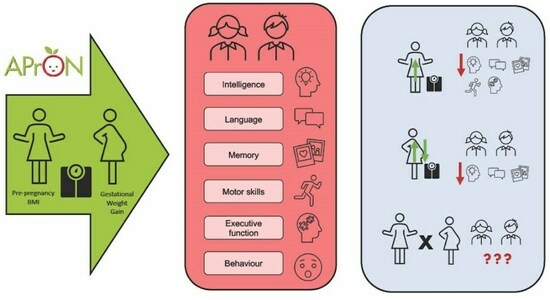
Maternal Pre-Pregnancy BMI and Gestational Weight Gain Are Associated with Preschool Children’s Neuropsychological Outcomes in the APrON Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Maternal Pre-Pregnancy Body Mass Index (BMI)
2.3. Maternal Gestational Weight Gain (GWG)
2.4. Child Neuropsychological Assessments
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Model 1: Relationship between Maternal Pre-Pregnancy BMI and Children’s Neurodevelopmental Outcomes
3.3. Model 2: Maternal GWG and Children’s Neurodevelopment
3.4. Model 3: Interactions between Maternal BMI and GWG on Children’s Neurodevelopment
3.5. Supplementary Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Statistics Canada. Overweight and Obese Adults; Statistics Canada: Ottawa, ON, Canada, 2018. [Google Scholar]
- Hales, C.; Carroll, M.; Fryer, C.; Ogden, C. Prevalence of Obesity and Severe Obesity among Adults: United States, 2017–2018; National Centre for Health Statistics: Hyattsvill, MD, USA, 2020. [Google Scholar]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-Analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, R.; Wen, S.W.; Tan, H.; Zhou, S.; Ye, C.; Shen, M.; Smith, G.N.; Walker, M.C. Association of Timing of Weight Gain in Pregnancy With Infant Birth Weight. JAMA Pediatr. 2018, 172, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal Pre-Pregnancy Obesity and Child Neurodevelopmental Outcomes: A Meta-Analysis. Obes. Rev. 2018, 19, 464–484. [Google Scholar] [CrossRef] [PubMed]
- Scher, M.S. “The First Thousand Days” Define a Fetal/Neonatal Neurology Program. Front. Pediatr. 2021, 9, 683138. [Google Scholar] [CrossRef]
- Van Lieshout, R.J.; Taylor, V.H.; Boyle, M.H. Pre-Pregnancy and Pregnancy Obesity and Neurodevelopmental Outcomes in Offspring: A Systematic Review. Obes. Rev. 2011, 12, e548–e559. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Tsang, V. Enduring Consequences of Maternal Obesity for Brain Inflammation and Behavior of Offspring. FASEB J. 2010, 24, 2104–2115. [Google Scholar] [CrossRef]
- Casas, M.; Chatzi, L.; Carsin, A.-E.; Amiano, P.; Guxens, M.; Kogevinas, M.; Koutra, K.; Lertxundi, N.; Murcia, M.; Rebagliato, M.; et al. Maternal Pre-Pregnancy Overweight and Obesity, and Child Neuropsychological Development: Two Southern European Birth Cohort Studies. Int. J. Epidemiol. 2013, 42, 506–517. [Google Scholar] [CrossRef]
- Adane, A.A.; Mishra, G.D.; Tooth, L.R. Maternal Pre-Pregnancy Obesity and Childhood Physical and Cognitive Development of Children: A Systematic Review. Int. J. Obes. 2016, 40, 1608–1618. [Google Scholar] [CrossRef]
- Girchenko, P.; Tuovinen, S.; Lahti-Pulkkinen, M.; Lahti, J.; Savolainen, K.; Heinonen, K.; Pyhälä, R.; Reynolds, R.M.; Hämäläinen, E.; Villa, P.M.; et al. Maternal Early Pregnancy Obesity and Related Pregnancy and Pre-Pregnancy Disorders: Associations with Child Developmental Milestones in the Prospective PREDO Study. Int. J. Obes. 2018, 42, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Zubrick, S.R.; Pennell, C.E.; Lieshout, R.J.V.; Jacoby, P.; Beilin, L.J.; Mori, T.A.; Stanley, F.J.; Newnham, J.P.; Oddy, W.H. Pre-Pregnancy Maternal Overweight and Obesity Increase the Risk for Affective Disorders in Offspring. J. Dev. Orig. Health Dis. 2013, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Van Lieshout, R.J.; Schmidt, L.A.; Robinson, M.; Niccols, A.; Boyle, M.H. Maternal Pre-Pregnancy Body Mass Index and Offspring Temperament and Behavior at 1 and 2 Years of Age. Child Psychiatry Hum. Dev. 2013, 44, 382–390. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Keim, S.; Li, L.; Zhang, L.; Zhang, J. Maternal Prepregnancy Obesity and Child Neurodevelopment in the Collaborative Perinatal Project. Int. J. Epidemiol. 2014, 43, 783–792. [Google Scholar] [CrossRef]
- Neggers, Y.H.; Goldenberg, R.L.; Ramey, S.L.; Cliver, S.P. Maternal Prepregnancy Body Mass Index and Psychomotor Development in Children. Acta Obstet. Gynecol. Scand. 2003, 82, 235–240. [Google Scholar] [CrossRef]
- Brion, M.-J.; Zeegers, M.; Jaddoe, V.; Verhulst, F.; Tiemeier, H.; Lawlor, D.A.; Smith, G.D. Intrauterine Effects of Maternal Prepregnancy Overweight on Child Cognition and Behavior in 2 Cohorts. Pediatrics 2011, 127, e202–e211. [Google Scholar] [CrossRef]
- Pugh, S.J.; Richardson, G.A.; Hutcheon, J.A.; Himes, K.P.; Brooks, M.M.; Day, N.L.; Bodnar, L.M. Maternal Obesity and Excessive Gestational Weight Gain Are Associated with Components of Child Cognition. J. Nutr. 2015, 145, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hortelano, J.A.; Álvarez-Bueno, C.; Cavero-Redondo, I.; Herráiz-Adillo, Á.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Gestational Weight Gain and Offspring’s Cognitive Skills: A Systematic Review and Meta-Analysis. BMC Pediatr. 2020, 20, 533. [Google Scholar] [CrossRef] [PubMed]
- Kouba, I.; Del Pozzo, J.; Lesser, M.L.; Shahani, D.; Gulersen, M.; Bracero, L.A.; Blitz, M.J. Socioeconomic and Clinical Factors Associated with Excessive Gestational Weight Gain. Arch Gynecol. Obstet. 2023. [Google Scholar] [CrossRef]
- Ng, S.-K.; Cameron, C.M.; Hills, A.P.; McClure, R.J.; Scuffham, P.A. Socioeconomic Disparities in Prepregnancy BMI and Impact on Maternal and Neonatal Outcomes and Postpartum Weight Retention: The EFHL Longitudinal Birth Cohort Study. BMC Pregnancy Childbirth 2014, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Heerman, W.J.; Bian, A.; Shintani, A.; Barkin, S.L. Interaction between Maternal Prepregnancy Body Mass Index and Gestational Weight Gain Shapes Infant Growth. Acad. Pediatr. 2014, 14, 463–470. [Google Scholar] [CrossRef]
- Zhang, M.; Gazimbi, M.M.; Chen, Z.; Zhang, B.; Chen, Y.; Yu, Y.; Tang, J. Association between Birth Weight and Neurodevelopment at Age 1–6 Months: Results from the Wuhan Healthy Baby Cohort. BMJ Open 2020, 10, e031916. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Giesbrecht, G.F.; Leung, B.M.Y.; Field, C.J.; Dewey, D.; Bell, R.C.; Manca, D.P.; O’Beirne, M.; Johnston, D.W.; Pop, V.J.; et al. The Alberta Pregnancy Outcomes and Nutrition (APrON) Cohort Study: Rationale and Methods. Matern. Child Nutr. 2014, 10, 44–60. [Google Scholar] [CrossRef]
- Shin, D.; Chung, H.; Weatherspoon, L.; Song, W.O. Validity of Prepregnancy Weight Status Estimated from Self-Reported Height and Weight. Matern. Child Health J. 2014, 18, 1667–1674. [Google Scholar] [CrossRef]
- World Health Organization. International Statistical Classification of Diseases and World Health Problems; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Health Canada. Prenatal Nutrition Guidelines for Health Professionals; Health Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, 4th ed.; Pearson: Toronto, ON, Canada, 2012. [Google Scholar]
- Syeda, M.M.; Climie, E.A. Test Review: Wechsler Preschool and Primary Scale of Intelligence–Fourth Edition. J. Psychoeduc. Assess. 2014, 32, 265–272. [Google Scholar] [CrossRef]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY®—Second Edition (NEPSY®—II); PsychCorp: San Antonio, TX, USA, 2007. [Google Scholar]
- Brooks, B.; Sherman, E.; Strauss, E. NEPSY-II: A Developmental Neuropsychological Assessment, Second Edition. Child Neuropsychol. 2010, 16, 80–101. [Google Scholar] [CrossRef]
- Henderson, S.E.; Sugden, D.; Barnett, A.L. Movement Assessment Battery for Children, 2nd ed.; Pearson: London, UK, 2007. [Google Scholar]
- Gerstadt, C.L.; Hong, Y.J.; Diamond, A. The Relationship between Cognition and Action: Performance of Children 3 1/2-7 Years Old on a Stroop-like Day-Night Test. Cognition 1994, 53, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.M. Developmentally Sensitive Measures of Executive Function in Preschool Children. Dev. Neuropsychol. 2005, 28, 595–616. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.I.; Müller, U.; Carpendale, J.I.M.; Bibok, M.B.; Liebermann-Finestone, D.P. The Effects of Parental Scaffolding on Preschoolers’ Executive Function. Dev. Psychol. 2012, 48, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cragg, L.; Nation, K. Self-Ordered Pointing as a Test of Working Memory in Typically Developing Children. Memory 2007, 15, 526–535. [Google Scholar] [CrossRef]
- Gioia, G.A.; Andrews, K.; Isquith, P.K. Behavior Rating Inventory of Executive Function—Preschool Version (BRIEF-P); PAR Inc.: Lutz, FL, USA, 2003. [Google Scholar]
- Reynold, C.R.; Kamphaus, R.W. Behavior Assessment System for Children—Second Edition (BASC—2); American Guidance Service: Circle Pines, MN, USA, 2004. [Google Scholar]
- Lourenço, V.M.; Pires, A.M.; Kirst, M. Robust Linear Regression Methods in Association Studies. Bioinformatics 2011, 27, 815–821. [Google Scholar] [CrossRef]
- Maechler, M. Sfsmisc: Utilities from “Seminar Fuer Statistik” ETH Zurich 2022. Available online: https://cran.r-project.org/web/packages/sfsmisc/sfsmisc.pdf (accessed on 26 April 2022).
- Lenth, R.V.; Beurkner, P.; Herve, M.; Love, J.; Miguez, F.; Riebl, H.; Sigmann, H. Emmeans: Estimated Marginal Means, Aka Least-Squares Means 2022. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 26 April 2022).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Flouri, E.; Papachristou, E.; Midouhas, E.; Ploubidis, G.B.; Lewis, G.; Joshi, H. Developmental Cascades of Internalising Symptoms, Externalising Problems and Cognitive Ability from Early Childhood to Middle Adolescence. Eur. Psychiatry 2019, 57, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Weeks, M.; Wild, T.C.; Ploubidis, G.B.; Naicker, K.; Cairney, J.; North, C.R.; Colman, I. Childhood Cognitive Ability and Its Relationship with Anxiety and Depression in Adolescence. J. Affect. Disord. 2014, 152–154, 139–145. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The Role of Systemic Inflammation Linking Maternal BMI to Neurodevelopment in Children. Pediatr. Res. 2016, 79, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Shook, L.L.; Kislal, S.; Edlow, A.G. Fetal Brain and Placental Programming in Maternal Obesity: A Review of Human and Animal Model Studies. Prenat. Diagn. 2020, 40, 1126–1137. [Google Scholar] [CrossRef]
- Gaillard, R.; Santos, S.; Duijts, L.; Felix, J.F. Childhood Health Consequences of Maternal Obesity during Pregnancy: A Narrative Review. Ann. Nutr. Metab. 2016, 69, 171–180. [Google Scholar] [CrossRef] [PubMed]
- West, Z.; Demchenko, I.; Clark, L.; White, M.; MacFarlane, A.J.; Fraser, W.D.; Arbuckle, T.E.; Connor, K.L.; MIREC Study Group. Relationships between Maternal Body Mass Index and Child Cognitive Outcomes at 3 Years of Age Are Buffered by Specific Early Environments in a Prospective Canadian Birth Cohort. J. Dev. Orig. Health Dis. 2023, 14, 42–52. [Google Scholar] [CrossRef]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 42–49. [Google Scholar] [CrossRef]
- Yu, S.M.; Nagey, D.A. Validity of Self-Reported Pregravid Weight. Ann. Epidemiol. 1992, 2, 715–721. [Google Scholar] [CrossRef]
- Eriksen, H.-L.F.; Kesmodel, U.S.; Underbjerg, M.; Kilburn, T.R.; Bertrand, J.; Mortensen, E.L. Predictors of Intelligence at the Age of 5: Family, Pregnancy and Birth Characteristics, Postnatal Influences, and Postnatal Growth. PLoS ONE 2013, 8, e79200. [Google Scholar] [CrossRef]
| Characteristics | N (%) |
|---|---|
| Maternal Age in years, M (SD) | 32.34 (3.76) |
| Pre-pregnancy BMI, M (SD) | 24.58 (5.06) |
| Underweight a | 11 (2.90) |
| Normal weight a | 237 (62.54) |
| Overweight a | 84 (22.16) |
| Obese a | 47 (12.40) |
| Birthplace | |
| Canadian Born | 315 (83.11) |
| Not Canadian Born | 57 (16.89) |
| Education | |
| High school diploma/trade school | 86 (22.69) |
| Undergraduate university degree or higher | 293 (77.31) |
| Annual household income | |
| <$70,000 CAD | 57 (15.04) |
| ≥$70,000 CAD | 322 (84.96) |
| Parity | |
| Primiparous | 201 (53.03) |
| Multiparous | 178 (46.97) |
| Delivery mode | |
| Vaginal | 293 (77.31) |
| Caesarean section | 86 (22.69) |
| GWG Class b | |
| Below | 69 (18.21) |
| Within | 126 (33.24) |
| Above | 186 (49.08) |
| Alcohol consumption during pregnancy | 7 (1.8%) |
| Smoking during pregnancy | 6 (1.6%) |
| Recreational drug use during pregnancy | 1 (0.03%) |
| Child | |
| Female | 183 (48.28) |
| Birth weight in grams, M (SD) | 3365.25 (529.90) |
| Age in years at Assessment, M (SD) | 4.27 (0.50) |
| WPPSI-IVCND FSIQ a B (95% CI) | WPPSI-IVCND VCI a B (95% CI) | WPPSI-IVCND VSI a B (95% CI) | WPPSI-IVCND WMI a B (95% CI) | NEPSY-II Phonological Processing b B (95% CI) | NEPSY-II Speeded Naming b B (95% CI) | |
|---|---|---|---|---|---|---|
| Model 1 c | ||||||
| Maternal BMI | −0.48 **‡ (−0.75, −0.21) | −0.47 **‡ (−0.75, −0.18) | −0.35 *‡ (−0.67, −0.02) | −0.36 *‡ (−0.67, −0.05) | −0.09 **‡ (−0.16, −0.03) | −0.03 (−0.09, 0.04) |
| Model 2 d | ||||||
| GWG Below | −4.04 * (−7.89, −0.11) | −1.46 (−5.58, 2.65) | −2.61 (−7.18, 1.97) | −4.27 (−8.68, 0.14) | −0.70 (−1.65, 0.24) | −0.54 (−1.42, 0.34) |
| GWG Met | Reference | Reference | Reference | Reference | Reference | Reference |
| GWG Above | −2.67 (−5.71, 0.38) | −1.67 (−4.85, 1.51) | −0.90 (−4.44, 2.64) | −1.78 (−5.18, 1.63) | −0.79 * (−1.52, −0.06) | 0.02 (−0.67, 0.70) |
| Model 3 e | ||||||
| Maternal BMI | −0.78 ** (−1.30, −0.26) | −0.26 (−0.81, 0.29) | −0.93 ** (−01.55, −0.31) | −0.92 ** (−1.50, −0.33) | −0.12 (−0.25, 0.004) | −0.10 (−0.22, 0.02) |
| GWG Below | −9.69 (−28.1, 8.68) | 9.44 (−9.84, 28.70) | −17.90 (−39.70, 3.89) | −18.9 (−39.50, 1.73) | −1.32 (−5.80, 3.16) | −3.41 (−7.52, 0.70) |
| GWG Met | Reference | Reference | Reference | Reference | Reference | Reference |
| GWG Above | −15.7 (−31.4, 0.07) | 2.52 (−14.00, 19.00) | −20.80 * (−39.50, −2.13) | −20.3 * (−37.90, −2.61) | −2.35 (−6.19, 1.49) | −1.86 (−5.38, 1.66) |
| Maternal BMI × GWG Below | 0.25 (−0.50, 1.01) | −0.45 (−1.24, 0.35) | 0.67 (−0.23, 1.57) | 0.64 (−0.21, 1.49) | 0.03 (−0.16, 0.21) | 0.13 (−0.05, 0.29) |
| Maternal BMI × GWG Met | Reference | Reference | Reference | Reference | Reference | Reference |
| Maternal BMI × GWG Above | 0.56 (−0.08, 1.20) | −0.15 (−0.82, 0.52) | 0.86 * (0.10, 1.62) | 0.80 * (0.09, 1.52) | 0.07 (−0.09, 0.23) | 0.08 (−0.06, 0.23) |
| NEPSY-II Memory for Design a B (95% CI) | NEPSY-II Narrative Memory a B (95% CI) | NEPSY-II Sentence Repetition a B (95% CI) | MABC-2 Total a B (95% CI) | MABC-2 Manual Dexterity a B (95% CI) | MABC-2 Aiming and Catching a B (95% CI) | MABC-2 Balance a B (95% CI) | BASC- 2 Externalizing Problems b B (95% CI) | BASC-2 Internalizing Problems b B (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 c | |||||||||
| Maternal BMI | −0.05 (−0.10, 0.005) | −0.06 (−0.12, 0.01) | −0.08 **‡ (−0.15, −0.02) | −0.08 *‡ (−0.14, −0.01) | −0.11 **‡ (−0.18, −0.05) | −0.03 (−0.10, 0.04) | −0.03 (−0.08, 0.03) | 0.15 ‡ (−0.02, 0.31) | 0.02 (−0.17, 0.20) |
| Model 2 d | |||||||||
| GWG Below | −0.31 (−1.09, 0.47) | −0.66 (−1.58, 0.26) | −0.75 (−1.65, 0.16) | −0.52 (−1.39, 0.36) | −0.52 (−1.49, 0.46) | 0.27 (−0.70, 1.23) | −0.45 (−1.23, 0.34) | 0.13 (−2.22, 2.48) | −2.42 (−5.07, 0.22) |
| GWG Met | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| GWG Above | −0.13 (−0.73, 0.47) | −0.93 ** (−1.64, −0.22) | −0.36 (−1.06, 0.34) | −0.35 (−1.03, 0.32) | −0.38 (−1.13, 0.37) | 0.20 (−0.54, 0.95) | −0.24 (−0.84, 0.37) | 0.82 (−1.00, 2.64) | −0.10 (−2.15, 1.95) |
| Model 3 e | |||||||||
| Maternal BMI | −0.06 (−0.16, 0.05) | −0.03 (−0.15, 0.10) | −0.06 (−0.19, 0.06) | −0.11 (−0.23, 0.004) | −0.12 (−0.25, 0.01) | −0.09 (−0.22, 0.04) | −0.08 (−0.18, 0.03) | 0.11 (−0.20, 0.43) | 0.09 (−0.27, 0.44) |
| GWG Below | −0.22 (−3.90, 3.47) | 1.22 (−3.14, 5.59) | 0.91 (−3.39, 5.21) | −0.13 (−4.26, 4.01) | 0.06 (−4.52, 4.65) | −0.41 (−4.93, 4.12) | −0.66 (−4.42, 3.11) | −8.27 (−19.30, 2.74) | −3.86 (−16.40, 8.70) |
| GWG Met | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| GWG Above | −0.51 (−3.67, 2.65) | −1.15 (−4.89, 2.59) | −0.10 (−3.79, 3.58) | −2.42 (−5.96, 1.12) | −0.96 (−4.88, 2.97) | −2.74 (−6.61, 1.14) | −2.54 (−5.77, 0.69) | 2.79 (−6.64, 12.20) | 4.60 (−6.15, 15.40) |
| Maternal BMI × GWG Below | −0.001 (−0.15, 0.15) | −0.08 (−0.26, 0.10) | −0.07 (−0.25, 0.11) | −0.01 (−0.18, 0.16) | −0.02 (−0.21, 0.17) | 0.03 (−0.15, 0.22) | 0.01 (−0.15, 0.17) | 0.36 (−0.09, 0.81) | 0.06 (−0.46, 0.58) |
| Maternal BMI × GWG Met | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Maternal BMI × GWG Above | 0.02 (−0.11, 0.15) | 0.01 (−0.14, 0.16) | −0.01 (−0.16, 0.14) | 0.09 (−0.06, 0.23) | 0.03 (−0.13, 0.19) | 0.12 (−0.03, 0.28) | 0.09 (−0.04, 0.23) | −0.08 (−0.47, 0.30) | −0.19 (−0.63, 0.25) |
| Boy/Girl Stroop a B (95% CI) | Less Is More a B (95% CI) | NEPSY-II Statue b B (95% CI) | SOPT c B (95% CI) | Spatial Span d B (95% CI) | BRIEF-P GEC e B (95% CI) | BRIEF-P ISC e B (95% CI) | BRIEF-P FLEX e B (95% CI) | BRIEF-P EMC e B (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Model 1 f | |||||||||
| Maternal BMI | −0.16 **‡ (−0.26, −0.07) | −0.09 *‡ (−0.17, −0.01) | −0.06 (−0.12, 0.01) | −0.01 (−0.04, 0.03) | −0.004 (−0.02, 0.01) | 0.37 **‡ (0.16, 0.58) | 0.24 *‡ (0.05, 0.43) | 0.12 (−0.05, 0.29) | 0.35 **‡ (0.23, 0.68) |
| Model 2 g | |||||||||
| GWG Below | −0.80 (−2.14, −0.55) | −0.31 (−6.83, 4.51) | −0.17 (−1.15, 0.81) | −0.31 (−0.81, 0.20 | 0.27 * (0.02, 0.52) | 0.87 (−2.15, 3.90) | 0.50 (−2.18, 3.19) | 0.66 (−1.79, 3.10) | 0.88 (−2.36, 4.12) |
| GWG Met | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| GWG Above | −0.38 (−1.42, −0.66) | 0.18 (−0.72, 1.08) | −0.05 (−0.70, 0.81) | −0.06 (−0.44, 0.33) | −0.02 (−0.22, 0.17) | 1.51 (−0.83, 3.85) | 1.78 ‡ (−0.30, 3.86) | 0.16 (−1.73, 2.04) | 1.57 (−0.94, 4.08) |
| Model 3 h | |||||||||
| Maternal BMI | −0.19 * (−0.37, −0.02) | −0.01 (−0.19, 0.16) | −0.15 * (−0.29, −0.02) | 0.03 (−0.04, 0.09) | 0.002 (−0.03, 0.04) | 0.31 (−0.10, 0.72) | 0.14 (−0.22, 0.50) | 0.16 (−0.17, 0.49) | 0.36 (−0.08, 0.80) |
| GWG Below | −0.95 (−7.14, 5.25) | 7.01 * (1.06, 13.00) | −0.75 (−5.43, 3.93) | −0.08 (−2.45, 2.29) | 1.11 ‡ (−0.13, 2.34) | −9.85 (−24.30, 4.58) | −12.90 * (−25.50, −3.70) | −7.01 (−18.60, 4.62) | −5.96 (−21.30, 9.39) |
| GWG Met | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| GWG Above | −2.34 (−7.65, 2.96) | −0.39 (−5.49, 4.71) | −3.78 (7.79, 0.23) | 1.34 (−0.69, 3.37) | −0.33 (−1.39, 0.72) | 3.30 (−9.07,15.70) | 3.19 (−7.56, 13.9) | 4.28 (−5.68, 14.2) | 0.24 (−12.90,13.4) |
| Maternal BMI × GWG Below | 0.03 (−0.23, 0.28) | −0.30 * (−0.54, −0.05) | 0.03 (−0.17, 0.22) | −0.01 (−0.11, 0.09) | −0.04 (−0.08, 0.02) | 0.46 (−0.14, 1.05) | 0.58 * (0.06, 1.09) | 0.33 (−0.15, 0.81) | 0.28 (−0.36, 0.91) |
| Maternal BMI × GWG Met | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Maternal BMI × GWG Above | 0.09 (−0.12, 0.31) | 0.02 (−0.19, 0.23) | 0.16 * (0.001, 0.33) | −0.06 (−0.14, 0.03) | 0.01 (−0.03, 0.05) | −0.09 (−0.60, 0.41) | −0.06 (−0.50, 0.37) | −0.17 (−0.58, 0.23) | 0.02 (−0.51, 0.56) |
| Interaction | B (SE) | 95% CI |
|---|---|---|
| Maternal BMI × GWG Class for WPPSI-IV VSI | ||
| GWG Below | −0.26 (0.34) | −0.92, 0.40 |
| GWG Met | −0.93 * (0.32) | −1.55, −0.31 |
| GWG Above | −0.07 (0.23) | −0.52, 0.39 |
| Maternal BMI × GWG Class for WPPSI-IV WMI | ||
| GWG Below | −0.28 (0.32) | −0.90, 0.35 |
| GWG Met | −0.92 * (0.30) | −1.50, −0.33 |
| GWG Above | −0.11 (0.22) | −0.54, 0.32 |
| Maternal BMI × GWG Class for NEPSY-II Statue subtest | ||
| GWG Below | −0.13 (0.07) | −0.27, 0.02 |
| GWG Met | −0.15 * (0.07) | −0.29, −0.02 |
| GWG Above | 0.01 (0.05) | −0.09, 0.11 |
| Maternal BMI × GWG Class for Less is More task | ||
| GWG Below | −0.31 * (0.09) | −0.49, −0.13 |
| GWG Met | −0.01 (0.09) | −0.19, 0.16 |
| GWG Above | 0.003 (0.06) | −0.12, 0.13 |
| Maternal BMI × GWG Class for BRIEF-P ISC index | ||
| GWG Below | 0.72 * (0.19) | 0.34, 1.01 |
| GWG Met | 0.14 (0.18) | −0.22, 0.50 |
| GWG Above | 0.08 (0.13) | −0.19, 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
England-Mason, G.; Anderson, A.; Bell, R.C.; Subhan, F.B.; Field, C.J.; Letourneau, N.; Giesbrecht, G.F.; Dewey, D.; The APrON Study Team. Maternal Pre-Pregnancy BMI and Gestational Weight Gain Are Associated with Preschool Children’s Neuropsychological Outcomes in the APrON Cohort. Children 2023, 10, 1849. https://doi.org/10.3390/children10121849
England-Mason G, Anderson A, Bell RC, Subhan FB, Field CJ, Letourneau N, Giesbrecht GF, Dewey D, The APrON Study Team. Maternal Pre-Pregnancy BMI and Gestational Weight Gain Are Associated with Preschool Children’s Neuropsychological Outcomes in the APrON Cohort. Children. 2023; 10(12):1849. https://doi.org/10.3390/children10121849
Chicago/Turabian StyleEngland-Mason, Gillian, Alida Anderson, Rhonda C. Bell, Fatheema B. Subhan, Catherine J. Field, Nicole Letourneau, Gerald F. Giesbrecht, Deborah Dewey, and The APrON Study Team. 2023. "Maternal Pre-Pregnancy BMI and Gestational Weight Gain Are Associated with Preschool Children’s Neuropsychological Outcomes in the APrON Cohort" Children 10, no. 12: 1849. https://doi.org/10.3390/children10121849