Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
2.3. Culture Maintenance
2.4. Preparation of Nanomaterial Stock Solutions
2.5. Cytotoxicity Assay
2.6. Proliferation Assay
2.7. Gene Expression
2.8. Statistical Analysis
3. Results
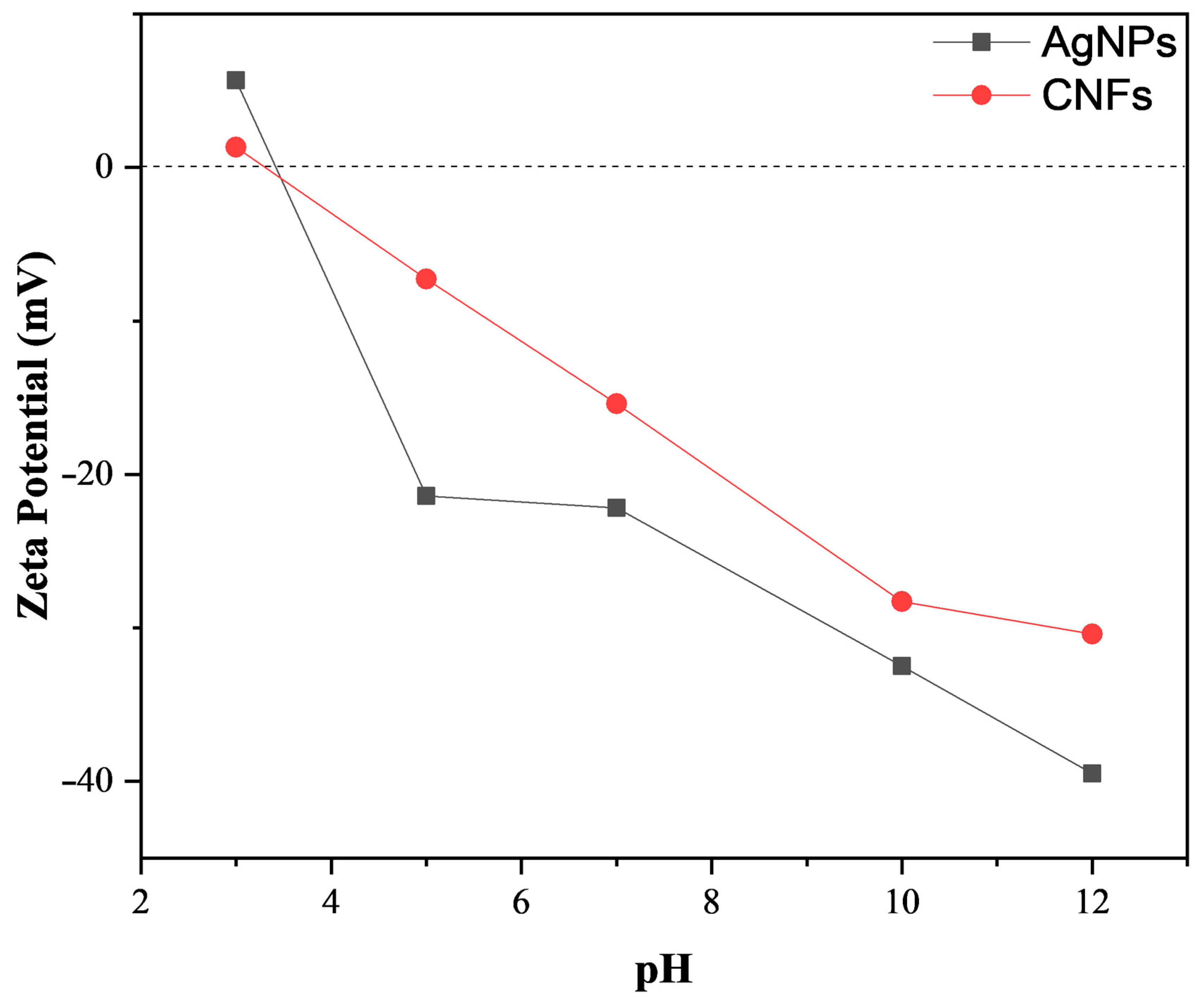
3.1. Material Characterization
3.2. Biological Properties
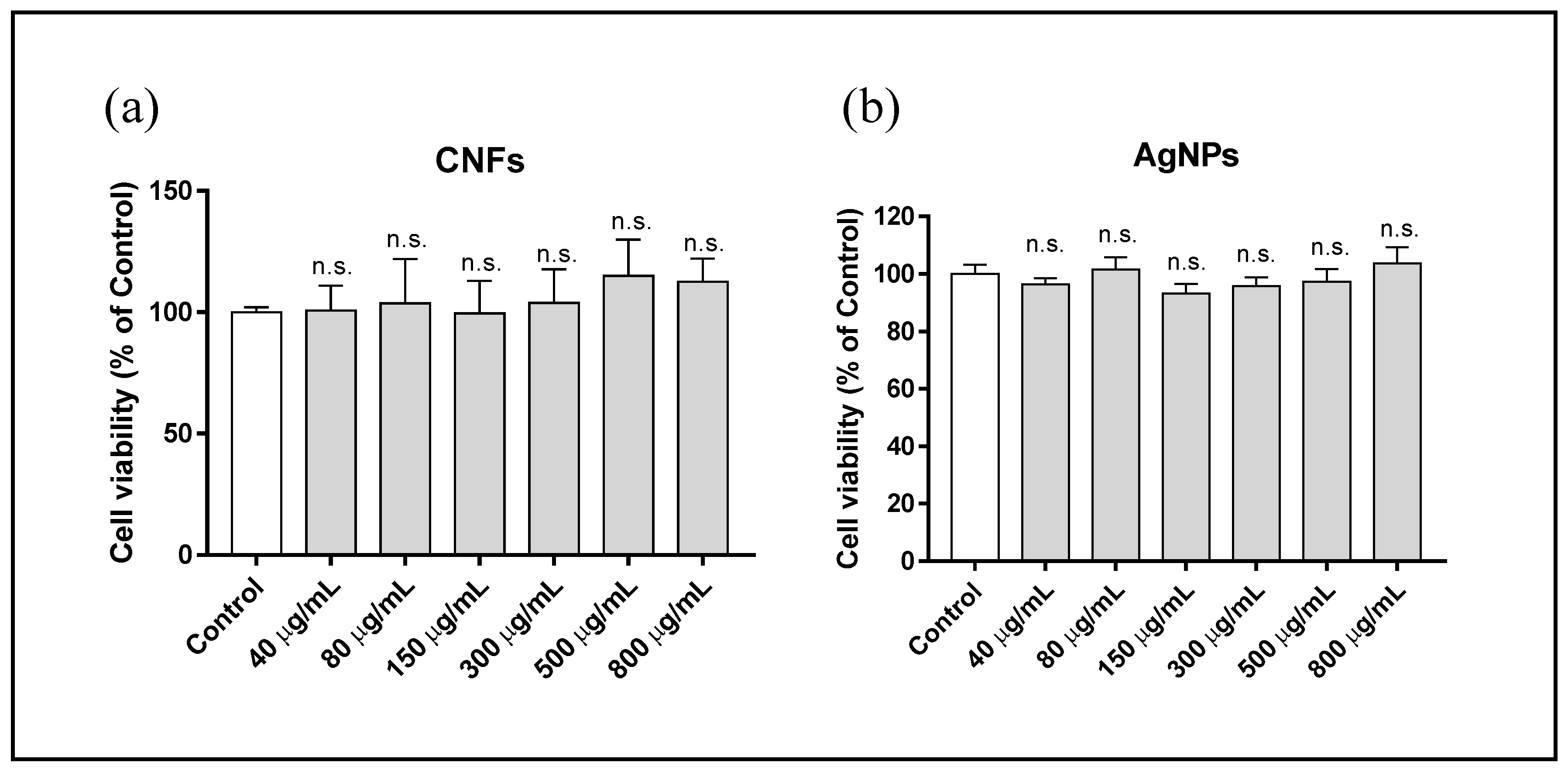
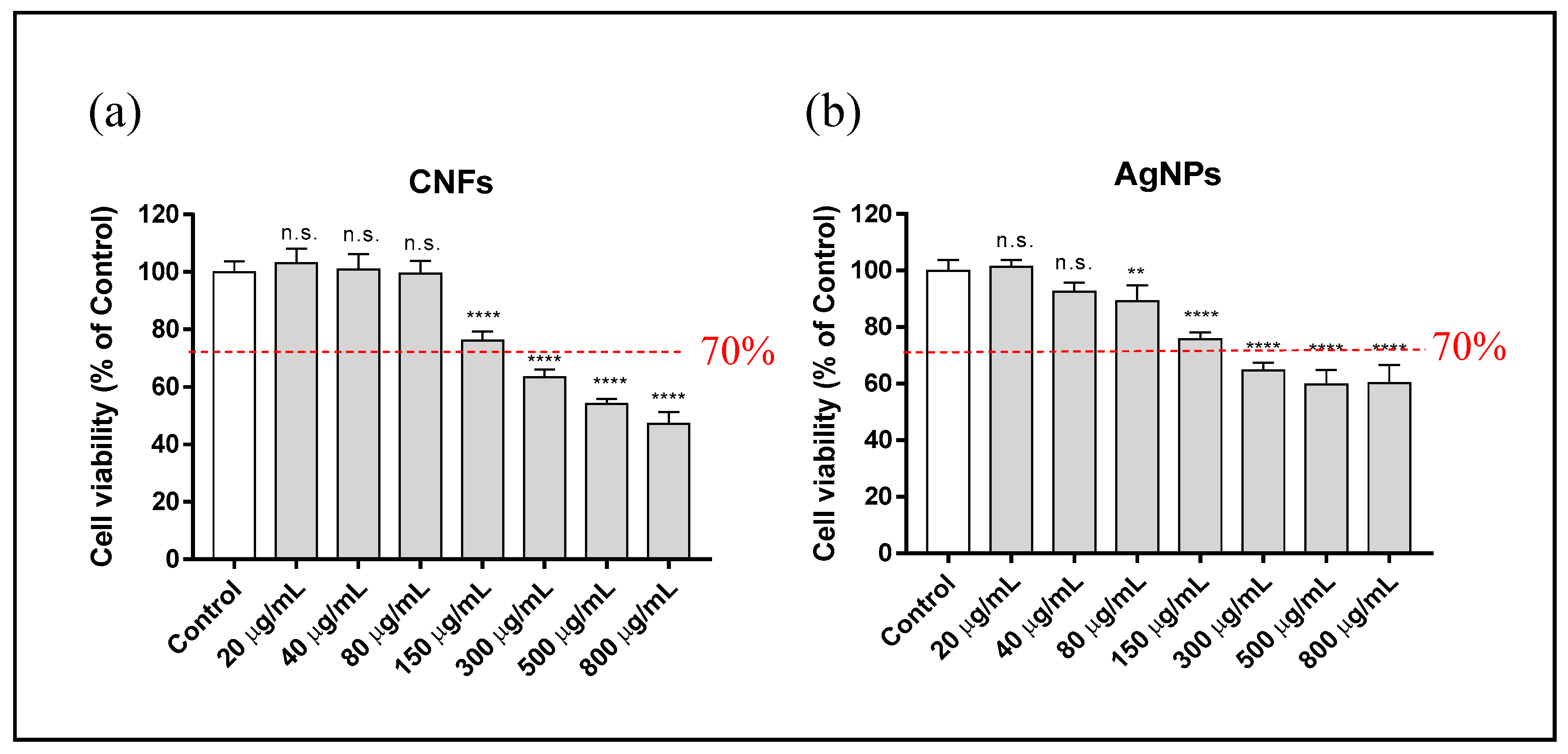
3.3. Cytotoxicity Assay
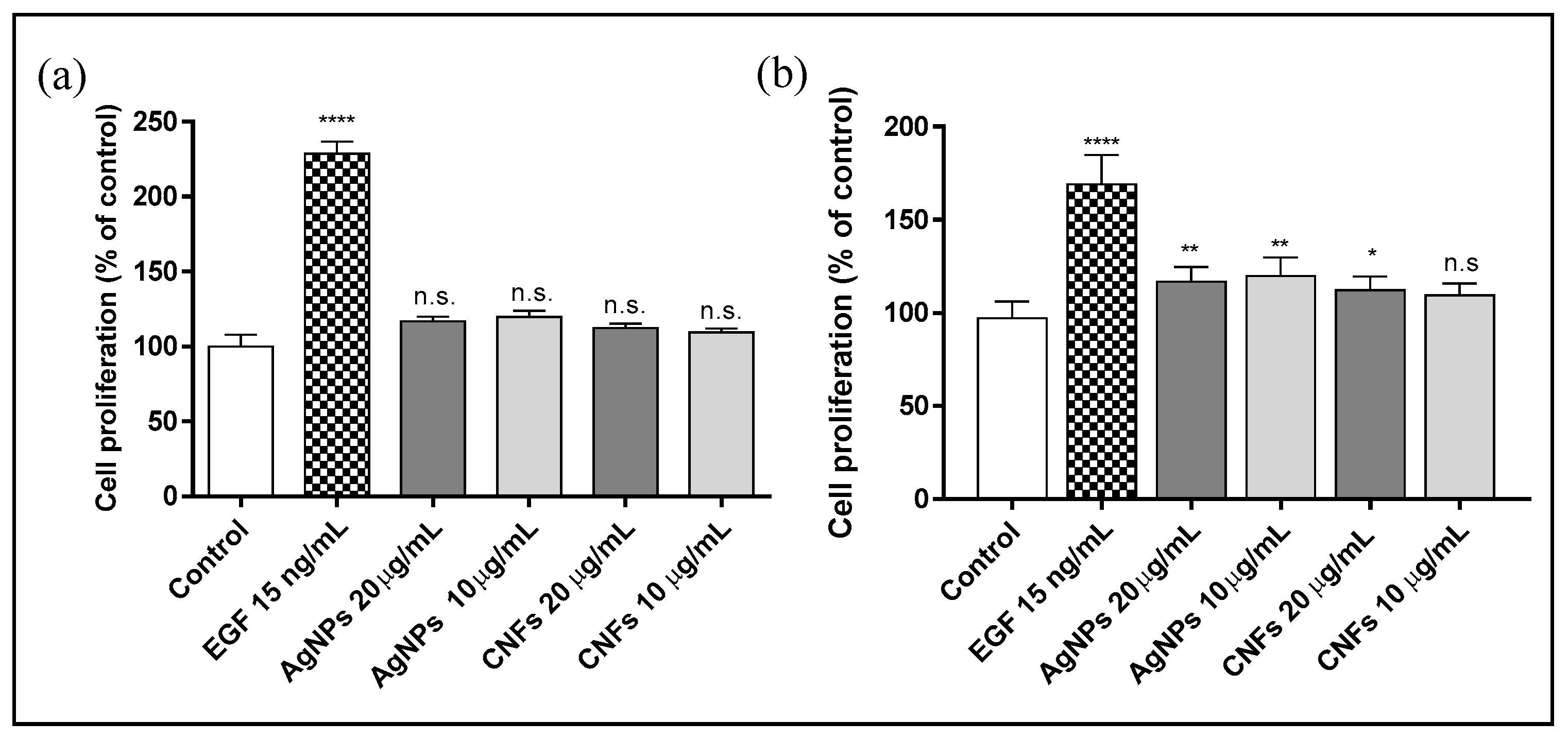
3.4. Proliferation Assay
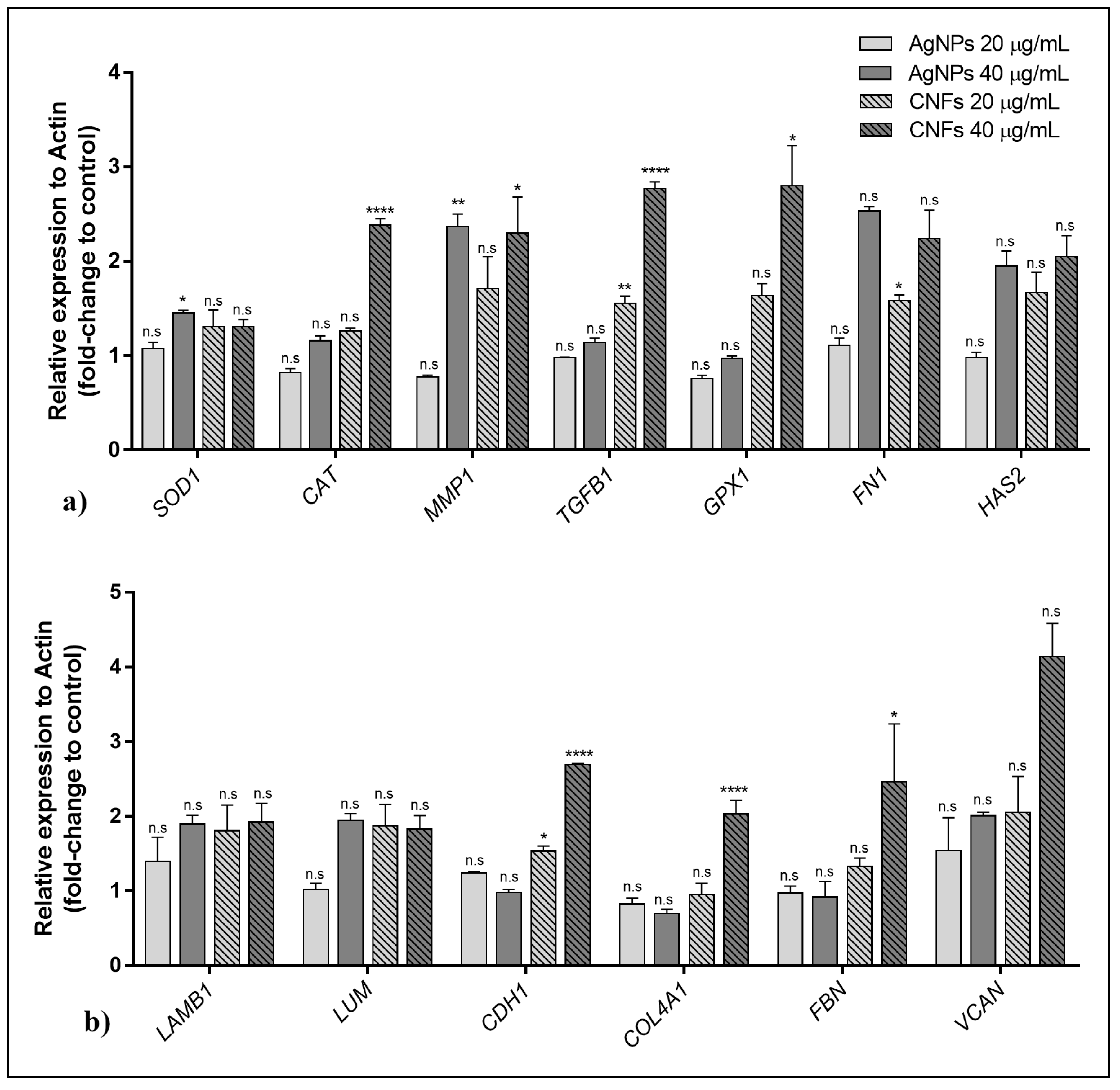
3.5. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene Symbol (Access Number) | Gene Name | Oligo Sequences | Function |
|---|---|---|---|
| ACTB (NM_001101) | Actin beta | 5′-CCATGCCCACCATCACGC-3′ | Highly conserved protein involved in cell motility, structure, and integrity |
| 5′-CACAGAGCCTCGCCTTTG-3′ | |||
| CAT (NM_001752) | Catalase | 5′-TGAATGAGGAACAGAGGAAACG-3′ | Encodes catalase, a key antioxidant enzyme in the body’s defense against oxidative stress |
| 5′-AGATCCGGACTGCACAAAG-3′ | |||
| MMP1 (NM_001145938) | Matrix metallopeptidase 1 | 5′-GGACCATGCCATTGAGAAAG-3′ | Involved in the breakdown of extracellular matrix in normal physiological processes |
| 5′-TCCTCCAGGTCCATCAAAAG-3′ | |||
| GPX1 (NM_000581) | Glutathione peroxidase 1 | 5′-TTTGGGCATCAGGAGAACGC-3′ | Catalyze the reduction of organic hydroperoxides and hydrogen peroxide by glutathione, and thereby protect cells against oxidative damage |
| 5′-ACCGTTCACCTCGCACTTC-3′ | |||
| COL4A1 (NM_000088) | Collagen type I alpha 1 | 5′-CAAGGGCGACAGAGGTTTGC-3′ | Abundant in bone, cornea, dermis, and tendon. Mutations in this gene are associated with osteogenesis imperfect types I-IV |
| 5′-AAAACTCACCAGGCTCCCCC-3′ | |||
| TGFB1 (NM_000660) | Transforming growth factor beta 1 | 5′-AGCTGTACATTGACTTCCGCA-3′ | Regulates cell proliferation, differentiation, and growth |
| 5′-TGTCCAGGCTCCAAATGTAGG-3′ | |||
| HAS2 (NM_005328) | Hyaluronan synthase 2 | 5′-CCGAGAATGGCTGTACAATGC-3′ | Serves a variety of functions, including space filling, lubrication of joints, and provision of a matrix through which cells can migrate |
| 5′-AGAGCTGGATTACTGTGGCAA-3′ | |||
| LAMB1 (NM_002291) | Laminin subunit beta 1 | 5′-CAGGGTGTGCAGTCAGGGAA-3′ | Implicated in a wide variety of biological processes including cell adhesion, differentiation, migration, signaling, neurite outgrowth, and metastasis |
| 5′-TGTGTCTGCGTTGAGGGTGT-3′ | |||
| LUM (NM_002345) | Lumican | 5′-ACTTGGGTAGCTTTCAGGGCA-3′ | Is the major keratan sulfate proteoglycan of the cornea but is also distributed in interstitial collagenous matrices throughout the body |
| 5′-TTCCTGGCATTGATTGGTGGT-3′ | |||
| FN1 (NM_001306129) | Fibronectin 1 | 5′-GGCCAGTCCTACAACCAGT-3′ | Involved in cell adhesion and migration processes including embryogenesis, wound healing, blood coagulation, host defense, and metastasis. |
| 5′-CGGGAATCTTCTCTGTCAGC-3′ | |||
| VCAN (NM_001126336) | Versican | 5′-CTGGTCTCCGCTGTATCCTG-3′ | Involved in cell adhesion, proliferation, migration, and angiogenesis and plays a central role in tissue morphogenesis and maintenance |
| 5′-ATCGCTGCAAAATGAACCCG-3′ | |||
| CDH1 (NM_001317184) | Cadherin 1 | 5′-AACAGCACGTACACAGCCCT-3′ | Loss of function of this gene is thought to contribute to cancer progression by increasing proliferation, invasion, and/or metastasis. |
| 5′-TCTGGTATGGGGGCGTTGTC-3′ | |||
| FBN (NM_000138) | Fibrillin 1 | 5′-ATCCAACCACGTGCATCAGT-3′ | Extracellular matrix glycoprotein that serves as a structural component of calcium-binding microfibrils, providing force-bearing structural support in elastic and nonelastic connective tissue throughout the body |
| 5′-AGAGCGGGTATCAACACAGC-3′ | |||
| SOD1 (NM_000454) | Superoxide dismutase 1 | 5′-GGTGTGGCCGATGTGTCT-3′ | The protein encoded by this gene binds copper and zinc ions and is one of two isozymes responsible for destroying free superoxide radicals in the body |
| 5′-TCCACCTTTGCCCAAGTCA-3′ |
References
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioff, N. Can nanotechnology and materials science help the fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, C.; Carriere, M.; Fusco, L.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Pasquali, M.; Pasquali, M.; Scott, J.A.; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef]
- El-Far, A.H.; Godugu, K.; Salaheldin, T.A.; Darwish, N.H.E.; Saddiq, A.A.; Mousa, S.A. Nanonutraceuticals: Anti-Cancer Activity and Improved Safety of Chemotherapy by Costunolide and Its Nanoformulation against Colon and Breast Cancer. Biomedicines 2021, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Salesa, B.; Llorens-Gámez, M.; Serrano-Aroca, Á. Study of 1D and 2D carbon nanomaterial in alginate films. Nanomaterials 2020, 10, 206. [Google Scholar] [CrossRef] [Green Version]
- Gardea, F.; Naraghi, M.; Lagoudas, D. Effect of thermal interface on heat flow in carbon nanofiber composites. ACS Appl. Mater. Interfaces 2014, 6, 1061–1072. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Sanmartín-Santos, I.; Gandía-Llop, S.; Salesa, B.; Martí, M.; Lillelund Aachmann, F.; Serrano-Aroca, Á. Enhancement of Antimicrobial Activity of Alginate Films with a Low Amount of Carbon Nanofibers (0.1% w/w). Appl. Sci. 2021, 11, 2311. [Google Scholar] [CrossRef]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly(lactic-co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef]
- Stout, D.A.; Yoo, J.; Santiago-Miranda, A.N.; Webster, T.J. Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular applications. Int. J. Nanomed. 2012, 7, 5653–5669. [Google Scholar] [CrossRef] [Green Version]
- Wood, W.; Li, B.; Zhong, W.-H. Influence of Phase Morphology on the Sliding Wear of Polyethylene Blends Filled with Carbon Nanofibers. Polym. Eng. Sci. 2010, 50, 613–623. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forró, L. Cellular toxicity of carbon-based nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef] [PubMed]
- Kisin, E.R.; Murray, A.R.; Sargent, L.; Lowry, D.; Chirila, M.; Siegrist, K.J.; Schwegler-Berry, D.; Leonard, S.; Castranova, V.; Fadeel, B.; et al. Genotoxicity of carbon nanofibers: Are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol. Appl. Pharmacol. 2011, 252, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabinski, C.; Hussain, S.; Lafdi, K.; Braydich-Stolle, L.; Schlager, J. Effect of particle dimension on biocompatibility of carbon nanomaterials. Carbon N. Y. 2007, 45, 2828–2835. [Google Scholar] [CrossRef]
- Salesa, B.; Martí, M.; Frígols, B.; Serrano-Aroca, Á. Carbon Nanofibers in Pure Form and in Calcium Alginate Composites Films: New Cost-Effective Antibacterial Biomaterials against the Life-Threatening Multidrug-Resistant Staphylococcus epidermidis. Polymers 2019, 11, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorens-Gámez, M.; Serrano-Aroca, Á. Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties. Polymers 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Llorens-Gámez, M.; Salesa, B.; Serrano-Aroca, Á. Physical and biological properties of alginate/carbon nanofibers hydrogel films. Int. J. Biol. Macromol. 2020, 151, 499–507. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Aachmann, F.L.; Moreno-Manzano, V.; Serrano-Aroca, Á. Graphene oxide nanosheets versus carbon nanofibers: Enhancement of physical and biological properties of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 2020, 143, 1000–1008. [Google Scholar] [CrossRef]
- Elias, L.; Taengua, R.; Frígols, B.; Salesa, B.; Serrano-Aroca, Á. Carbon Nanomaterials and LED Irradiation as Antibacterial Strategies against Gram-Positive Multidrug-Resistant Pathogens. Int. J. Mol. Sci. 2019, 20, 3603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [Green Version]
- Zorraquín-Peña, I.; Cueva, C.; de Llano, D.G.; Bartolomé, B.; Moreno-Arribas, M.V. Glutathione-stabilized silver nanoparticles: Antibacterial activity against periodontal bacteria, and cytotoxicity and inflammatory response in oral cells. Biomedicines 2020, 8, 375. [Google Scholar] [CrossRef]
- Dorovskikh, S.I.; Vikulova, E.S.; Chepeleva, E.V.; Vasilieva, M.B.; Nasimov, D.A.; Maksimovskii, E.A.; Tsygankova, A.R.; Basova, T.V.; Sergeevichev, D.S.; Morozova, N.B. Noble Metals for Modern Implant Materials: MOCVD of Film Structures and Cytotoxical, Antibacterial, and Histological Studies. Biomedicines 2021, 9, 851. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabakar, D.; Jacob, J.M.; Karuppusamy, I.; Saratale, R.G. Synthesis and characterization of silver nanoparticles using Gelidium amansii and its antimicrobial property against various pathogenic bacteria. Microb. Pathog. 2018, 114, 41–45. [Google Scholar] [CrossRef]
- Lee, Y.H.; Cheng, F.Y.; Chiu, H.W.; Tsai, J.C.; Fang, C.Y.; Chen, C.W.; Wang, Y.J. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials 2014, 35, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Stern, J.M.; Vanni, A.J.; Kelley, R.S.; Baumgart, E.; Field, D.; Libertino, J.A.; Summerhayes, I.C. In vitro analysis of a nanocrystalline silver-coated surgical mesh. Surg. Infect. 2007, 8, 397–403. [Google Scholar] [CrossRef]
- Lee, H.Y.; Park, H.K.; Lee, Y.M.; Kim, K.; Park, S.B. A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem. Commun. 2007, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef]
- Ahlberg, S.; Meinke, M.C.; Werner, L.; Epple, M.; Diendorf, J.; Blume-Peytavi, U.; Lademann, J.; Vogt, A.; Rancan, F. Comparison of silver nanoparticles stored under air or argon with respect to the induction of intracellular free radicals and toxic effects toward keratinocytes. Eur. J. Pharm. Biopharm. 2014, 88, 651–657. [Google Scholar] [CrossRef]
- Tian, J.; Wong, K.K.Y.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K.H. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef]
- Holmes, A.M.; Lim, J.; Studier, H.; Roberts, M.S. Varying the morphology of silver nanoparticles results in differential toxicity against micro-organisms, HaCaT keratinocytes and affects skin deposition. Nanotoxicology 2016, 10, 1503–1514. [Google Scholar] [CrossRef]
- Lu, W.; Senapati, D.; Wang, S.; Tovmachenko, O.; Singh, A.K.; Yu, H.; Ray, P.C. Effect of surface coating on the toxicity of silver nanomaterials on human skin keratinocytes. Chem. Phys. Lett. 2010, 487, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palanki, R.; Arora, S.; Tyagi, N.; Rusu, L.; Singh, A.P.; Palanki, S.; Carter, J.E.; Singh, S. Size is an essential parameter in governing the UVB-protective efficacy of silver nanoparticles in human keratinocytes. BMC Cancer 2015, 15, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szmyd, R.; Goralczyk, A.G.; Skalniak, L.; Cierniak, A.; Lipert, B.; Filon, F.L.; Crosera, M.; Borowczyk, J.; Laczna, E.; Drukala, J.; et al. Effect of silver nanoparticles on human primary keratinocytes. Biol. Chem. 2013, 394, 113–123. [Google Scholar] [CrossRef]
- Franková, J.; Pivodová, V.; Vágnerová, H.; Juráňová, J.; Ulrichová, J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J. Appl. Biomater. Funct. Mater. 2016, 14, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H.; Williams, K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997, 179, 6127–6132. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Sun, Q.; Duan, M.; Liu, D.; Fan, W. Establishment and characterization of silver-resistant Enterococcus faecalis. Folia Microbiol. 2020, 65, 721–733. [Google Scholar] [CrossRef]
- Kunkalekar, R.K. Role of Oxides (Fe3O4, MnO2) in the Antibacterial Action of Ag-Metal Oxide Hybrid Nanoparticles. In Noble Metal-Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 303–312. ISBN 9780128141359. [Google Scholar]
- Serrano-Aroca, Á.; Takayama, K.; Tuñón-Molina, A.; Seyran, M.; Hassan, S.S.; Pal Choudhury, P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palù, G.; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef]
- NCBI Primer Designing Tool. Available online: https://www.ncbi.nlm.nih.gov/tools/primer-blast/ (accessed on 5 August 2021).
- Aguilar, T.; Sani, E.; Mercatelli, L.; Carrillo-Berdugo, I.; Torres, E.; Navas, J. Exfoliated graphene oxide-based nanofluids with enhanced thermal and optical properties for solar collectors in concentrating solar power. J. Mol. Liq. 2020, 306, 112862. [Google Scholar] [CrossRef]
- Roy, D.; Kanojia, S.; Mukhopadhyay, K.; Eswara Prasad, N. Analysis of carbon-based nanomaterials using Raman spectroscopy: Principles and case studies. Bull. Mater. Sci. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Gouadec, G.; Colomban, P. Raman Spectroscopy of nanomaterials: How spectra relate to disorder, particle size and mechanical properties. Prog. Cryst. Growth Charact. Mater. 2007, 53, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Tomankova, K.; Horakova, J.; Harvanova, M.; Malina, L.; Soukupova, J.; Hradilova, S.; Kejlova, K.; Malohlava, J.; Licman, L.; Dvorakova, M.; et al. Cytotoxicity, cell uptake and microscopic analysis of titanium dioxide and silver nanoparticles in vitro. Food Chem. Toxicol. 2015, 82, 106–115. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, C.; Wang, J. Raman spectra of carbon nanotubes and nanofibers prepared by ethanol flames. J. Mater. Sci. 2004, 39, 1091–1094. [Google Scholar] [CrossRef]
- Salesa, B.; Serrano-Aroca, Á. Multi-Layer Graphene Oxide in Human Keratinocytes: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Coatings 2021, 11, 414. [Google Scholar] [CrossRef]
- Steffens, S.; Schrader, A.J.; Vetter, G.; Eggers, H.; Blasig, H.; Becker, J.; Kuczyk, M.A.; Serth, J. Fibronectin 1 protein expression in clear cell renal cell carcinoma. Oncol. Lett. 2012, 3, 787–790. [Google Scholar] [CrossRef]
- Duan, D.; Derynck, R. Transforming growth factor-β (TGF-β)-induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response. J. Biol. Chem. 2019, 294, 8490–8504. [Google Scholar] [CrossRef]
- Erlebacher, A.; Filvaroff, E.H.; Ye, J.Q.; Derynck, R. Osteoblastic responses to TGF-β during bone remodeling. Mol. Biol. Cell 1998, 9, 1903–1918. [Google Scholar] [CrossRef]
- Afaq, F.; Mukhtar, H. Effects of solar radiation on cutaneous detoxification pathways. J. Photochem. Photobiol. B Biol. 2001, 63, 61–69. [Google Scholar] [CrossRef]
- Song, X.; Mosby, N.; Yang, J.; Xu, A.; Abdel-Malek, Z.; Kadekaro, A.L. α-MSH activates immediate defense responses to UV-induced oxidative stress in human melanocytes. Pigment. Cell Melanoma Res. 2009, 22, 809–818. [Google Scholar] [CrossRef]
- Panich, U.; Tangsupa-A-Nan, V.; Onkoksoong, T.; Kongtaphan, K.; Kasetsinsombat, K.; Akarasereenont, P.; Wongkajornsilp, A. Inhibition of UVA-mediated melanogenesis by ascorbic acid through modulation of antioxidant defense and nitric oxide system. Arch. Pharm. Res. 2011, 34, 811–820. [Google Scholar] [CrossRef]
- Pluemsamran, T.; Onkoksoong, T.; Panich, U. Caffeic acid and ferulic acid inhibit UVA-induced matrix metalloproteinase-1 through regulation of antioxidant defense system in keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 961–968. [Google Scholar] [CrossRef]
- Pećina-Šlaus, N. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int. 2003, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Crosstalk between microrna and oxidative stress in primary open-angle glaucoma. Int. J. Mol. Sci. 2021, 22, 2421. [Google Scholar] [CrossRef]
- Chen, D.; Lu, Y.; Yu, W.; Luo, J.; Xiao, Z.; Xiao, F.; Wang, X. Clinical value of decreased superoxide dismutase 1 in patients with epilepsy. Seizure 2012, 21, 508–511. [Google Scholar] [CrossRef] [Green Version]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Colombo, R.; Milzani, A. S-glutathionylation in protein redox regulation. Free Radic. Biol. Med. 2007, 43, 883–898. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Chen, L.; Wu, M.; Jiang, S.; Zhang, Y.; Li, R.; Lu, Y.; Liu, L.; Wu, G.; Liu, Y.; Xie, L.; et al. Skin toxicity assessment of silver nanoparticles in a 3D epidermal model compared to 2D keratinocytes. Int. J. Nanomed. 2019, 14, 9707–9719. [Google Scholar] [CrossRef] [Green Version]
- Carrola, J.; Bastos, V.; Ferreira De Oliveira, J.M.P.; Oliveira, H.; Santos, C.; Gil, A.M.; Duarte, I.F. Insights into the impact of silver nanoparticles on human keratinocytes metabolism through NMR metabolomics. Arch. Biochem. Biophys. 2016, 589, 53–61. [Google Scholar] [CrossRef]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Material | DLS (nm) | PdI | ||
|---|---|---|---|---|
| Water | DMEM | Water | DMEM | |
| AgNPs | 461.3 | 1693 | 0.471 | 0.533 |
| CNFs | 811.2 | 1142 | 0.586 | 0.615 |
| Nanomaterial | EC50 (µg/mL) | 95% CI | R Square |
|---|---|---|---|
| AgNPs | 581.9 | 515.2–670.4 | 0.9037 |
| CNFs | 608.1 | 531.4–709.5 | 0.9308 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salesa, B.; Assis, M.; Andrés, J.; Serrano-Aroca, Á. Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Biomedicines 2021, 9, 1155. https://doi.org/10.3390/biomedicines9091155
Salesa B, Assis M, Andrés J, Serrano-Aroca Á. Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Biomedicines. 2021; 9(9):1155. https://doi.org/10.3390/biomedicines9091155
Chicago/Turabian StyleSalesa, Beatriz, Marcelo Assis, Juan Andrés, and Ángel Serrano-Aroca. 2021. "Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression" Biomedicines 9, no. 9: 1155. https://doi.org/10.3390/biomedicines9091155
APA StyleSalesa, B., Assis, M., Andrés, J., & Serrano-Aroca, Á. (2021). Carbon Nanofibers versus Silver Nanoparticles: Time-Dependent Cytotoxicity, Proliferation, and Gene Expression. Biomedicines, 9(9), 1155. https://doi.org/10.3390/biomedicines9091155







