Maternal Exercise during Pregnancy Impacts Motor Performance in 9-Year-Old Children: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.1.1. Intervention during Pregnancy
2.1.2. Follow-Up
2.2. Anthropometry
2.2.1. Baseline Maternal and Fetal Anthropometric Data
2.2.2. Child Anthropometric Data at Follow-Up
2.3. Laboratory Parameters
2.4. Motor Skills
2.5. Statistics, Data Management, and Data Analysis
3. Results
3.1. Mothers’ and Children’s Baseline Characteristics
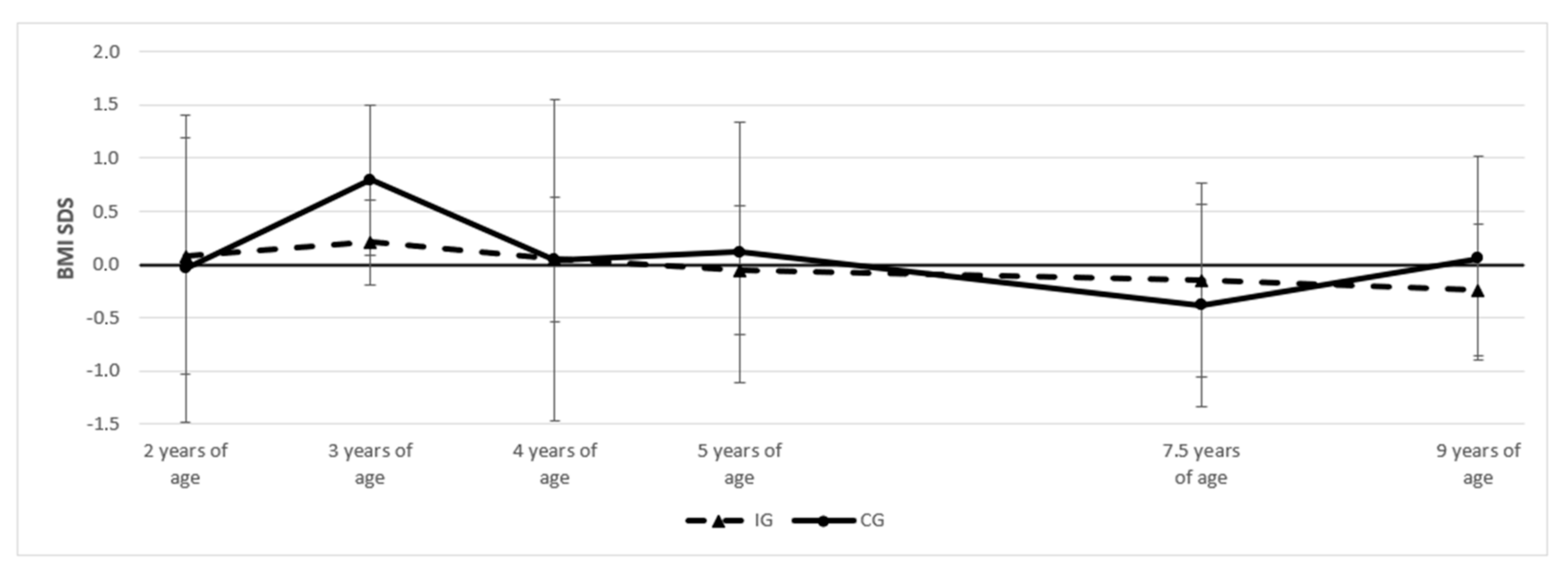
3.2. Anthropometry of Children at Follow-Up
3.3. Motor Performance
Factors Associated with Motor Performance at the Second Follow-Up
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nascimento, S.L.; Surita, F.G.; Cecatti, J.G. Physical exercise during pregnancy: A systematic review. Curr. Opin. Obstet. Gynecol. 2012, 24, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.H.; Meah, V.L.; Ruchat, S.M.; Davies, G.A.; Skow, R.J.; Barrowman, N.; Adamo, K.B.; Poitras, V.J.; Gray, C.E.; Jaramillo Garcia, A.; et al. Impact of prenatal exercise on neonatal and childhood outcomes: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 1386–1396. [Google Scholar] [CrossRef]
- Stafne, S.N.; Salvesen, K.A.; Romundstad, P.R.; Eggebo, T.M.; Carlsen, S.M.; Morkved, S. Regular exercise during pregnancy to prevent gestational diabetes: A randomized controlled trial. Obstet. Gynecol. 2012, 119, 29–36. [Google Scholar] [CrossRef]
- Di Mascio, D.; Magro-Malosso, E.R.; Saccone, G.; Marhefka, G.D.; Berghella, V. Exercise during pregnancy in normal-weight women and risk of preterm birth: A systematic review and meta-analysis of randomized controlled trials. Am. J. Obstet. Gynecol. 2016, 215, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, G.; Hu, Y.; Yang, Q.; Deavila, J.M.; Zhu, M.J.; Du, M. Effects of Maternal Exercise During Pregnancy on Perinatal Growth and Childhood Obesity Outcomes: A Meta-analysis and Meta-regression. Sports Med. 2021, 51, 2329–2347. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F., 3rd; Simonian, S.; Lopez, B.; Appleby-Wineberg, S.; Harcar-Sevcik, R. The one-year morphometric and neurodevelopmental outcome of the offspring of women who continued to exercise regularly throughout pregnancy. Am. J. Obstet. Gynecol. 1998, 178, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Labonte-Lemoyne, E.; Curnier, D.; Ellemberg, D. Exercise during pregnancy enhances cerebral maturation in the newborn: A randomized controlled trial. J. Clin. Exp. Neuropsychol. 2017, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Hellenes, O.M.; Vik, T.; Lohaugen, G.C.; Salvesen, K.A.; Stafne, S.N.; Morkved, S.; Evensen, K.A. Regular moderate exercise during pregnancy does not have an adverse effect on the neurodevelopment of the child. Acta Paediatr. 2015, 104, 285–291. [Google Scholar] [CrossRef] [PubMed]
- McMillan, A.G.; May, L.E.; Gaines, G.G.; Isler, C.; Kuehn, D. Effects of Aerobic Exercise during Pregnancy on 1-Month Infant Neuromotor Skills. Med. Sci. Sports Exerc. 2019, 51, 1671–1676. [Google Scholar] [CrossRef]
- Clapp, J.F., 3rd; Lopez, B.; Harcar-Sevcik, R. Neonatal behavioral profile of the offspring of women who continued to exercise regularly throughout pregnancy. Am. J. Obstet. Gynecol. 1999, 180, 91–94. [Google Scholar] [CrossRef]
- Nakahara, K.; Michikawa, T.; Morokuma, S.; Ogawa, M.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Shimono, M.; Kawamoto, T.; et al. Influence of physical activity before and during pregnancy on infant’s sleep and neurodevelopment at 1-year-old. Sci. Rep. 2021, 11, 8099. [Google Scholar] [CrossRef] [PubMed]
- Domingues, M.R.; Matijasevich, A.; Barros, A.J.; Santos, I.S.; Horta, B.L.; Hallal, P.C. Physical activity during pregnancy and offspring neurodevelopment and IQ in the first 4 years of life. PLoS ONE 2014, 9, e110050. [Google Scholar] [CrossRef] [PubMed]
- Parnpiansil, P.; Jutapakdeegul, N.; Chentanez, T.; Kotchabhakdi, N. Exercise during pregnancy increases hippocampal brain-derived neurotrophic factor mRNA expression and spatial learning in neonatal rat pup. Neurosci. Lett. 2003, 352, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Bick-Sander, A.; Steiner, B.; Wolf, S.A.; Babu, H.; Kempermann, G. Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proc. Natl. Acad. Sci. USA 2006, 103, 3852–3857. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Kim, H.; Lee, J.W.; Kim, Y.S.; Yang, H.Y.; Chang, H.K.; Lee, T.H.; Shin, M.C.; Lee, M.H.; Shin, M.S.; et al. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev. 2006, 28, 147–154. [Google Scholar] [CrossRef]
- Aksu, I.; Baykara, B.; Ozbal, S.; Cetin, F.; Sisman, A.R.; Dayi, A.; Gencoglu, C.; Tas, A.; Buyuk, E.; Gonenc-Arda, S.; et al. Maternal treadmill exercise during pregnancy decreases anxiety and increases prefrontal cortex VEGF and BDNF levels of rat pups in early and late periods of life. Neurosci. Lett. 2012, 516, 221–225. [Google Scholar] [CrossRef]
- Gomes da Silva, S.; de Almeida, A.A.; Fernandes, J.; Lopim, G.M.; Cabral, F.R.; Scerni, D.A.; de Oliveira-Pinto, A.V.; Lent, R.; Arida, R.M. Maternal Exercise during Pregnancy Increases BDNF Levels and Cell Numbers in the Hippocampal Formation but Not in the Cerebral Cortex of Adult Rat Offspring. PLoS ONE 2016, 11, e0147200. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, N.; Bae-Gartz, I.; Bauer, C.; Janoschek, R.; Koxholt, I.; Mahabir, E.; Appel, S.; Alejandre Alcazar, M.A.; Grossmann, N.; Vohlen, C.; et al. Exercise during pregnancy and its impact on mothers and offspring in humans and mice. J. Dev. Orig. Health Dis. 2018, 9, 63–76. [Google Scholar] [CrossRef]
- Kodomari, I.; Wada, E.; Nakamura, S.; Wada, K. Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 2009, 54, 95–98. [Google Scholar] [CrossRef]
- Vega, S.R.; Kleinert, J.; Sulprizio, M.; Hollmann, W.; Bloch, W.; Struder, H.K. Responses of serum neurotrophic factors to exercise in pregnant and postpartum women. Psychoneuroendocrinology 2011, 36, 220–227. [Google Scholar] [CrossRef]
- Clapp, J.F., 3rd. Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J. Pediatr. 1996, 129, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Ellingsen, M.S.; Pettersen, A.; Stafne, S.N.; Morkved, S.; Salvesen, K.A.; Evensen, K. Neurodevelopmental outcome in 7-year-old children is not affected by exercise during pregnancy: Follow up of a multicentre randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity and Exercise During Pregnancy and the Postpartum Period: ACOG Committee Opinion Summary, Number 804. Obstet. Gynecol. 2020, 135, 991–993. [CrossRef] [PubMed]
- Ferrari, N.; Schmitz, L.; Schmidt, N.; Mahabir, E.; Van de Vondel, P.; Merz, W.M.; Lehmacher, W.; Stock, S.; Brockmeier, K.; Ensenauer, R.; et al. A lifestyle intervention during pregnancy to reduce obesity in early childhood: The study protocol of ADEBAR—A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. (Eds.) Weight Gain During Pregnancy: Reexamining the Guidelines; Reports funded by National Institutes of Health; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Stolzenberg, H.; Kahl, H.; Bergmann, K.E. Body measurements of children and adolescents in Germany. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007, 50, 659–669. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschrift Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Wabitsch, M.; Kunze, D. Evidenzbasierte (S3-) Leitlinie der Arbeitsgemeinschaft Adipositas im Kindes- und Jugendalter (AGA) der Deutschen Adipositas-Gesellschaft (DAG) und der Deutschen Gesellschaft für Kinder-und Jugendmedizin (DGKJ). 2019. Available online: https://register.awmf.org/de/leitlinien/detail/050-002 (accessed on 6 February 2023).
- DataInput. Nutriguard-MS Gebrauchsanleitung: Version 2.0. Available online: https://data-input.de/bia/deutsch/service/downloads.php (accessed on 2 November 2023).
- DataInput. Nutri Plus Gebrauchsanleitung: Software zur Bestimmung von Körperzusammensetzung und Ernährungszustand aus BIA Messungen. Available online: https://data-input.de/media/pdf_deutsch_2020/Data_Input_NutriPlus_Gebrauchsanleitung_2022_DE.pdf (accessed on 2 November 2023).
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef]
- Klein, D.; Koch, B.; Dordel, S.; Strüder, H.K.; Graf, C. The KiMo test: A motor screening for pre-school children aged 3–6 years. Gazz. Medica Ital. Arch. Per Le Sci. Mediche 2012, 171, 13–26. [Google Scholar]
- Dordel, S.; Koch, B. Basistests zur Erfassung der Motorischen Leistungsfähigkeit von Kindern und Jugendlichen—Dordel-Koch-Test (DKT). Available online: https://fitnessolympiade.de/pdfs/manual-dordel-koch-test.pdf (accessed on 6 February 2023).
- Schlag, E.; Ferrari, N.; Koch, B.; Dordel, S.; Joisten, C. Secular trends in motor performance of children and adolescents between 2010 and 2020. Transl. Sports Med. 2021, 4, 882–891. [Google Scholar] [CrossRef]
- Wessely, S.; Ferrari, N.; Friesen, D.; Grauduszus, M.; Klaudius, M.; Joisten, C. Changes in Motor Performance and BMI of Primary School Children over Time-Influence of the COVID-19 Confinement and Social Burden. Int. J. Environ. Res. Public Health 2022, 19, 4565. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.A.; Baldi, J.C.; Cutfield, W.S.; McCowan, L.; Hofman, P.L. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. J. Clin. Endocrinol. Metab. 2010, 95, 2080–2088. [Google Scholar] [CrossRef]
- Bell, R.J.; Palma, S.M.; Lumley, J.M. The effect of vigorous exercise during pregnancy on birth-weight. Aust. N. Zealand J. Obstet. Gynaecol. 1995, 35, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Magann, E.F.; Evans, S.F.; Weitz, B.; Newnham, J. Antepartum, intrapartum, and neonatal significance of exercise on healthy low-risk pregnant working women. Obstet. Gynecol. 2002, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Leao, O.A.A.; Domingues, M.R.; Bertoldi, A.D.; Ricardo, L.I.C.; Muller, W.A.; Tornquist, L.; Martins, R.C.; Murray, J.; Silveira, M.F.; Crochemore-Silva, I.; et al. Effects of Regular Exercise During Pregnancy on Early Childhood Neurodevelopment: The Physical Activity for Mothers Enrolled in Longitudinal Analysis Randomized Controlled Trial. J. Phys. Act. Health 2022, 19, 203–210. [Google Scholar] [CrossRef]
- Domin, R.; Dadej, D.; Pytka, M.; Zybek-Kocik, A.; Ruchala, M.; Guzik, P. Effect of Various Exercise Regimens on Selected Exercise-Induced Cytokines in Healthy People. Int. J. Environ. Res. Public Health 2021, 18, 1261. [Google Scholar] [CrossRef]
- Guinhouya, B.C.; Duclos, M.; Enea, C.; Storme, L. Beneficial Effects of Maternal Physical Activity during Pregnancy on Fetal, Newborn, and Child Health: Guidelines for Interventions during the Perinatal Period from the French National College of Midwives. J. Midwifery Women’s Health 2022, 67 (Suppl. 1), S149–S157. [Google Scholar] [CrossRef] [PubMed]
- Klintsova, A.Y.; Dickson, E.; Yoshida, R.; Greenough, W.T. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004, 1028, 92–104. [Google Scholar] [CrossRef]
- Sasaki, R.; Watanabe, H.; Miyaguchi, S.; Otsuru, N.; Ohno, K.; Sakurai, N.; Kodama, N.; Onishi, H. Contribution of the brain-derived neurotrophic factor and neurometabolites to the motor performance. Behav. Brain Res. 2021, 412, 113433. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Korte, M. Form follows function: BDNF and its involvement in sculpting the function and structure of synapses. Neuropharmacology 2014, 76 Pt C, 628–638. [Google Scholar] [CrossRef]
- Porcher, C.; Hatchett, C.; Longbottom, R.E.; McAinch, K.; Sihra, T.S.; Moss, S.J.; Thomson, A.M.; Jovanovic, J.N. Positive feedback regulation between gamma-aminobutyric acid type A (GABA(A)) receptor signaling and brain-derived neurotrophic factor (BDNF) release in developing neurons. J. Biol. Chem. 2011, 286, 21667–21677. [Google Scholar] [CrossRef] [PubMed]
- Vaz, S.H.; Jorgensen, T.N.; Cristovao-Ferreira, S.; Duflot, S.; Ribeiro, J.A.; Gether, U.; Sebastiao, A.M. Brain-derived neurotrophic factor (BDNF) enhances GABA transport by modulating the trafficking of GABA transporter-1 (GAT-1) from the plasma membrane of rat cortical astrocytes. J. Biol. Chem. 2011, 286, 40464–40476. [Google Scholar] [CrossRef] [PubMed]
- Mega, F.; de Meireles, A.L.F.; Piazza, F.V.; Spindler, C.; Segabinazi, E.; Dos Santos Salvalaggio, G.; Achaval, M.; Marcuzzo, S. Paternal physical exercise demethylates the hippocampal DNA of male pups without modifying the cognitive and physical development. Behav. Brain Res. 2018, 348, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Riyahi, J.; Abdoli, B.; Gelfo, F.; Petrosini, L.; Khatami, L.; Meftahi, G.H.; Haghparast, A. Multigenerational effects of paternal spatial training are lasting in the F1 and F2 male offspring. Behav. Pharmacol. 2022, 33, 342–354. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Total Population Mean ± SD/% | Ig Mean ± SD/% | CG Mean ± SD/% | p-Value |
|---|---|---|---|---|
| Baseline characteristics of the mother | ||||
| Age (year) | 30.9 ± 3.5 (n = 19) | 31.4 ± 4.1 (n = 11) | 30.2 ± 2.7 (n = 8) | 0.484 + |
| Height (m) | 1.68 ± 0.05 (n = 19) | 1.69 ± 0.06 (n = 11) | 1.68 ± 0.05 (n = 8) | 0.752 + |
| Weight before pregnancy (kg) | 63.6 ± 7.8 (n = 19) | 62.5 ± 7.1 (n = 11) | 65.1 ± 9.1 (n = 8) | 0.516 + |
| BMI before pregnancy (kg/m2) | 22.2 ± 2.1 (n = 19) | 21.8 ± 2.0 (n = 11) | 22.9 ± 2.3 (n = 8) | 0.308 + |
| BMI classification before pregnancy | ||||
| Underweight | 0.0% (n = 0) | 0.0% (n = 0) | 0.0% (n = 0) | 0.080 § |
| Normal weight | 89.5% (n = 17) | 100.0% (n = 11) | 75.0% (n = 6) | |
| Overweight | 10.5% (n = 2) | 0.0% (n = 0) | 25.0% (n = 2) | |
| Obese | 0.0% (n = 0) | 0.0% (n = 0) | 0.0% (n = 0) | |
| Nationality (German) | 94.7% (n = 18) | 90.9% (n = 10) | 100.0% (n = 8) | 0.381 § |
| Primipara | 84.2% (n = 16) | 90.9% (n = 10) | 75.0% (n = 6) | 0.348 § |
| Obstetric data of the mother | ||||
| Weight gain during pregnancy (kg) | 17.7 ± 6.5 (n = 18) | 17.5 ± 5.0 (n = 11) | 18.1 ± 8.9 (n = 7) | 0.883 + |
| Classification of weight gain (IOM) | 0.429 § | |||
| Below recommendation | 11.2% (n = 2) | 18.1% (n = 2) | 0.0% (n = 0) | |
| Within recommendation | 44.4% (n = 8) | 36.4% (n = 4) | 57.1% (n = 4) | |
| Above recommendation | 44.4% (n = 8) | 45.5% (n = 5) | 42.9% (n = 3) | |
| Mode of delivery | ||||
| Vaginal delivery (normal) | 49.9% (n = 9) | 40.0% (n= 4) | 62.5% (n = 5) | |
| Vaginal delivery (instrumental) | 16.7% (n = 3) | 20.0% (n = 2) | 12.5% (n = 1) | 0.813 § |
| Caesarean section (elective) | 16.7% (n = 3) | 20.0% (n = 2) | 12.5% (n = 1) | |
| Caesarean section (emergency) | 16.7% (n = 3) | 20.0% (n = 2) | 12.5% (n = 1) | |
| Maternal laboratory parameter at 36 weeks of gestation | ||||
| BDNF | 5175.4 ± 2698.9 (n = 18) | 6284.6 ± 2468.5 (n = 11) | 3432.4 ± 2164.0 (n = 7) | 0.022 + |
| Data of the newborn | ||||
| Female sex | 63.2% (n = 12) | 72.7% (n = 8) | 50.0% (n = 4) | 0.377 § |
| Birth weight (g) | 3482.6 ± 407.5 (n = 19) | 3505.9 ± 338.1 (n = 11) | 3450.6 ± 511.4 (n = 8) | 0.795 + |
| Birth length (cm) | 51.7 ± 2.6 (n = 19) | 51.9 ± 2.7 (n = 11) | 51.4 ± 2.5 (n = 8) | 0.665 + |
| Classification of birth weight according to gestational age | ||||
| SGA (<10. Percentile) | 0.0% (n = 0) | 0.0% (n = 0) | 0.0% (n = 0) | |
| Appropriate for gestational age (10–90 Percentile) | 100.0% (n = 19) | 100.0% (n = 11) | 100.0% (n = 8) | - |
| LGA (>90 Percentile) | 0.0% (n = 0) | 0.0% (n = 0) | 0.0% (n = 0) |
| Parameter | All | IG | CG | p-Value |
|---|---|---|---|---|
| Age (years) 2nd follow-up | 9.4 ± 0.7 (n = 19) | 9.9 ± 0.2 (n = 11) | 8.7 ± 0.5 (n = 8) | ≤0.001 + |
| Height (meters) 2nd follow-up | 1.38 ± 0.07 (n = 19) | 1.41 ± 0.06 (n = 11) | 1.33 ± 0.06 (n = 8) | 0.005 + |
| Weight (kg) 2nd follow-up | 31.5 ± 5.1 (n = 19) | 32.9 ± 4.3 (n = 11) | 29.4 ± 5.7 (n = 8) | 0.151 + |
| BMI (kg/m2) 2nd follow-up | 16.5 ± 1.7 (n = 19) | 16.3 ± 1.4 (n = 11) | 16.7 ± 2.0 (n = 8) | 0.656 + |
| BMI-SDS 2nd follow-up | −0.1 ± 0.8 (n = 19) | −0.2 ± 0.6 (n = 11) | 0.06 ± 1.0 (n = 8) | 0.422 + |
| Fat mass (kg) 2nd follow-up | 5.0 ± 2.4 (n = 18) | 5.4 ± 2.0 (n = 11) | 4.3 ± 2.9 (n = 7) | 0.360 + |
| Fat mass (%) 2nd follow-up | 15.4 ± 6.0 (n = 18) | 16.4 ± 5.2 (n = 11) | 13.8 ± 7.2 (n = 7) | 0.596 # |
| Lean body mass (kg) 2nd follow-up | 26.7 ± 3.7 (n = 18) | 27.4 ± 3.5 (n = 11) | 25.5 ± 3.9 (n = 7) | 0.295 + |
| Lean body mass (%) 2nd follow-up | 84.6 ± 5.9 (n = 18) | 83.5 ± 5.1 (n = 11) | 86.2 ± 7.1 (n = 7) | 0.367 + |
| Skeletal muscle mass (kg) 2nd follow-up | 17.2 ± 2.6 (n = 18) | 17.2 ± 3.1 (n = 11) | 17.1 ± 1.7 (n = 7) | 0.937 + |
| Skeletal muscle mass (%) 2nd follow-up | 54.9 ± 9.0 (n = 18) | 52.5 ± 7.6 (n = 11) | 58.8 ± 10.3 (n = 8) | 0.154 + |
| Parameter | All | IG | CG | p-Value |
|---|---|---|---|---|
| Lateral jumping (counts) 1st follow-up | 11.2 ± 7.7 (n = 19) | 11.6 ± 8.2 (n = 12) | 10.6 ± 7.3 (n = 7) | 0.916 * |
| Sit and reach (cm) 1st follow-up | 3.8 ± 3.9 (n = 19) | 4.3 ± 4.1 (n = 12) | 3.1 ± 3.4 (n = 7) | 0.344 * |
| Standing long jump (cm) 1st follow-up | 57.5 ± 9.4 (n = 19) | 55.9 ± 9.4 (n = 12) | 60.1 ± 9.5 (n = 7) | 0.069 * |
| One leg stand (counts) 1st follow-up | 22.2 ± 7.6 (n = 13) | 25.6 ± 5.4 (n = 8) | 16.6 ± 7.7 (n = 5) | 0.024 * |
| Shuttle run test (seconds) 1st follow-up | 14.0 ± 2.3 (n = 19) | 14.2 ± 1.8 (n = 12) | 13.6 ± 3.1 (n = 7) | 0.456 * |
| Lateral jumping (counts) 2nd follow-up | 60.0 ± 10.4 (n = 19) | 62.3 ± 8.6 (n = 11) | 56.9 ± 12.3 (n = 8) | 0.436 * |
| Sit and reach (cm) 2nd follow-up | 1.3 ± 7.4 (n = 19) | 1.3 ± 8.1 (n = 11) | 1.3 ± 6.9 (n = 8) | 0.790 * |
| Standing long jump (cm) 2nd follow-up | 138.4 ± 16.2 (n = 19) | 138.2 ± 14.4 (n = 11) | 138.6 ± 19.5 (n = 8) | 0.164 * |
| Sit ups (counts) 2nd follow-up | 16.3 ± 5.9 (n = 19) | 17.8 ± 4.5 (n = 11) | 14.3 ± 7.3 (n = 8) | 0.834 * |
| One leg stand (counts) 2nd follow-up | 0.8 ± 1.4 (n = 19) | 0.5 ± 1.2 (n = 11) | 1.1 ± 1.6 (n = 8) | 0.387 # |
| Push-ups (counts) 2nd follow-up | 4.3 ± 4.0 (n = 19) | 4.1 ± 2.9 (n = 11) | 4.7 ± 5.4 (n = 8) | 0.735 # |
| 6-min run (meters) 2nd follow-up | 1142.8 ± 111.1 (n = 17) | 1165.2 ± 106.0 (n = 11) | 1101.8 ± 117.9 (n = 6) | 0.198 * |
| Model | β-Coefficient | p-Value | R2 | |
|---|---|---|---|---|
| 1 | Age of child [year] | −1.757 | 0.002 | 0.833 |
| Group | −1.546 | 0.004 | ||
| Gender | −0.470 | 0.049 | ||
| BMI-SDS of child | −0.088 | 0.578 | ||
| Lean body mass of child [%] | 0.155 | 0.377 | ||
| Maternal BDNF at 36 weeks of gestation [pg/mL] | 0.294 | 0.104 | ||
| 3 | Age of child [year] | −1.865 | ≤0.001 | 0.806 |
| Group | −1.689 | ≤0.001 | ||
| Gender | −0.412 | 0.052 | ||
| Maternal BDNF at 36 weeks of gestation [pg/mL] | 0.290 | 0.088 | ||
| Model | β-Coefficient | p-Value | R2 | |
|---|---|---|---|---|
| 1 | Age of child [year] | 0.031 | 0.970 | 0.246 |
| Group | −0.451 | 0.563 | ||
| Gender | 0.105 | 0.740 | ||
| BMI-SDS of child | −0.030 | 0.909 | ||
| Lean body mass of child [%] | −0.232 | 0.507 | ||
| Maternal BDNF at 36 weeks of gestation [pg/mL] | −0.207 | 0.548 | ||
| 6 | Group | −0.415 | 0.098 | 0.172 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, N.; Schmidt, N.; Bae-Gartz, I.; Vohlen, C.; Alcazar, M.A.A.; Brockmeier, K.; Dötsch, J.; Mahabir, E.; Joisten, C. Maternal Exercise during Pregnancy Impacts Motor Performance in 9-Year-Old Children: A Pilot Study. Children 2023, 10, 1797. https://doi.org/10.3390/children10111797
Ferrari N, Schmidt N, Bae-Gartz I, Vohlen C, Alcazar MAA, Brockmeier K, Dötsch J, Mahabir E, Joisten C. Maternal Exercise during Pregnancy Impacts Motor Performance in 9-Year-Old Children: A Pilot Study. Children. 2023; 10(11):1797. https://doi.org/10.3390/children10111797
Chicago/Turabian StyleFerrari, Nina, Nikola Schmidt, Inga Bae-Gartz, Christina Vohlen, Miguel A Alejandre Alcazar, Konrad Brockmeier, Jörg Dötsch, Esther Mahabir, and Christine Joisten. 2023. "Maternal Exercise during Pregnancy Impacts Motor Performance in 9-Year-Old Children: A Pilot Study" Children 10, no. 11: 1797. https://doi.org/10.3390/children10111797
APA StyleFerrari, N., Schmidt, N., Bae-Gartz, I., Vohlen, C., Alcazar, M. A. A., Brockmeier, K., Dötsch, J., Mahabir, E., & Joisten, C. (2023). Maternal Exercise during Pregnancy Impacts Motor Performance in 9-Year-Old Children: A Pilot Study. Children, 10(11), 1797. https://doi.org/10.3390/children10111797







