. Is Hard Tissue Formation in the Dental Pulp after the Death of the Primary Odontoblasts a Regenerative or a Reparative Process? J. Dent. 2014, 42, 1156–1170. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Li, Y.; Tay, F.R. Vital Pulp Therapy: Histopathology and Histobacteriology-Based Guidelines to Treat Teeth with Deep Caries and Pulp Exposure. J. Dent. 2019, 86, 41–52. [Google Scholar] [CrossRef]
- Cooper, P.R.; Takahashi, Y.; Graham, L.W.; Simon, S.; Imazato, S.; Smith, A.J. Inflammation-Regeneration Interplay in the Dentine-Pulp Complex. J. Dent. 2010, 38, 687–697. [Google Scholar] [CrossRef]
- Farges, J.C.; Alliot-Licht, B.; Renard, E.; Ducret, M.; Gaudin, A.; Smith, A.J.; Cooper, P.R. Dental Pulp Defence and Repair Mechanisms in Dental Caries. Mediat. Inflamm. 2015, 2015, 230251. [Google Scholar] [CrossRef]
- Staquet, M.J.; Durand, S.H.; Colomb, E.; Romeas, A.; Vincent, C.; Bleicher, F.; Lebecque, S.; Farges, J.C. Different Roles of Odontoblasts and Fibroblasts in Immunity. J. Dent. Res. 2008, 87, 256–261. [Google Scholar] [CrossRef]
- Chogle, S.M.; Goodis, H.E.; Kinaia, B.M. Pulpal and Periradicular Response to Caries: Current Management and Regenerative Options. Dent. Clin. 2012, 56, 521–536. [Google Scholar] [CrossRef]
- Nowicka, A.; Lipski, M.; Parafiniuk, M.; Sporniak-Tutak, K.; Lichota, D.; Kosierkiewicz, A.; Kaczmarek, W.; Buczkowska-Radlinska, J. Response of Human Dental Pulp Capped with Biodentine and Mineral Trioxide Aggregate. J. Endod. 2013, 39, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Neves, V.C.; Babb, R.; Chandrasekaran, D.; Sharpe, P.T. Promotion of Natural Tooth Repair by Small Molecule GSK3 Antagonists. Sci. Rep. 2017, 7, 39654. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, L.K.; Banu, A.; Walther, A.R.; Chandrasekaran, D.; Babb, R.C.; Salzlechner, C.; Hedegaard, M.A.B.; Gentleman, E.; Sharpe, P.T. Translation Approach for Dentine Regeneration Using GSK-3 Antagonists. J. Dent. Res. 2020, 99, 544–551. [Google Scholar] [CrossRef]
- Wolters, W.J.; Duncan, H.F.; Tomson, P.L.; Karim, I.E.; McKenna, G.; Dorri, M.; Stangvaltaite, L.; Van der Sluis, L.W.M. Minimally Invasive Endodontics: A New Diagnostic System for Assessing Pulpitis and Subsequent Treatment Needs. Int. Endod. J. 2017, 50, 825–829. [Google Scholar] [CrossRef]
- Elmsmari, F.; Ruiz, X.F.; Miro, Q.; Feijoo-Pato, N.; Duran-Sindreu, F.; Olivieri, J.G. Outcome of Partial Pulpotomy in Cariously Exposed Posterior Permanent Teeth: A Systematic Review and Meta-Analysis. J. Endod. 2019, 45, 1296–1306.e3. [Google Scholar] [CrossRef] [PubMed]
- Cvek, M. Prognosis of Luxated Non-Vital Maxillary Incisors Treated with Calcium Hydroxide and Filled with Gutta-Percha. A Retrospective Clinical Study. Endod. Dent. Traumatol. 1992, 8, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, C.; Marce-Nogue, J.; Galler, K.M.; Widbiller, M. Biomechanical Performance of an Immature Maxillary Central Incisor after Revitalization: A Finite Element Analysis. Int. Endod. J. 2019, 52, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, S.I.; Ikawa, M.; Kubota, M. Revascularization of an Immature Permanent Tooth with Apical Periodontitis and Sinus Tract. Dent. Traumatol. 2001, 17, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Banchs, F.; Trope, M. Revascularization of Immature Permanent Teeth with Apical Periodontitis: New Treatment Protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Ruparel, N.B. Regenerative Endodontic Procedures: Clinical Outcomes. Dent. Clin. 2017, 61, 111–125. [Google Scholar] [CrossRef]
- Chan, E.K.; Desmeules, M.; Cielecki, M.; Dabbagh, B.; Dos Santos, B.F. Longitudinal Cohort Study of Regenerative Endodontic Treatment for Immature Necrotic Permanent Teeth. J. Endod. 2017, 43, 395–400. [Google Scholar] [CrossRef]
- Ong, T.K.; Lim, G.S.; Singh, M.; Fial, A.V. Quantitative Assessment of Root Development after Regenerative Endodontic Therapy: A Systematic Review and Meta-Analysis. J. Endod. 2020, 46, 1856–1866.e2. [Google Scholar] [CrossRef] [PubMed]
- Digka, A.; Sakka, D.; Lyroudia, K. Histological Assessment of Human Regenerative Endodontic Procedures (REP) of Immature Permanent Teeth with Necrotic Pulp/Apical Periodontitis: A Systematic Review. Aust. Endod. J. 2020, 46, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Diogenes, A.; Ruparel, N.B.; Shiloah, Y.; Hargreaves, K.M. Regenerative Endodontics: A Way Forward. J. Am. Dent. Assoc. 2016, 147, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology Position Statement: Revitalization Procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete Pulp Regeneration after Pulpectomy by Transplantation of CD105+ Stem Cells with Stromal Cell-Derived Factor-1. Tissue. Eng. Part. A. 2011, 17, 1911–1920. [Google Scholar] [CrossRef]
- Nakashima, M.; Iohara, K. Regeneration of Dental Pulp by Stem Cells. Adv. Dent. Res. 2011, 23, 313–319. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Hirono, H.; Nakahara, T.; Ishikawa, H. Dental Pulp Cell Bank as a Possible Future Source of Individual Hepatocytes. World J. Hepatol. 2018, 10, 702–707. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M. Perspectives for Cell-Homing Approaches to Engineer Dental Pulp. J. Endod. 2017, 43 (Suppl. S9), S40–S45. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M. Cell-Free Approaches for Dental Pulp Tissue Engineering. J. Endod. 2020, 46 (Suppl. S9), S143–S149. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Signaling Molecules and Pulp Regeneration. J. Endod. 2017, 43 (Suppl. S9), S7–S11. [Google Scholar] [CrossRef]
- Duncan, H.F.; Kobayashi, Y.; Shimizu, E. Growth Factors and Cell Homing in Dental Tissue Regeneration. Curr. Oral Health Rep. 2018, 5, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.A.; Marques, M.M.; Tedesco, T.K.; Carvalho, G.L.; Goncalves, F.; Caballero-Flores, H.; Morimoto, S.; Moreira, M.S. Screening of Hydrogel-Based Scaffolds for Dental Pulp Regeneration-A Systematic Review. Arch. Oral Biol. 2019, 98, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Driesen, R.B.; Eidt, A.; Lambrichts, I.; Hiller, K.A.; Buchalla, W.; Schmalz, G.; Galler, K.M. Cell Homing for Pulp Tissue Engineering with Endogenous Dentin Matrix Proteins. J. Endod. 2018, 44, 956–962.e2. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Brandl, F.P.; Kirchhof, S.; Widbiller, M.; Eidt, A.; Buchalla, W.; Gopferich, A.; Schmalz, G. Suitability of Different Natural and Synthetic Biomaterials for Dental Pulp Tissue Engineering. Tissue Eng. Part A 2018, 24, 234–244. [Google Scholar] [CrossRef]
- Song, J.S.; Takimoto, K.; Jeon, M.; Vadakekalam, J.; Ruparel, N.B.; Diogenes, A. Decellularized Human Dental Pulp as a Scaffold for Regenerative Endodontics. J. Dent. Res. 2017, 96, 640–646. [Google Scholar] [CrossRef]
- Torabinejad, M.; Alexander, A.; Vahdati, S.A.; Grandhi, A.; Baylink, D.; Shabahang, S. Effect of Residual Dental Pulp Tissue on Regeneration of Dentin-Pulp Complex: An In Vivo Investigation. J. Endod. 2018, 44, 1796–1801. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.C.; Narayanan, R.; Alapati, S.; Ravindran, S. Exosomes as Biomimetic Tools for Stem Cell Differentiation: Applications in Dental Pulp Tissue Regeneration. Biomaterials 2016, 111, 103–115. [Google Scholar] [CrossRef]
- Altanerova, U.; Benejova, K.; Altanerova, V.; Tyciakova, S.; Rychly, B.; Szomolanyi, P.; Ciampor, F.; Cihova, M.; Repiska, V.; Ondicova, K.; et al. Dental Pulp Mesenchymal Stem/Stromal Cells Labeled with Iron Sucrose Release Exosomes and Cells Applied Intra-Nasally Migrate to Intracerebral Glioblastoma. Neoplasma 2016, 63, 925–933. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; Garcia, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepulveda, P. Hypoxia Inducible Factor-1alpha Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Venugopal, C.; Shamir, C.; Senthilkumar, S.; Babu, J.V.; Sonu, P.K.; Nishtha, K.J.; Rai, K.S.; Dhanushkodi, A. Dosage and Passage Dependent Neuroprotective Effects of Exosomes Derived from Rat Bone Marrow Mesenchymal Stem Cells: An In Vitro Analysis. Curr. Gene Ther. 2017, 17, 379–390. [Google Scholar] [CrossRef]
- Xian, X.; Gong, Q.; Li, C.; Guo, B.; Jiang, H. Exosomes with Highly Angiogenic Potential for Possible Use in Pulp Regeneration. J. Endod. 2018, 44, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ju, Y.; Liu, S.; Fu, Y.; Zhao, S. Exosomes Derived from Lipopolysaccharide-Preconditioned Human Dental Pulp Stem Cells Regulate Schwann Cell Migration and Differentiation. Connect. Tissue Res. 2019, 62, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of Immunomodulatory Properties of Exosomes Derived from Bone Marrow Mesenchymal Stem Cells and Dental Pulp Stem Cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Ivica, A.; Ghayor, C.; Zehnder, M.; Valdec, S.; Weber, F.E. Pulp-Derived Exosomes in a Fibrin-Based Regenerative Root Filling Material. J. Clin. Med. 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Ji, L.; Jiang, H.; Liu, Y.; Liu, X.; Bi, J.; Zhao, W.; Ding, Z.; Chen, X. Exosomes Derived from Stem Cells from the Apical Papilla Promote Dentine-Pulp Complex Regeneration by Inducing Specific Dentinogenesis. Stem Cells Int. 2020, 2020, 5816723. [Google Scholar] [CrossRef]
- Wang, H.S.; Yang, F.H.; Wang, Y.J.; Pei, F.; Chen, Z.; Zhang, L. Odontoblastic Exosomes Attenuate Apoptosis in Neighboring Cells. J. Dent. Res. 2019, 98, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Widbiller, M.; Schmalz, G. Endodontic Regeneration: Hard Shell, Soft Core. Odontology 2021, 109, 303–312. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46 (Suppl. S9), S161–S174. [Google Scholar] [CrossRef]
- Zayed, M.; Iohara, K.; Watanabe, H.; Ishikawa, M.; Tominaga, M.; Nakashima, M. Characterization of stable hypoxia-preconditioned dental pulp stem cells compared with mobilized dental pulp stem cells for application for pulp regenerative therapy. Stem Cell Res. Ther. 2021, 12, 302. [Google Scholar] [CrossRef]
| Name | Source | Regeneration Role |
|---|---|---|
| DPSCs | Dental Pulp | angiogenic potential [30], formation of dentin-pulp-like complex in empty root canal spaces [31,32,33], dentin repair [34], bone formation [39,40,41,42] |
| SHED | Exfoliated deciduous teeth | formation of dentin-like or pulp-like tissue [13], differentiation into endothelial cells [49,50], angiogenic ability [51,52], osteoinductive and osteogenic potential [53,54] |
| PDLSCs | Periodontal ligament | regeneration of PDL tissue [14,15,77], lower osteoinductive potential than DPSCs and SHED [56,57,58] |
| SCAPs | Apical papilla | remodeling and differentiation into dentin [15,16], root development and regeneration [62] |
| DFSCs | Dental follicle | odontogenic potential: dentin, root regeneration [67], and periodontal differentiation [17] |
| GMSCs | Gingiva | osteogenic potential in vitro [68,71], gingival lesion treatment [72,78], periodontal ligament regeneration in rats [48] |
| BFPSCs | Buccal fat pad | osteogenic potential [73,74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roato, I.; Chinigò, G.; Genova, T.; Munaron, L.; Mussano, F. Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry. Biomedicines 2021, 9, 1085. https://doi.org/10.3390/biomedicines9091085
Roato I, Chinigò G, Genova T, Munaron L, Mussano F. Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry. Biomedicines. 2021; 9(9):1085. https://doi.org/10.3390/biomedicines9091085
Chicago/Turabian StyleRoato, Ilaria, Giorgia Chinigò, Tullio Genova, Luca Munaron, and Federico Mussano. 2021. "Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry" Biomedicines 9, no. 9: 1085. https://doi.org/10.3390/biomedicines9091085
APA StyleRoato, I., Chinigò, G., Genova, T., Munaron, L., & Mussano, F. (2021). Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry. Biomedicines, 9(9), 1085. https://doi.org/10.3390/biomedicines9091085










