Pathophysiological Implication of Pattern Recognition Receptors in Fetal Membranes Rupture: RAGE and NLRP Inflammasome
Abstract
:1. Fetal Membranes: A Human Barrier Essential to Childbirth
1.1. Structure of Fetal Membranes
1.2. A Multifunctional Barrier: Different Biological Roles of FM during Pregnancy
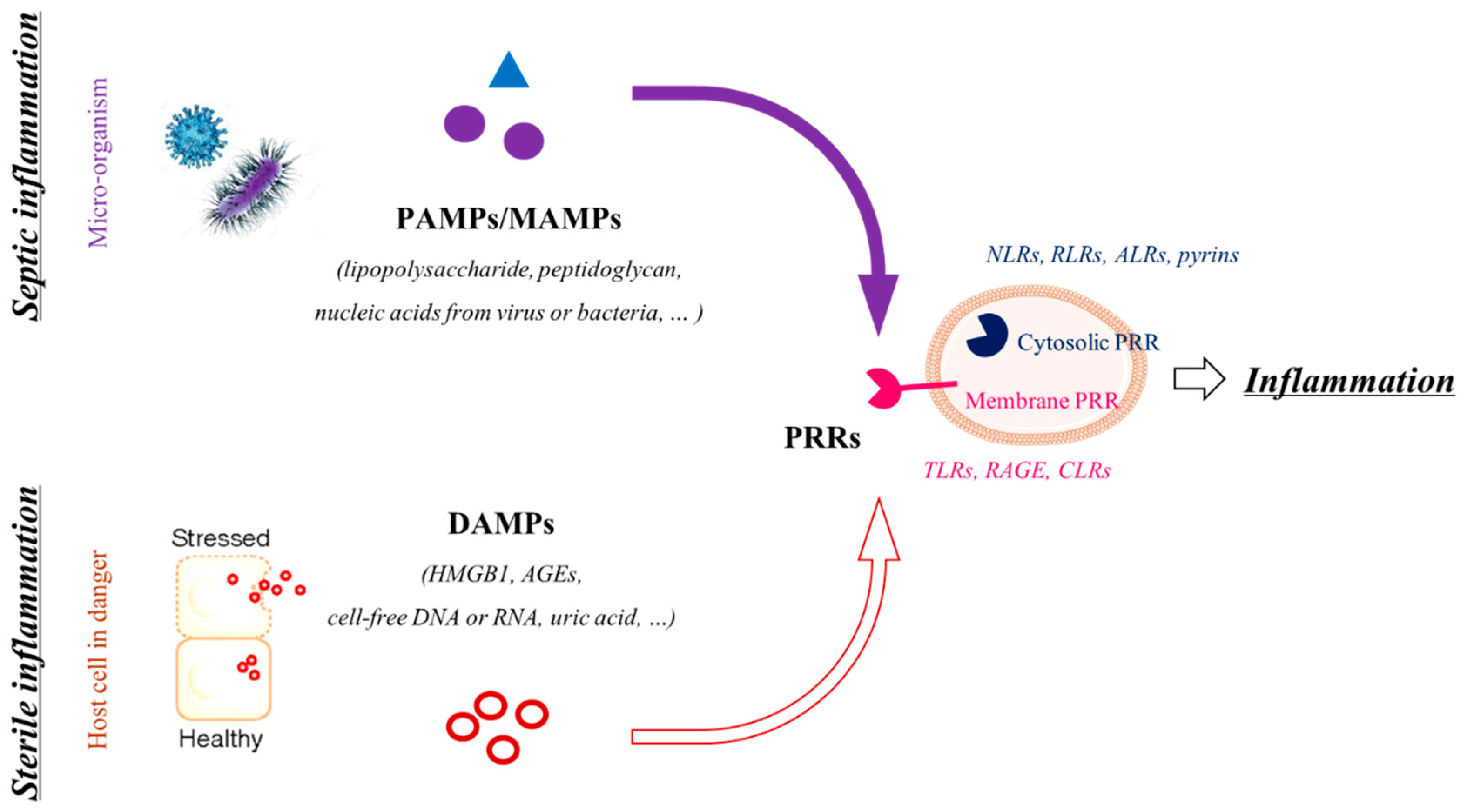
2. Inflammation or How Does the FM Rupture Occur Right on Time or Too Early?
2.1. Happy Ending: Well-Balanced Inflammation for a Well-Prepared Rupture of the FM at Term
2.2. Preterm Prelabor Rupture of Fetal Membranes (pPROM)
2.2.1. Definition and Consequences of the pPROM
2.2.2. Pathophysiology of pPROM and Inflammation
3. The Receptor for Advanced Glycation End Products (RAGE)
3.1. RAGE Protein Structure
3.2. RAGE, Ligands and Signaling
3.3. RAGE and Inflammation
3.4. RAGE and Fetal Membranes Rupture
4. Inflammasomes
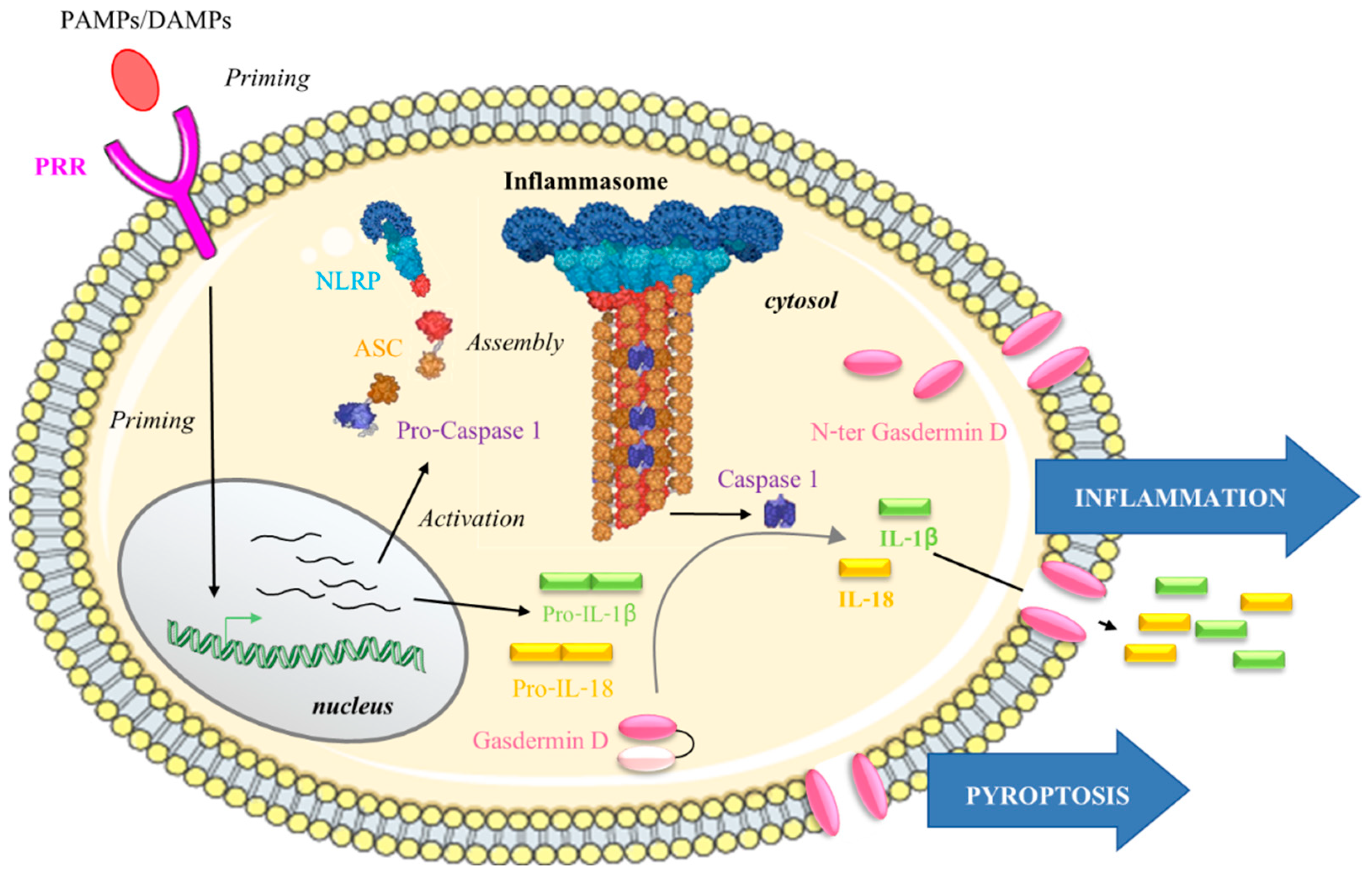
4.1. What Are Inflammasomes?
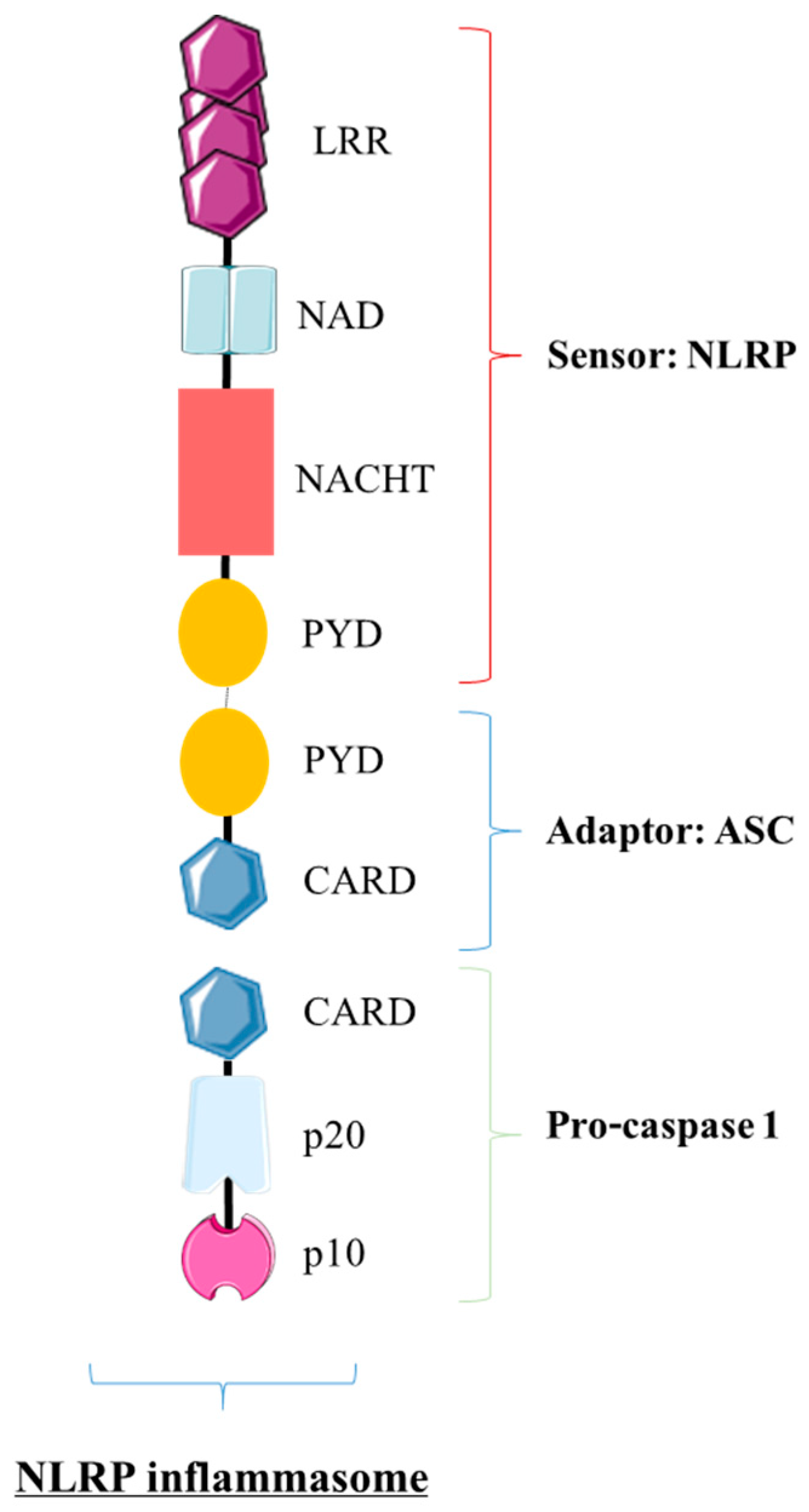
4.1.1. The NLRP Inflammasome: The Trio NLRP/ASC/pro-Caspase 1
4.1.2. NLRP Inflammasome Activation
4.2. NLRP Inflammasomes, FM Diseases and pPROM
4.3. Focus on NLRP7
4.4. Does NLRP7 Play a Role in FM Rupture?
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naeye, R.L.; Peters, E.C. Causes and Consequences of Premature Rupture of Fetal Membranes. Lancet 1980, 1, 192–194. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Kusanovic, J.P.; Gotsch, F.; Hassan, S.; Erez, O.; Chaiworapongsa, T.; Mazor, M. The Preterm Parturition Syndrome. BJOG 2006, 113 (Suppl. 3), 17–42. [Google Scholar] [CrossRef]
- Menon, R. Human Fetal Membranes at Term: Dead Tissue or Signalers of Parturition? Placenta 2016, 44, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, L.S.; Vargas, G.; Brown, T.; Ochoa, L.; Sheller-Miller, S.; Saade, G.R.; Taylor, R.N.; Menon, R. Discovery and Characterization of Human Amniochorionic Membrane Microfractures. Am. J. Pathol. 2017, 187, 2821–2830. [Google Scholar] [CrossRef] [Green Version]
- Lavery, J.P.; Miller, C.E. The Viscoelastic Nature of Chorioamniotic Membranes. Obstet. Gynecol. 1977, 50, 467–472. [Google Scholar]
- Oyen, M.L.; Calvin, S.E.; Landers, D.V. Premature Rupture of the Fetal Membranes: Is the Amnion the Major Determinant? Am. J. Obstet. Gynecol. 2006, 195, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W.; Oyen, M.L.; Phillips, A.T.M.; Nowlan, N.C. Function and Failure of the Fetal Membrane: Modelling the Mechanics of the Chorion and Amnion. PLoS ONE 2017, 12, e0171588. [Google Scholar] [CrossRef] [Green Version]
- Prat, C.; Blanchon, L.; Borel, V.; Gallot, D.; Herbet, A.; Bouvier, D.; Marceau, G.; Sapin, V. Ontogeny of Aquaporins in Human Fetal Membranes. Biol. Reprod. 2012, 86, 48. [Google Scholar] [CrossRef]
- Prat, C.; Bouvier, D.; Comptour, A.; Marceau, G.; Belville, C.; Clairefond, G.; Blanc, P.; Gallot, D.; Blanchon, L.; Sapin, V. All-Trans-Retinoic Acid Regulates Aquaporin-3 Expression and Related Cellular Membrane Permeability in the Human Amniotic Environment. Placenta 2015, 36, 881–887. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yasui, M. Cellular and Subcellular Localization of Aquaporins 1, 3, 8, and 9 in Amniotic Membranes during Pregnancy in Mice. Cell Tissue Res. 2010, 342, 307–316. [Google Scholar] [CrossRef]
- Ashraf, H.; Font, K.; Powell, C.; Schurr, M. Antimicrobial Activity of an Amnion-Chorion Membrane to Oral Microbes. Int. J. Dent. 2019, 2019, 1269534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szukiewicz, D.; Alkhalayla, H.; Pyzlak, M.; Watroba, M.; Szewczyk, G.; Wejman, J. Human Beta-Defensin 1, 2 and 3 Production by Amniotic Epithelial Cells with Respect to Human Papillomavirus (HPV) Infection, HPV Oncogenic Potential and the Mode of Delivery. Microb. Pathog. 2016, 97, 154–165. [Google Scholar] [CrossRef]
- Zare-Bidaki, M.; Sadrinia, S.; Erfani, S.; Afkar, E.; Ghanbarzade, N. Antimicrobial Properties of Amniotic and Chorionic Membranes: A Comparative Study of Two Human Fetal Sacs. J. Reprod. Infertil. 2017, 18, 218–224. [Google Scholar]
- Kobayashi, K.; Miwa, H.; Yasui, M. Inflammatory Mediators Weaken the Amniotic Membrane Barrier through Disruption of Tight Junctions. J. Physiol. 2010, 588, 4859–4869. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kadohira, I.; Tanaka, M.; Yoshimura, Y.; Ikeda, K.; Yasui, M. Expression and Distribution of Tight Junction Proteins in Human Amnion during Late Pregnancy. Placenta 2010, 31, 158–162. [Google Scholar] [CrossRef]
- Caughey, A.B.; Robinson, J.N.; Norwitz, E.R. Contemporary Diagnosis and Management of Preterm Premature Rupture of Membranes. Rev. Obstet. Gynecol. 2008, 1, 11–22. [Google Scholar] [PubMed]
- Moore, R.M.; Mansour, J.M.; Redline, R.W.; Mercer, B.M.; Moore, J.J. The Physiology of Fetal Membrane Rupture: Insight Gained from the Determination of Physical Properties. Placenta 2006, 27, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- El Khwad, M.; Stetzer, B.; Moore, R.M.; Kumar, D.; Mercer, B.; Arikat, S.; Redline, R.W.; Mansour, J.M.; Moore, J.J. Term Human Fetal Membranes Have a Weak Zone Overlying the Lower Uterine Pole and Cervix Before Onset of Labor1. Biol. Reprod. 2005, 72, 720–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, M.; Barker, G.; Menon, R.; Lappas, M. Increased Oxidative Stress in Human Fetal Membranes Overlying the Cervix from Term Non-Labouring and Post Labour Deliveries. Placenta 2012, 33, 604–610. [Google Scholar] [CrossRef]
- Chai, M.; Walker, S.P.; Riley, C.; Rice, G.E.; Permezel, M.; Lappas, M. Effect of Supracervical Apposition and Spontaneous Labour on Apoptosis and Matrix Metalloproteinases in Human Fetal Membranes. Biomed. Res. Int. 2013, 2013, 316146. [Google Scholar] [CrossRef] [Green Version]
- Colatto, B.N.; de Souza, I.F.; Schinke, L.A.A.; Noda-Nicolau, N.M.; da Silva, M.G.; Morceli, G.; Menon, R.; Polettini, J. Telomere Length and Telomerase Activity in Foetal Membranes from Term and Spontaneous Preterm Births. Reprod. Sci. 2020, 27, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. SMAD7 Regulates Proinflammatory and Prolabor Mediators in Amnion and Myometrium. Biol. Reprod. 2017, 97, 288–301. [Google Scholar] [CrossRef] [Green Version]
- Richardson, L.S.; Taylor, R.N.; Menon, R. Reversible EMT and MET Mediate Amnion Remodeling during Pregnancy and Labor. Sci. Signal. 2020, 13, eaay1486. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, L.; Schmitz, T.; Messaoudene, M.; Chader, D.; Parizot, C.; Jacques, S.; Delaire, J.; Gogusev, J.; Schmitt, A.; Lesaffre, C.; et al. Immune Modifications in Fetal Membranes Overlying the Cervix Precede Parturition in Humans. J. Immunol. 2017, 198, 1345–1356. [Google Scholar] [CrossRef]
- Nhan-Chang, C.-L.; Romero, R.; Tarca, A.L.; Mittal, P.; Kusanovic, J.P.; Erez, O.; Mazaki-Tovi, S.; Chaiworapongsa, T.; Hotra, J.; Than, N.G.; et al. Characterization of the Transcriptome of Chorioamniotic Membranes at the Site of Rupture in Spontaneous Labor at Term. Am. J. Obstet. Gynecol. 2010, 202, 462.e1–462.e41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.M.; Casey, M.L.; MacDonald, P.C. Accumulation of Interleukin-1beta and Interleukin-6 in Amniotic Fluid: A Sequela of Labour at Term and Preterm. Hum. Reprod. Update 1997, 3, 517–527. [Google Scholar] [CrossRef]
- Maymon, E.; Ghezzi, F.; Edwin, S.S.; Mazor, M.; Yoon, B.H.; Gomez, R.; Romero, R. The Tumor Necrosis Factor Alpha and Its Soluble Receptor Profile in Term and Preterm Parturition. Am. J. Obstet. Gynecol. 1999, 181, 1142–1148. [Google Scholar] [CrossRef]
- Menon, R.; Lombardi, S.J.; Fortunato, S.J. TNF-Alpha Promotes Caspase Activation and Apoptosis in Human Fetal Membranes. J. Assist. Reprod. Genet. 2002, 19, 201–204. [Google Scholar] [CrossRef]
- Ito, A.; Nakamura, T.; Uchiyama, T.; Hirose, K.; Hirakawa, S.; Sasaguri, Y.; Mori, Y. Stimulation of the Biosynthesis of Interleukin 8 by Interleukin 1 and Tumor Necrosis Factor Alpha in Cultured Human Chorionic Cells. Biol. Pharm. Bull. 1994, 17, 1463–1467. [Google Scholar] [CrossRef] [Green Version]
- Trautman, M.S.; Dudley, D.J.; Edwin, S.S.; Collmer, D.; Mitchell, M.D. Amnion Cell Biosynthesis of Interleukin-8: Regulation by Inflammatory Cytokines. J. Cell Physiol. 1992, 153, 38–43. [Google Scholar] [CrossRef]
- Kumar, D.; Fung, W.; Moore, R.M.; Pandey, V.; Fox, J.; Stetzer, B.; Mansour, J.M.; Mercer, B.M.; Redline, R.W.; Moore, J.J. Proinflammatory Cytokines Found in Amniotic Fluid Induce Collagen Remodeling, Apoptosis, and Biophysical Weakening of Cultured Human Fetal Membranes1. Biol. Reprod. 2006, 74, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Schatz, F.; Moore, R.; Mercer, B.; Rangaswamy, N.; Mansour, J.; Lockwood, C.; Moore, J. The Effects of Thrombin and Cytokines upon the Biomechanics and Remodeling of Isolated Amnion Membrane, In Vitro. Placenta 2011, 32, 206–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheller-Miller, S.; Trivedi, J.; Yellon, S.M.; Menon, R. Exosomes Cause Preterm Birth in Mice: Evidence for Paracrine Signaling in Pregnancy. Sci. Rep. 2019, 9, 608. [Google Scholar] [CrossRef] [Green Version]
- Bredeson, S.; Papaconstantinou, J.; Deford, J.H.; Kechichian, T.; Syed, T.A.; Saade, G.R.; Menon, R. HMGB1 Promotes a P38MAPK Associated Non-Infectious Inflammatory Response Pathway in Human Fetal Membranes. PLoS ONE 2014, 9, e113799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappas, M.; Permezel, M.; Rice, G.E. Advanced Glycation Endproducts Mediate Pro-Inflammatory Actions in Human Gestational Tissues via Nuclear Factor- B and Extracellular Signal-Regulated Kinase 1/2. J. Endocrinol. 2007, 193, 269–277. [Google Scholar] [CrossRef]
- Olive, C. Pattern Recognition Receptors: Sentinels in Innate Immunity and Targets of New Vaccine Adjuvants. Expert Rev. Vaccines 2012, 11, 237–256. [Google Scholar] [CrossRef]
- Hangai, S.; Kimura, Y.; Taniguchi, T.; Yanai, H. Signal Transducing Innate Receptors in Tumor Immunity. Cancer Sci. 2021, 112, 2578–2591. [Google Scholar] [CrossRef] [PubMed]
- Blanchon, L.; Accoceberry, M.; Belville, C.; Delabaere, A.; Prat, C.; Lemery, D.; Sapin, V.; Gallot, D. Rupture of membranes: Pathophysiology, diagnosis, consequences and management. J. Gynecol. Obstet. Biol. Reprod. 2013, 42, 105–116. [Google Scholar] [CrossRef]
- Menon, R.; Richardson, L.S. Preterm Prelabor Rupture of the Membranes: A Disease of the Fetal Membranes. Semin. Perinatol. 2017, 41, 409–419. [Google Scholar] [CrossRef]
- Furman, B.; Shoham-Vardi, I.; Bashiri, A.; Erez, O.; Mazor, M. Clinical Significance and Outcome of Preterm Prelabor Rupture of Membranes: Population-Based Study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 92, 209–216. [Google Scholar] [CrossRef]
- Ananth, C.V.; Oyelese, Y.; Srinivas, N.; Yeo, L.; Vintzileos, A.M. Preterm Premature Rupture of Membranes, Intrauterine Infection, and Oligohydramnios: Risk Factors for Placental Abruption. Obstet. Gynecol. 2004, 104, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Middleton, P.; Shepherd, E.; Flenady, V.; McBain, R.D.; Crowther, C.A. Planned Early Birth versus Expectant Management (Waiting) for Prelabour Rupture of Membranes at Term (37 Weeks or More). Cochrane Database Syst. Rev. 2017, 1, CD005302. [Google Scholar] [CrossRef]
- Newton, E.R. Preterm Labor, Preterm Premature Rupture of Membranes, and Chorioamnionitis. Clin. Perinatol. 2005, 32, 571–600. [Google Scholar] [CrossRef]
- Platt, M.J. Outcomes in Preterm Infants. Public Health 2014, 128, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, D.; Forest, J.-C.; Blanchon, L.; Bujold, E.; Pereira, B.; Bernard, N.; Gallot, D.; Sapin, V.; Giguère, Y. Risk Factors and Outcomes of Preterm Premature Rupture of Membranes in a Cohort of 6968 Pregnant Women Prospectively Recruited. J. Clin. Med. 2019, 8, 1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forde, B.; Habli, M. Unique Considerations: Preterm Prelabor Rupture of Membranes in the Setting of Fetal Surgery and Higher Order Pregnancies. Obstet. Gynecol. Clin. N. Am. 2020, 47, 653–669. [Google Scholar] [CrossRef] [PubMed]
- Modi, B.P.; Teves, M.E.; Pearson, L.N.; Parikh, H.I.; Haymond-Thornburg, H.; Tucker, J.L.; Chaemsaithong, P.; Gomez-Lopez, N.; York, T.P.; Romero, R.; et al. Mutations in Fetal Genes Involved in Innate Immunity and Host Defense against Microbes Increase Risk of Preterm Premature Rupture of Membranes (PPROM). Mol. Genet. Genom. Med. 2017, 5, 720–729. [Google Scholar] [CrossRef] [Green Version]
- Dorfeuille, N.; Morin, V.; Tétu, A.; Demers, S.; Laforest, G.; Gouin, K.; Piedboeuf, B.; Bujold, E. Vaginal Fluid Inflammatory Biomarkers and the Risk of Adverse Neonatal Outcomes in Women with PPROM. Am. J. Perinatol. 2016, 33, 1003–1007. [Google Scholar] [CrossRef]
- Galaz, J.; Romero, R.; Slutsky, R.; Xu, Y.; Motomura, K.; Para, R.; Pacora, P.; Panaitescu, B.; Hsu, C.-D.; Kacerovsky, M.; et al. Cellular Immune Responses in Amniotic Fluid of Women with Preterm Prelabor Rupture of Membranes. J. Perinat. Med. 2020, 48, 222–233. [Google Scholar] [CrossRef]
- Romero, R.; Miranda, J.; Chaemsaithong, P.; Chaiworapongsa, T.; Kusanovic, J.P.; Dong, Z.; Ahmed, A.I.; Shaman, M.; Lannaman, K.; Yoon, B.H.; et al. Sterile and Microbial-Associated Intra-Amniotic Inflammation in Preterm Prelabor Rupture of Membranes. J. Matern.-Fetal Neonatal Med. 2015, 28, 1394–1409. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Lombardi, S.J.; Fortunato, S.J. IL-18, a Product of Choriodecidual Cells, Increases during Premature Rupture of Membranes but Fails to Turn on the Fas-FasL-Mediated Apoptosis Pathway. J. Assist. Reprod. Genet. 2001, 18, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Shobokshi, A.; Shaarawy, M. Maternal Serum and Amniotic Fluid Cytokines in Patients with Preterm Premature Rupture of Membranes with and without Intrauterine Infection. Int. J. Gynaecol. Obstet. 2002, 79, 209–215. [Google Scholar] [CrossRef]
- Vajrychová, M.; Stráník, J.; Pimková, K.; Barman, M.; Kukla, R.; Zedníková, P.; Bolehovská, R.; Plíšková, L.; Hornychová, H.; Andrýs, C.; et al. Comprehensive Proteomic Investigation of Infectious and Inflammatory Changes in Late Preterm Prelabour Rupture of Membranes. Sci. Rep. 2020, 10, 17696. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lopez, N.; Romero, R.; Leng, Y.; Xu, Y.; Slutsky, R.; Levenson, D.; Pacora, P.; Jung, E.; Panaitescu, B.; Hsu, C.-D. The Origin of Amniotic Fluid Monocytes/Macrophages in Women with Intra-Amniotic Inflammation or Infection. J. Perinat. Med. 2019, 47, 822–840. [Google Scholar] [CrossRef]
- Ronzoni, S.; Steckle, V.; D’Souza, R.; Murphy, K.E.; Lye, S.; Shynlova, O. Cytokine Changes in Maternal Peripheral Blood Correlate With Time-to-Delivery in Pregnancies Complicated by Premature Prelabor Rupture of the Membranes. Reprod. Sci. 2019, 26, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Raba, G.; Kacerovsky, M.; Laudański, P. Eotaxin-2 as a Potential Marker of Preterm Premature Rupture of Membranes: A Prospective, Cohort, Multicenter Study. Adv. Clin. Exp. Med. 2021, 30, 197–202. [Google Scholar] [CrossRef]
- Seliger, G.; Bergner, M.; Haase, R.; Stepan, H.; Schleußner, E.; Zöllkau, J.; Seeger, S.; Kraus, F.B.; Hiller, G.G.R.; Wienke, A.; et al. Daily Monitoring of Vaginal Interleukin 6 as a Predictor of Intraamniotic Inflammation after Preterm Premature Rupture of Membranes—A New Method of Sampling Studied in a Prospective Multicenter Trial. J. Perinat. Med. 2021, 49, 572–582. [Google Scholar] [CrossRef]
- Li, W.; Zhao, X.; Li, S.; Chen, X.; Cui, H.; Chang, Y.; Zhang, R. Upregulation of TNF-α and IL-6 Induces Preterm Premature Rupture of Membranes by Activation of ADAMTS-9 in Embryonic Membrane Cells. Life Sci. 2020, 260, 118237. [Google Scholar] [CrossRef]
- Zaga, V.; Estrada-Gutierrez, G.; Beltran-Montoya, J.; Maida-Claros, R.; Lopez-Vancell, R.; Vadillo-Ortega, F. Secretions of Interleukin-1beta and Tumor Necrosis Factor Alpha by Whole Fetal Membranes Depend on Initial Interactions of Amnion or Choriodecidua with Lipopolysaccharides or Group B Streptococci. Biol. Reprod. 2004, 71, 1296–1302. [Google Scholar] [CrossRef]
- Zaga-Clavellina, V.; Garcia-Lopez, G.; Flores-Herrera, H.; Espejel-Nuñez, A.; Flores-Pliego, A.; Soriano-Becerril, D.; Maida-Claros, R.; Merchant-Larios, H.; Vadillo-Ortega, F. In Vitro Secretion Profiles of Interleukin (IL)-1beta, IL-6, IL-8, IL-10, and TNF Alpha after Selective Infection with Escherichia Coli in Human Fetal Membranes. Reprod. Biol. Endocrinol. 2007, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Romero, R.; Miranda, J.; Chaiworapongsa, T.; Korzeniewski, S.J.; Chaemsaithong, P.; Gotsch, F.; Dong, Z.; Ahmed, A.I.; Yoon, B.H.; Hassan, S.S.; et al. Prevalence and Clinical Significance of Sterile Intra-Amniotic Inflammation in Patients with Preterm Labor and Intact Membranes. Am. J. Reprod. Immunol. 2014, 72, 458–474. [Google Scholar] [CrossRef] [Green Version]
- Baumbusch, M.A.; Buhimschi, C.S.; Oliver, E.A.; Zhao, G.; Thung, S.; Rood, K.; Buhimschi, I.A. High Mobility Group-Box 1 (HMGB1) Levels Are Increased in Amniotic Fluid of Women with Intra-Amniotic Inflammation-Determined Preterm Birth, and the Source May Be the Damaged Fetal Membranes. Cytokine 2016, 81, 82–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Zhu, L.; Zhang, Z.; Li, H.; Li, P.; Wang, Y.; Leng, M. HMGB1-RAGE Signaling Pathway in PPROM. Taiwan. J. Obstet. Gynecol. 2018, 57, 211–216. [Google Scholar] [CrossRef]
- Chaiworapongsa, T.; Erez, O.; Kusanovic, J.P.; Vaisbuch, E.; Mazaki-Tovi, S.; Gotsch, F.; Than, N.G.; Mittal, P.; Kim, Y.M.; Camacho, N.; et al. Amniotic Fluid Heat Shock Protein 70 Concentration in Histologic Chorioamnionitis, Term and Preterm Parturition. J. Matern. Fetal. Neonatal Med. 2008, 21, 449–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friel, L.A.; Romero, R.; Edwin, S.; Nien, J.K.; Gomez, R.; Chaiworapongsa, T.; Kusanovic, J.P.; Tolosa, J.E.; Hassan, S.S.; Espinoza, J. The Calcium Binding Protein, S100B, Is Increased in the Amniotic Fluid of Women with Intra-Amniotic Infection/Inflammation and Preterm Labor with Intact or Ruptured Membranes. J. Perinat. Med. 2007, 35, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Chaiworapongsa, T.; Savasan, Z.A.; Xu, Y.; Hussein, Y.; Dong, Z.; Kusanovic, J.P.; Kim, C.J.; Hassan, S.S. Damage-Associated Molecular Patterns (DAMPs) in Preterm Labor with Intact Membranes and Preterm PROM: A Study of the Alarmin HMGB1. J. Matern. Fetal. Neonatal Med. 2011, 24, 1444–1455. [Google Scholar] [CrossRef]
- van Boeckel, S.R.; Davidson, D.J.; Norman, J.E.; Stock, S.J. Cell-Free Fetal DNA and Spontaneous Preterm Birth. Reproduction 2018, 155, R137–R145. [Google Scholar] [CrossRef] [Green Version]
- Golubinskaya, V.; Puttonen, H.; Fyhr, I.-M.; Rydbeck, H.; Hellström, A.; Jacobsson, B.; Nilsson, H.; Mallard, C.; Sävman, K. Expression of S100A Alarmins in Cord Blood Monocytes Is Highly Associated With Chorioamnionitis and Fetal Inflammation in Preterm Infants. Front. Immunol. 2020, 11, 1194. [Google Scholar] [CrossRef] [PubMed]
- Kacerovsky, M.; Vlkova, B.; Musilova, I.; Andrys, C.; Pliskova, L.; Zemlickova, H.; Stranik, J.; Halada, P.; Jacobsson, B.; Celec, P. Amniotic Fluid Cell-Free DNA in Preterm Prelabor Rupture of Membranes. Prenat. Diagn. 2018, 38, 1086–1095. [Google Scholar] [CrossRef]
- Menon, R.; Behnia, F.; Polettini, J.; Saade, G.R.; Campisi, J.; Velarde, M. Placental Membrane Aging and HMGB1 Signaling Associated with Human Parturition. Aging 2016, 8, 216–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padron, J.G.; Saito Reis, C.A.; Kendal-Wright, C.E. The Role of Danger Associated Molecular Patterns in Human Fetal Membrane Weakening. Front. Physiol. 2020, 11, 602. [Google Scholar] [CrossRef]
- Sheller-Miller, S.; Radnaa, E.; Yoo, J.-K.; Kim, E.; Choi, K.; Kim, Y.; Kim, Y.N.; Richardson, L.; Choi, C.; Menon, R. Exosomal Delivery of NF-ΚB Inhibitor Delays LPS-Induced Preterm Birth and Modulates Fetal Immune Cell Profile in Mouse Models. Sci. Adv. 2021, 7, eabd3865. [Google Scholar] [CrossRef]
- Bongarzone, S.; Savickas, V.; Luzi, F.; Gee, A.D. Targeting the Receptor for Advanced Glycation Endproducts (RAGE): A Medicinal Chemistry Perspective. J. Med. Chem. 2017, 60, 7213–7232. [Google Scholar] [CrossRef] [Green Version]
- Barbezier, N.; Tessier, F.J.; Chango, A. Receptor of advanced glycation endproducts RAGE/AGER: An integrative view for clinical applications. Ann. Biol. Clin. 2014, 72, 669–680. [Google Scholar] [CrossRef]
- Kierdorf, K.; Fritz, G. RAGE Regulation and Signaling in Inflammation and Beyond. J. Leukoc. Biol. 2013, 94, 55–68. [Google Scholar] [CrossRef]
- Sorci, G.; Riuzzi, F.; Giambanco, I.; Donato, R. RAGE in Tissue Homeostasis, Repair and Regeneration. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2013, 1833, 101–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, B.I.; Kalea, A.Z.; Del Mar Arriero, M.; Harja, E.; Boulanger, E.; D’Agati, V.; Schmidt, A.M. Interaction of the RAGE Cytoplasmic Domain with Diaphanous-1 Is Required for Ligand-Stimulated Cellular Migration through Activation of Rac1 and Cdc42. J. Biol. Chem. 2008, 283, 34457–34468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaguchi, M.; Murata, H.; Yamamoto, K.; Ono, T.; Sakaguchi, Y.; Motoyama, A.; Hibino, T.; Kataoka, K.; Huh, N. TIRAP, an Adaptor Protein for TLR2/4, Transduces a Signal from RAGE Phosphorylated upon Ligand Binding. PLoS ONE 2011, 6, e23132. [Google Scholar] [CrossRef] [Green Version]
- Raucci, A.; Cugusi, S.; Antonelli, A.; Barabino, S.M.; Monti, L.; Bierhaus, A.; Reiss, K.; Saftig, P.; Bianchi, M.E. A Soluble Form of the Receptor for Advanced Glycation Endproducts (RAGE) Is Produced by Proteolytic Cleavage of the Membrane-Bound Form by the Sheddase a Disintegrin and Metalloprotease 10 (ADAM10). FASEB J. 2008, 22, 3716–3727. [Google Scholar] [CrossRef]
- Zhang, L.; Bukulin, M.; Kojro, E.; Roth, A.; Metz, V.V.; Fahrenholz, F.; Nawroth, P.P.; Bierhaus, A.; Postina, R. Receptor for Advanced Glycation End Products Is Subjected to Protein Ectodomain Shedding by Metalloproteinases. J. Biol. Chem. 2008, 283, 35507–35516. [Google Scholar] [CrossRef] [Green Version]
- Teissier, T.; Boulanger, É. The Receptor for Advanced Glycation End-Products (RAGE) Is an Important Pattern Recognition Receptor (PRR) for Inflammaging. Biogerontology 2019, 20, 279–301. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Yan, S.F.; Stern, D.M. The Biology of the Receptor for Advanced Glycation End Products and Its Ligands. Biochim. Biophys. Acta 2000, 1498, 99–111. [Google Scholar] [CrossRef] [Green Version]
- Sakaguchi, M.; Kinoshita, R.; Putranto, E.W.; Ruma, I.M.W.; Sumardika, I.W.; Youyi, C.; Tomonobu, N.; Yamamoto, K.-I.; Murata, H. Signal Diversity of Receptor for Advanced Glycation End Products. Acta Med. Okayama 2017, 71, 459–465. [Google Scholar] [CrossRef]
- Tsoporis, J.N.; Izhar, S.; Leong-Poi, H.; Desjardins, J.-F.; Huttunen, H.J.; Parker, T.G. S100B Interaction With the Receptor for Advanced Glycation End Products (RAGE): A Novel Receptor-Mediated Mechanism for Myocyte Apoptosis Postinfarction. Circ. Res. 2010, 106, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating Mitochondrial DAMPs Cause Inflammatory Responses to Injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, M.A.; Drury, S.; Fu, C.; Qu, W.; Taguchi, A.; Lu, Y.; Avila, C.; Kambham, N.; Bierhaus, A.; Nawroth, P.; et al. RAGE Mediates a Novel Proinflammatory Axis: A Central Cell Surface Receptor for S100/Calgranulin Polypeptides. Cell 1999, 97, 889–901. [Google Scholar]
- Xie, J.; Méndez, J.D.; Méndez-Valenzuela, V.; Aguilar-Hernández, M.M. Cellular Signalling of the Receptor for Advanced Glycation End Products (RAGE). Cell. Signal. 2013, 25, 2185–2197. [Google Scholar] [CrossRef]
- Liliensiek, B.; Weigand, M.A.; Bierhaus, A.; Nicklas, W.; Kasper, M.; Hofer, S.; Plachky, J.; Gröne, H.-J.; Kurschus, F.C.; Schmidt, A.M.; et al. Receptor for Advanced Glycation End Products (RAGE) Regulates Sepsis but Not the Adaptive Immune Response. J. Clin. Investig. 2004, 113, 1641–1650. [Google Scholar] [CrossRef]
- Chen, Y.; Akirav, E.M.; Chen, W.; Henegariu, O.; Moser, B.; Desai, D.; Shen, J.M.; Webster, J.C.; Andrews, R.C.; Mjalli, A.M.; et al. RAGE Ligation Affects T Cell Activation and Controls T Cell Differentiation. J. Immunol. 2008, 181, 4272–4278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuah, Y.K.; Basir, R.; Talib, H.; Tie, T.H.; Nordin, N. Receptor for Advanced Glycation End Products and Its Involvement in Inflammatory Diseases. Int. J. Inflamm. 2013, 2013, 403460. [Google Scholar] [CrossRef] [Green Version]
- Collison, K.S.; Parhar, R.S.; Saleh, S.S.; Meyer, B.F.; Kwaasi, A.A.; Hammami, M.M.; Schmidt, A.M.; Stern, D.M.; Al-Mohanna, F.A. RAGE-Mediated Neutrophil Dysfunction Is Evoked by Advanced Glycation End Products (AGEs). J. Leukoc. Biol. 2002, 71, 433–444. [Google Scholar] [PubMed]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced Cellular Oxidant Stress by the Interaction of Advanced Glycation End Products with Their Receptors/Binding Proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [CrossRef]
- Li, J.; Schmidt, A.M. Characterization and Functional Analysis of the Promoter of RAGE, the Receptor for Advanced Glycation End Products. J. Biol. Chem. 1997, 272, 16498–16506. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, M.E. DAMPs, PAMPs and Alarmins: All We Need to Know about Danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef]
- Bopp, C.; Bierhaus, A.; Hofer, S.; Bouchon, A.; Nawroth, P.P.; Martin, E.; Weigand, M.A. Bench-to-Bedside Review: The Inflammation-Perpetuating Pattern-Recognition Receptor RAGE as a Therapeutic Target in Sepsis. Crit. Care 2007, 12, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, R.; Espinoza, J.; Hassan, S.; Gotsch, F.; Kusanovic, J.P.; Avila, C.; Erez, O.; Edwin, S.; Schmidt, A.M. Soluble Receptor for Advanced Glycation End Products (SRAGE) and Endogenous Secretory RAGE (EsRAGE) in Amniotic Fluid: Modulation by Infection and Inflammation. J. Perinat. Med. 2008, 36, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, I.A.; Zhao, G.; Pettker, C.M.; Bahtiyar, M.O.; Magloire, L.K.; Thung, S.; Fairchild, T.; Buhimschi, C.S. The Receptor for Advanced Glycation End Products (RAGE) System in Women with Intraamniotic Infection and Inflammation. Am. J. Obstet. Gynecol. 2007, 196, 181.e1–181.e13. [Google Scholar] [CrossRef]
- Rzepka, R.; Dołęgowska, B.; Sałata, D.; Rajewska, A.; Budkowska, M.; Domański, L.; Kwiatkowski, S.; Mikołajek-Bedner, W.; Torbé, A. Soluble Receptors for Advanced Glycation End Products and Receptor Activator of NF-ΚB Ligand Serum Levels as Markers of Premature Labor. BMC Pregnancy Childbirth 2015, 15, 134. [Google Scholar] [CrossRef] [Green Version]
- Bouvier, D.; Giguère, Y.; Blanchon, L.; Bujold, E.; Pereira, B.; Bernard, N.; Gallot, D.; Sapin, V.; Forest, J.-C. Study of SRAGE, HMGB1, AGE, and S100A8/A9 Concentrations in Plasma and in Serum-Extracted Extracellular Vesicles of Pregnant Women With Preterm Premature Rupture of Membranes. Front. Physiol. 2020, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-Y.; Sun, L.; Han, X.-L.; Chang, Y.; Cheng, L.; Yin, L.-R. Alarmin High Mobility Group Box-1 in Maternal Serum as a Potential Biomarker of Chorioamnionitis-Associated Preterm Birth. Gynecol. Endocrinol. 2017, 33, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Kansu-Celik, H.; Tasci, Y.; Karakaya, B.K.; Cinar, M.; Candar, T.; Caglar, G.S. Maternal Serum Advanced Glycation End Products Level as an Early Marker for Predicting Preterm Labor/PPROM: A Prospective Preliminary Study. J. Matern. Fetal. Neonatal Med. 2019, 32, 2758–2762. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, S.D.; Hesse, L.; Faiz, A.; Lubbers, J.; Bodha, P.K.; ten Hacken, N.H.T.; van Oosterhout, A.J.M.; Nawijn, M.C.; Heijink, I.H. Susceptibility for Cigarette Smoke-Induced DAMP Release and DAMP-Induced Inflammation in COPD. Am. J. Physiol. -Lung Cell. Mol. Physiol. 2016, 311, L881–L892. [Google Scholar] [CrossRef] [Green Version]
- Choltus, H.; Lavergne, M.; Belville, C.; Gallot, D.; Minet-Quinard, R.; Durif, J.; Blanchon, L.; Sapin, V. Occurrence of a RAGE-Mediated Inflammatory Response in Human Fetal Membranes. Front. Physiol. 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.A.; Hutchinson, M.R.; Rice, K.C.; Chin, P.; Moldenhauer, L.M.; Stark, M.J.; Olson, D.M.; Keelan, J.A. Targeting Toll-like Receptor-4 to Tackle Preterm Birth and Fetal Inflammatory Injury. Clin. Transl. Immunol. 2020, 9, e1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plazyo, O.; Romero, R.; Unkel, R.; Balancio, A.; Mial, T.N.; Xu, Y.; Dong, Z.; Hassan, S.S.; Gomez-Lopez, N. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol. Reprod. 2016, 95, 130. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-Beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Gomez, A.; Serrano, A.; Salero, E.; Tovar, A.; Amescua, G.; Galor, A.; Keane, R.W.; Pablo de Rivero Vaccari, J.; Sabater, A.L. Tumor Necrosis Factor-Alpha and Interferon-Gamma Induce Inflammasome-Mediated Corneal Endothelial Cell Death. Exp. Eye Res. 2021, 207, 108574. [Google Scholar] [CrossRef]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.-K. An Update on the Regulatory Mechanisms of NLRP3 Inflammasome Activation. Cell Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef]
- de Vasconcelos, N.M.; Lamkanfi, M. Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb Perspect. Biol. 2020, 12, a036392. [Google Scholar] [CrossRef] [PubMed]
- Dubois, H.; Wullaert, A.; Lamkanfi, M. General Strategies in Inflammasome Biology. Curr Top. Microbiol. Immunol. 2016, 397, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.; Biswas, A.; Zimmer, N.; Messaed, C.; Oldenburg, J.; Slim, R.; El-Maarri, O. NLRP7 Inter-Domain Interactions: The NACHT-Associated Domain Is the Physical Mediator for Oligomeric Assembly. Mol. Hum. Reprod 2014, 20, 990–1001. [Google Scholar] [CrossRef] [Green Version]
- Istomin, A.Y.; Godzik, A. Understanding Diversity of Human Innate Immunity Receptors: Analysis of Surface Features of Leucine-Rich Repeat Domains in NLRs and TLRs. BMC Immunol. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeretssian, G. Effector Functions of NLRs in the Intestine: Innate Sensing, Cell Death, and Disease. Immunol. Res. 2012, 54, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kanneganti, T.-D. Inflammasome Activation and Assembly at a Glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, M.S.; Sborgi, L.; Rühl, S.; Hiller, S.; Broz, P. ASC Filament Formation Serves as a Signal Amplification Mechanism for Inflammasomes. Nat. Commun. 2016, 7, 11929. [Google Scholar] [CrossRef] [Green Version]
- Pop, C.; Salvesen, G.S. Human Caspases: Activation, Specificity, and Regulation. J. Biol. Chem. 2009, 284, 21777–21781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, D.; Monteleone, M.; Coll, R.C.; Chen, K.W.; Ross, C.M.; Teo, J.L.; Gomez, G.A.; Holley, C.L.; Bierschenk, D.; Stacey, K.J.; et al. Caspase-1 Self-Cleavage Is an Intrinsic Mechanism to Terminate Inflammasome Activity. J. Exp. Med. 2018, 215, 827–840. [Google Scholar] [CrossRef]
- Kesavardhana, S.; Malireddi, R.K.S.; Kanneganti, T.-D. Caspases in Cell Death, Inflammation, and Pyroptosis. Annu. Rev. Immunol. 2020, 38, 567–595. [Google Scholar] [CrossRef] [Green Version]
- Groslambert, M.; Py, B.F. Spotlight on the NLRP3 Inflammasome Pathway. J. Inflamm Res. 2018, 11, 359–374. [Google Scholar] [CrossRef] [Green Version]
- Xiang, P.; Chen, T.; Mou, Y.; Wu, H.; Xie, P.; Lu, G.; Gong, X.; Hu, Q.; Zhang, Y.; Ji, H. NZ Suppresses TLR4/NF-ΚB Signalings and NLRP3 Inflammasome Activation in LPS-Induced RAW264.7 Macrophages. Inflamm. Res. 2015, 64, 799–808. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non-Transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. J. Biol. Chem. 2012, 287, 36617–36622. [Google Scholar] [CrossRef] [Green Version]
- Kesavardhana, S.; Kanneganti, T.-D. Mechanisms Governing Inflammasome Activation, Assembly and Pyroptosis Induction. Int. Immunol. 2017, 29, 201–210. [Google Scholar] [CrossRef]
- Evavold, C.L.; Ruan, J.; Tan, Y.; Xia, S.; Wu, H.; Kagan, J.C. The Pore-Forming Protein Gasdermin D Regulates Interleukin-1 Secretion from Living Macrophages. Immunity 2018, 48, 35–44.e6. [Google Scholar] [CrossRef] [Green Version]
- Monteleone, M.; Stanley, A.C.; Chen, K.W.; Brown, D.L.; Bezbradica, J.S.; von Pein, J.B.; Holley, C.L.; Boucher, D.; Shakespear, M.R.; Kapetanovic, R.; et al. Interleukin-1β Maturation Triggers Its Relocation to the Plasma Membrane for Gasdermin-D-Dependent and -Independent Secretion. Cell Rep. 2018, 24, 1425–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D Is an Executor of Pyroptosis and Required for Interleukin-1β Secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Chan, A.H.; Schroder, K. Inflammasome Signaling and Regulation of Interleukin-1 Family Cytokines. J. Exp. Med. 2019, 217, e20190314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Li, C.; Zhang, S.-Y. NLRP3 Inflammasome: A New Therapeutic Target for High-Risk Reproductive Disorders? Chin. Med. J. 2020, 134, 20–27. [Google Scholar] [CrossRef]
- Lahooti, B.; Chhibber, T.; Bagchi, S.; Varahachalam, S.P.; Jayant, R.D. Therapeutic Role of Inflammasome Inhibitors in Neurodegenerative Disorders. Brain. Behav. Immun. 2021, 91, 771–783. [Google Scholar] [CrossRef]
- Sarmah, D.; Datta, A.; Raut, S.; Sarkar, A.; Shah, B.; Bohra, M.; Singh, U.; Jagtap, P.; Baidya, F.; Kalia, K.; et al. The Role of Inflammasomes in Atherosclerosis and Stroke Pathogenesis. Curr. Pharm. Des. 2020, 26, 4234–4245. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Moradkhani, F.; Asghari, N.; Fallah, M.; Asghari, M.H.; Moghadamnia, A.A.; Abdollahi, M. NLRP Inflammasome as a Key Role Player in the Pathogenesis of Environmental Toxicants. Life Sci. 2019, 231, 116585. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Do, H.T.T.; Her, J.; Kim, Y.; Seo, D.; Rhee, I. Inflammasome as a Promising Therapeutic Target for Cancer. Life Sci. 2019, 231, 116593. [Google Scholar] [CrossRef] [PubMed]
- Soellner, L.; Kopp, K.M.; Mütze, S.; Meyer, R.; Begemann, M.; Rudnik, S.; Rath, W.; Eggermann, T.; Zerres, K. NLRP Genes and Their Role in Preeclampsia and Multi-Locus Imprinting Disorders. J. Perinat. Med. 2018, 46, 169–173. [Google Scholar] [CrossRef]
- Stødle, G.S.; Silva, G.B.; Tangerås, L.H.; Gierman, L.M.; Nervik, I.; Dahlberg, U.E.; Sun, C.; Aune, M.H.; Thomsen, L.C.V.; Bjørge, L.; et al. Placental Inflammation in Pre-Eclampsia by Nod-like Receptor Protein (NLRP)3 Inflammasome Activation in Trophoblasts. Clin. Exp. Immunol. 2018, 193, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Murthi, P.; Pinar, A.A.; Dimitriadis, E.; Samuel, C.S. Inflammasomes—A Molecular Link for Altered Immunoregulation and Inflammation Mediated Vascular Dysfunction in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weel, I.C.; Romão-Veiga, M.; Matias, M.L.; Fioratti, E.G.; Peraçoli, J.C.; Borges, V.T.; Araujo, J.P.; Peraçoli, M.T. Increased Expression of NLRP3 Inflammasome in Placentas from Pregnant Women with Severe Preeclampsia. J. Reprod. Immunol. 2017, 123, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Mulla, M.J.; Myrtolli, K.; Potter, J.; Boeras, C.; Kavathas, P.B.; Sfakianaki, A.K.; Tadesse, S.; Norwitz, E.R.; Guller, S.; Abrahams, V.M. Uric Acid Induces Trophoblast IL-1β Production via the Inflammasome: Implications for the Pathogenesis of Preeclampsia. Am. J. Reprod. Immunol. 2011, 65, 542–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulla, M.J.; Salmon, J.E.; Chamley, L.W.; Brosens, J.J.; Boeras, C.M.; Kavathas, P.B.; Abrahams, V.M. A Role for Uric Acid and the Nalp3 Inflammasome in Antiphospholipid Antibody-Induced IL-1β Production by Human First Trimester Trophoblast. PLoS ONE 2013, 8, e65237. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Garcia-Flores, V.; Leng, Y.; Panaitescu, B.; Miller, D.; Abrahams, V.M.; Hassan, S.S. Inflammasome Assembly in the Chorioamniotic Membranes during Spontaneous Labor at Term. Am. J. Reprod. Immunol. 2017, 77, e12648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Lopez, N.; Romero, R.; Xu, Y.; Plazyo, O.; Unkel, R.; Leng, Y.; Than, N.G.; Chaiworapongsa, T.; Panaitescu, B.; Dong, Z.; et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod. Sci. 2017, 24, 1382–1401. [Google Scholar] [CrossRef] [PubMed]
- Gotsch, F.; Romero, R.; Chaiworapongsa, T.; Erez, O.; Vaisbuch, E.; Espinoza, J.; Kusanovic, J.P.; Mittal, P.; Mazaki-Tovi, S.; Kim, C.J.; et al. Evidence of the Involvement of Caspase-1 under Physiologic and Pathologic Cellular Stress during Human Pregnancy: A Link between the Inflammasome and Parturition. J. Matern. Fetal. Neonatal Med. 2008, 21, 605–616. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Lopez, N.; Romero, R.; Tarca, A.L.; Miller, D.; Panaitescu, B.; Schwenkel, G.; Gudicha, D.W.; Hassan, S.S.; Pacora, P.; Jung, E.; et al. Gasdermin D: Evidence of Pyroptosis in Spontaneous Preterm Labor with Sterile Intra-Amniotic Inflammation or Intra-Amniotic Infection. Am. J. Reprod. Immunol. 2019, 82, e13184. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Miller, D.; Hassan, S.S.; Hsu, C.-D.; Panaitescu, B. Inhibition of the NLRP3 Inflammasome Can Prevent Sterile Intra-Amniotic Inflammation, Preterm Labor/Birth, and Adverse Neonatal Outcomes. Biol. Reprod. 2019, 100, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Romero, R.; Garcia-Flores, V.; Leng, Y.; Xu, Y.; Galaz, J.; Slutsky, R.; Levenson, D.; Gomez-Lopez, N. The Alarmin Interleukin-1α Causes Preterm Birth through the NLRP3 Inflammasome. Mol. Hum. Reprod. 2020, 26, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Faro, J.; Romero, R.; Schwenkel, G.; Garcia-Flores, V.; Arenas-Hernandez, M.; Leng, Y.; Xu, Y.; Miller, D.; Hassan, S.S.; Gomez-Lopez, N. Intra-Amniotic Inflammation Induces Preterm Birth by Activating the NLRP3 Inflammasome. Biol. Reprod. 2019, 100, 1290–1305. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Zhu, Y.; Zhang, W.; Zhu, J. Expression of NOD1 and Downstream Factors in Placenta, Fetal Membrane and Plasma from Pregnancies with Premature Rupture of Membranes and Their Significance. Int. J. Clin. Exp. Pathol. 2018, 11, 5745–5754. [Google Scholar]
- Theis, K.R.; Romero, R.; Motomura, K.; Galaz, J.; Winters, A.D.; Pacora, P.; Miller, D.; Slutsky, R.; Florova, V.; Levenson, D.; et al. Microbial Burden and Inflammasome Activation in Amniotic Fluid of Patients with Preterm Prelabor Rupture of Membranes. J. Perinat. Med. 2020, 48, 115–131. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Ma, C.; Luan, X.; Li, J.; Peng, F.; Huang, L. Inflammasome Components and ADAMTS4 in Premature Rupture of Membranes. Mol. Med. Rep. 2021, 23, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shah, S.Z.A.; Yang, L.; Zhang, Z.; Zhou, X.; Zhao, D. Virulent Mycobacterium Bovis Beijing Strain Activates the NLRP7 Inflammasome in THP-1 Macrophages. PLoS ONE 2016, 11, e0152853. [Google Scholar] [CrossRef] [Green Version]
- Bednash, J.S.; Weathington, N.; Londino, J.; Rojas, M.; Gulick, D.L.; Fort, R.; Han, S.; McKelvey, A.C.; Chen, B.B.; Mallampalli, R.K. Targeting the Deubiquitinase STAMBP Inhibits NALP7 Inflammasome Activity. Nat. Commun. 2017, 8, 15203. [Google Scholar] [CrossRef] [Green Version]
- Abi Nahed, R.; Reynaud, D.; Borg, A.J.; Traboulsi, W.; Wetzel, A.; Sapin, V.; Brouillet, S.; Dieudonné, M.N.; Dakouane-Giudicelli, M.; Benharouga, M.; et al. NLRP7 Is Increased in Human Idiopathic Fetal Growth Restriction and Plays a Critical Role in Trophoblast Differentiation. J. Mol. Med. 2019, 97, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.-Y.; Li, Y.-J.; Zhen, L.; Jiang, F.; Gu, C.-M.; Li, D.-Z. Detection of Parental Contribution to Molar Genome Leads to Diagnosis of Recurrent Hydatidiform Mole in a Family with NLRP7 Variants. Fetal Pediatr Pathol 2020. [Google Scholar] [CrossRef]
- Rezaei, M.; Suresh, B.; Bereke, E.; Hadipour, Z.; Aguinaga, M.; Qian, J.; Bagga, R.; Fardaei, M.; Hemida, R.; Jagadeesh, S.; et al. Novel Pathogenic Variants in NLRP7, NLRP5, and PADI6 in Patients with Recurrent Hydatidiform Moles and Reproductive Failure. Clin. Genet. 2021, 99, 823–828. [Google Scholar] [CrossRef]
- Qian, J.; Nguyen, N.M.P.; Rezaei, M.; Huang, B.; Tao, Y.; Zhang, X.; Cheng, Q.; Yang, H.; Asangla, A.; Majewski, J.; et al. Biallelic PADI6 Variants Linking Infertility, Miscarriages, and Hydatidiform Moles. Eur. J. Hum. Genet. 2018, 26, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Zhu, X.; Yu, X.; Huang, B.; Jiang, T.; Zhang, X.; Yang, H.; Qian, J. Abnormal Processing of IL-1β in NLRP7-Mutated Monocytes in Hydatidiform Mole Patients. Clin. Exp. Immunol. 2020, 202, 72–79. [Google Scholar] [CrossRef]
- Fallahi, J.; Razban, V.; Momtahan, M.; Akbarzadeh-Jahromi, M.; Namavar-Jahromi, B.; Anvar, Z.; Fardaei, M. A Novel Mutation in NLRP7 Related to Recurrent Hydatidiform Mole and Reproductive Failure. Int. J. Fertil Steril 2019, 13, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.-Y.; Chen, K.-R.; Li, Y.-C.; Kuo, P.-L. NLRP7 Is Involved in the Differentiation of the Decidual Macrophages. Int. J. Mol. Sci. 2019, 20, 5994. [Google Scholar] [CrossRef] [Green Version]
- Lavergne, M.; Belville, C.; Choltus, H.; Gross, C.; Minet-Quinard, R.; Gallot, D.; Sapin, V.; Blanchon, L. Human Amnion Epithelial Cells (AECs) Respond to the FSL-1 Lipopeptide by Engaging the NLRP7 Inflammasome. Front. Immunol. 2020, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Dorfleutner, A.; Bryan, N.B.; Yun, C.; Radian, A.D.; de Almeida, L.; Rojanasakul, Y.; Stehlik, C. An NLRP7-Containing Inflammasome Mediates Recognition of Microbial Lipopeptides in Human Macrophages. Immunity 2012, 36, 464–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibata, K.; Hasebe, A.; Into, T.; Yamada, M.; Watanabe, T. The N-Terminal Lipopeptide of a 44-KDa Membrane-Bound Lipoprotein of Mycoplasma Salivarium Is Responsible for the Expression of Intercellular Adhesion Molecule-1 on the Cell Surface of Normal Human Gingival Fibroblasts. J. Immunol. 2000, 165, 6538–6544. [Google Scholar] [CrossRef] [Green Version]
- Mühlradt, P.F.; Kiess, M.; Meyer, H.; Süssmuth, R.; Jung, G. Isolation, Structure Elucidation, and Synthesis of a Macrophage Stimulatory Lipopeptide from Mycoplasma Fermentans Acting at Picomolar Concentration. J. Exp. Med. 1997, 185, 1951–1958. [Google Scholar] [CrossRef] [Green Version]
- Lappas, M. A20, an Essential Component of the Ubiquitin-Editing Protein Complex, Is a Negative Regulator of Inflammation in Human Myometrium and Foetal Membranes. Mol. Hum. Reprod. 2017, 23, 628–645. [Google Scholar] [CrossRef]
- Lim, R.; Barker, G.; Liong, S.; Nguyen-Ngo, C.; Tong, S.; Kaitu’u-Lino, T.; Lappas, M. ATF3 Is a Negative Regulator of Inflammation in Human Fetal Membranes. Placenta 2016, 47, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. Pellino 1 Is a Novel Regulator of TNF and TLR Signalling in Human Myometrial and Amnion Cells. J. Reprod. Immunol. 2018, 127, 24–35. [Google Scholar] [CrossRef]
- Goradia, P.; Lim, R.; Lappas, M. DREAM Is Involved in the Genesis of Inflammation-Induced Prolabour Mediators in Human Myometrial and Amnion Cells. Biomed. Res. Int. 2018, 2018, 8237087. [Google Scholar] [CrossRef] [Green Version]
- Lim, R.; Barker, G.; Lappas, M. PARK7 Regulates Inflammation-Induced pro-Labour Mediators in Myometrial and Amnion Cells. Reproduction 2018, 155, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Lappas, M. The TLR2 Ligand FSL-1 and the TLR5 Ligand Flagellin Mediate pro-Inflammatory and pro-Labour Response via MyD88/TRAF6/NF-ΚB-Dependent Signalling. Am. J. Reprod. Immunol. 2014, 71, 401–417. [Google Scholar] [CrossRef]
- Ariza, M.-E.; Glaser, R.; Kaumaya, P.T.P.; Jones, C.; Williams, M.V. The EBV-Encoded DUTPase Activates NF-Kappa B through the TLR2 and MyD88-Dependent Signaling Pathway. J. Immunol. 2009, 182, 851–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, R.; Tran, H.T.; Liong, S.; Barker, G.; Lappas, M. The Transcription Factor Interferon Regulatory Factor-1 (IRF1) Plays a Key Role in the Terminal Effector Pathways of Human Preterm Labor. Biol. Reprod. 2016, 94, 32. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Lappas, M. A Novel Role for GSK3 in the Regulation of the Processes of Human Labour. Reproduction 2015, 149, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lappas, M. Effect of Spontaneous Term Labour on the Expression of the NR4A Receptors Nuclear Receptor Related 1 Protein (Nurr1), Neuron-Derived Clone 77 (Nur77) and Neuron-Derived Orphan Receptor 1 (NOR1) in Human Fetal Membranes and Myometrium. Reprod. Fertil Dev. 2016, 28, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Xu, Y.; Plazyo, O.; Chaemsaithong, P.; Chaiworapongsa, T.; Unkel, R.; Than, N.G.; Chiang, P.J.; Dong, Z.; Xu, Z.; et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am. J. Reprod. Immunol. 2018, 79, e12440. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Pliskova, L.; Bolehovska, R.; Musilova, I.; Hornychova, H.; Tambor, V.; Jacobsson, B. The Microbial Load with Genital Mycoplasmas Correlates with the Degree of Histologic Chorioamnionitis in Preterm PROM. Am. J. Obstet. Gynecol. 2011, 205, 213.E1–213.E7. [Google Scholar] [CrossRef] [PubMed]
- Kacerovský, M.; Pavlovský, M.; Tosner, J. Preterm Premature Rupture of the Membranes and Genital Mycoplasmas. Acta Med. 2009, 52, 117–120. [Google Scholar] [CrossRef] [Green Version]
- Paramel Jayaprakash, T.; Wagner, E.C.; van Schalkwyk, J.; Albert, A.Y.K.; Hill, J.E.; Money, D.M.; PPROM Study Group. High Diversity and Variability in the Vaginal Microbiome in Women Following Preterm Premature Rupture of Membranes (PPROM): A Prospective Cohort Study. PLoS ONE 2016, 11, e0166794. [Google Scholar] [CrossRef]
- Musilova, I.; Pliskova, L.; Kutova, R.; Hornychova, H.; Jacobsson, B.; Kacerovsky, M. Ureaplasma Species and Mycoplasma Hominis in Cervical Fluid of Pregnancies Complicated by Preterm Prelabor Rupture of Membranes. J. Matern. Fetal Neonatal Med. 2016, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Latino, M.A.; Botta, G.; Badino, C.; Maria, D.D.; Petrozziello, A.; Sensini, A.; Leli, C. Association between Genital Mycoplasmas, Acute Chorioamnionitis and Fetal Pneumonia in Spontaneous Abortions. J. Perinat. Med. 2018, 46, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kacerovský, M.; Boudys, L. Preterm premature rupture of membranes and Ureaplasma urealyticum. Ceska Gynekol 2008, 73, 154–159. [Google Scholar]
- Kwak, D.-W.; Hwang, H.-S.; Kwon, J.-Y.; Park, Y.-W.; Kim, Y.-H. Co-Infection with Vaginal Ureaplasma Urealyticum and Mycoplasma Hominis Increases Adverse Pregnancy Outcomes in Patients with Preterm Labor or Preterm Premature Rupture of Membranes. J. Matern. Fetal Neonatal Med. 2014, 27, 333–337. [Google Scholar] [CrossRef]
- Cox, C.; Saxena, N.; Watt, A.P.; Gannon, C.; McKenna, J.P.; Fairley, D.J.; Sweet, D.; Shields, M.D.; Cosby, S.L.; Coyle, P.V. The Common Vaginal Commensal Bacterium Ureaplasma Parvum Is Associated with Chorioamnionitis in Extreme Preterm Labor. J. Matern. Fetal Neonatal Med. 2016, 29, 3646–3651. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| NLRP | ASC | Caspase-1 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 12 | 14 | |||
| Term Amnion | + | + | + | Ø | Ø | + | + | Ø | + | + | Ø | Ø | Ø | + | + | + |
| Term Chorion | + | + | + | Ø | Ø | + | + | Ø | + | + | Ø | + | Ø | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choltus, H.; Lavergne, M.; De Sousa Do Outeiro, C.; Coste, K.; Belville, C.; Blanchon, L.; Sapin, V. Pathophysiological Implication of Pattern Recognition Receptors in Fetal Membranes Rupture: RAGE and NLRP Inflammasome. Biomedicines 2021, 9, 1123. https://doi.org/10.3390/biomedicines9091123
Choltus H, Lavergne M, De Sousa Do Outeiro C, Coste K, Belville C, Blanchon L, Sapin V. Pathophysiological Implication of Pattern Recognition Receptors in Fetal Membranes Rupture: RAGE and NLRP Inflammasome. Biomedicines. 2021; 9(9):1123. https://doi.org/10.3390/biomedicines9091123
Chicago/Turabian StyleCholtus, Helena, Marilyne Lavergne, Coraline De Sousa Do Outeiro, Karen Coste, Corinne Belville, Loïc Blanchon, and Vincent Sapin. 2021. "Pathophysiological Implication of Pattern Recognition Receptors in Fetal Membranes Rupture: RAGE and NLRP Inflammasome" Biomedicines 9, no. 9: 1123. https://doi.org/10.3390/biomedicines9091123






