Monitoring of Auditory Function in Newborns of Women Infected by SARS-CoV-2 during Pregnancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
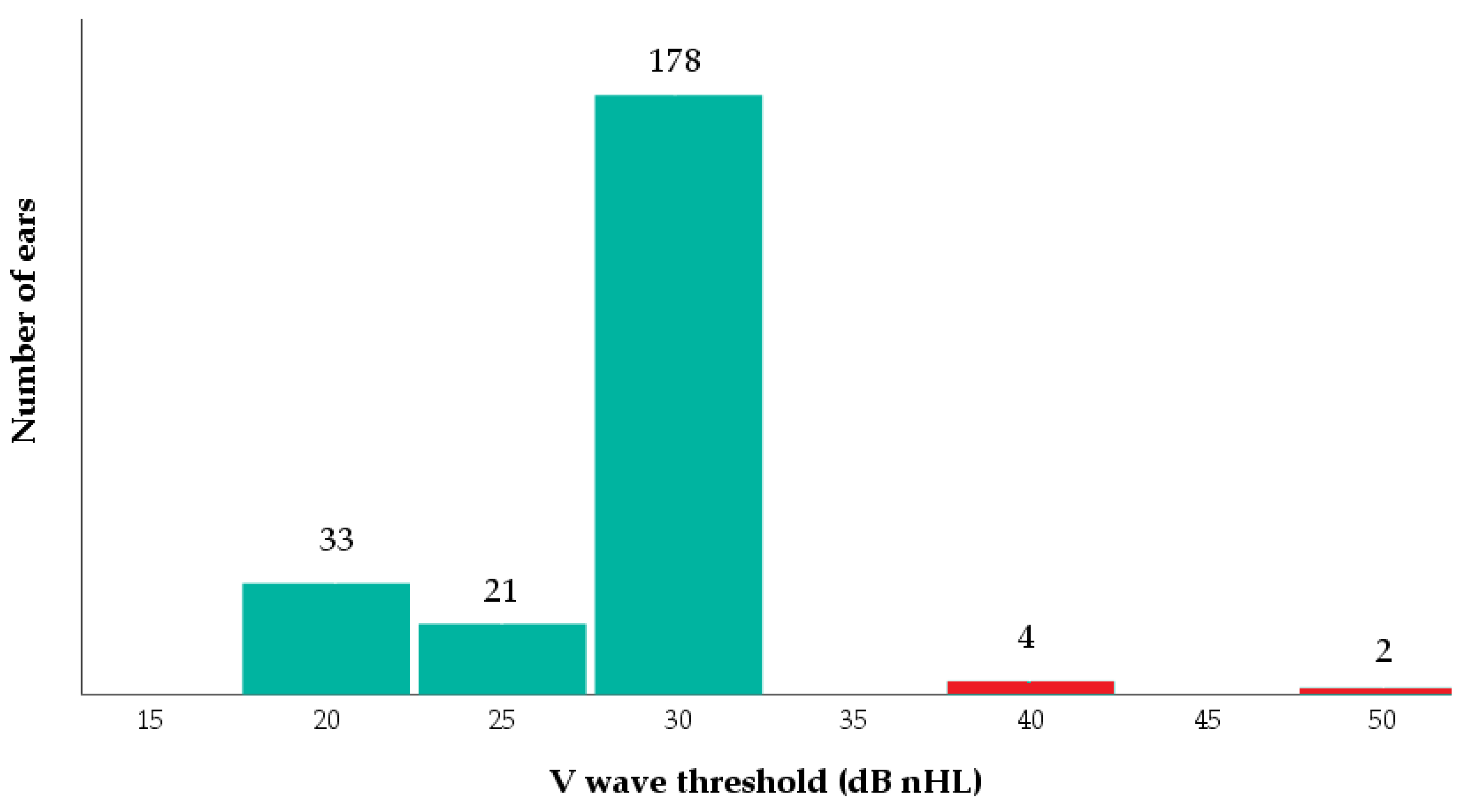
3. Results
3.1. Participants
3.2. Newborn Hearing Function
3.3. Follow-Up at 1 Year
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Rahman, S.A.; Ehtesham, N.Z.; Hira, S.; Hasnain, S.E. SARS-CoV-2 variants of concern are emerging in India. Nat. Med. 2021, 27, 1131–1133. [Google Scholar] [CrossRef]
- Li, M.; Chen, L.; Zhang, J.; Xiong, C.; Li, X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE 2020, 15, e0230295. [Google Scholar] [CrossRef]
- Wastnedge, E.A.N.; Reynolds, R.M.; Van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS–CoV-2 infection of the placenta. J. Clin. Investig. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 1–34. [Google Scholar] [CrossRef]
- Tolu, L.B.; Ezeh, A.; Feyissa, G.T. Vertical transmission of Severe Acute Respiratory Syndrome Coronavirus 2: A scoping review. PLoS ONE 2021, 16, e0250196. [Google Scholar] [CrossRef]
- Meppiel, E.; Peiffer-Smadja, N.; Maury, A.; Bekri, I.; Delorme, C.; Desestret, V.; Gorza, L.; Hautecloque-Raysz, G.; Landre, S.; Lannuzel, A.; et al. Neurologic manifestations associated with COVID-19: A multicentre registry. Clin. Microbiol. Infect. 2021, 27, 458–466. [Google Scholar] [CrossRef]
- Maury, A.; Lyoubi, A.; Peiffer-Smadja, N.; de Broucker, T.; Meppiel, E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians. Rev. Neurol. 2021, 177, 51–64. [Google Scholar] [CrossRef]
- Celik, T.; Simsek, A.; Koca, C.F.; Aydin, S.; Yasar, S. Evaluation of cochlear functions in infants exposed to SARS-CoV-2 intrauterine. Am. J. Otolaryngol. 2021, 42, 102982. [Google Scholar] [CrossRef]
- Ricciardiello, F.; Pisani, D.; Viola, P.; Cristiano, E.; Scarpa, A.; Giannone, A.; Longo, G.; Russo, G.; Bocchetti, M.; Coppola, C.; et al. Sudden Sensorineural Hearing Loss in Mild COVID-19: Case Series and Analysis of the Literature. Audiol. Res. 2021, 11, 313–326. [Google Scholar] [CrossRef]
- Korkmaz, M.O.; Eğilmez, O.K.; Özçelik, M.A.; Güven, M. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Eur. Arch. Oto-Rhino-Laryngology 2021, 278, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Hearing Loss, Tinnitus, and Dizziness in COVID-19: A Systematic Review and Meta-Analysis. Can. J. Neurol. Sci. 2022, 49, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Muldoon, K.M.; Fowlerb, K.B.; Peschc, M.H.; Schleissd, M.R. SARS-CoV-2: Is it the newest spark in the TORCH? J. Clin. Virol. 2020, 127, 104372. [Google Scholar] [CrossRef] [PubMed]
- Uranaka, T.; Kashio, A.; Ueha, R.; Sato, T.; Bing, H.; Ying, G.; Kinoshita, M.; Kondo, K.; Yamasoba, T. Expression of ACE2, TMPRSS2, and furin in mouse ear tissue, and the implications for SARS-CoV-2 infection. Laryngoscope 2021, 131, E2013–E2017. [Google Scholar] [CrossRef]
- Mostafa, B.E.; Mostafa, A.; El Fiky, L.M.; Omara, A.; Teaima, A. Maternal COVID-19 and neonatal hearing loss: A multicentric survey. Eur. Arch. Oto-Rhino--Laryngol. 2021, 279, 3435–3438. [Google Scholar] [CrossRef]
- Buonsenso, D.; Costa, S.; Giordano, L.; Priolo, F.; Colonna, A.T.; Morini, S.; Sbarbati, M.; Pata, D.; Acampora, A.; Conti, G.; et al. Short- and mid-term multidisciplinary outcomes of newborns exposed to SARS-CoV-2 in utero or during the perinatal period: Preliminary findings. Eur. J. Pediatr. 2022, 181, 1507–1520. [Google Scholar] [CrossRef]
- Ghiselli, S.; Laborai, A.; Biasucci, G.; Carvelli, M.; Salsi, D.; Cuda, D. Auditory evaluation of infants born to COVID19 positive mothers. Am. J. Otolaryngol. 2022, 43, 103379. [Google Scholar] [CrossRef]
- Alan, M.A.; Alan, C. Hearing screening outcomes in neonates of SARS-CoV-2 positive pregnant women. Int. J. Pediatr. Otorhinolaryngol. 2021, 146, 110754. [Google Scholar] [CrossRef]
- Oskovi-Kaplan, Z.A.; Ozgu-Erdinc, A.S.; Buyuk, G.N.; Sert-Dinc, U.Y.; Ali-Algan, C.; Demir, B.; Sahin, D.; Keskin, H.L.; Tayman, C.; Moraloglu-Tekin, Ö. Newborn Hearing Screening Results of Infants Born to Mothers Who Had COVID-19 Disease During Pregnancy: A Retrospective Cohort Study. Ear Hear. 2022, 43, 41–44. [Google Scholar] [CrossRef]
- Yıldız, G.; Kurt, D.; Mat, E.; Yıldız, P.; Başol, G.; Gündogdu, E.C.; Kuru, B.; Topcu, B.; Kale, A. Hearing test results of newborns born from the coronavirus disease 2019 (COVID-19) infected mothers: A tertiary center experience in Turkey. J. Obstet. Gynaecol. Res. 2022, 48, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Emilia-Romagna, R. Screening Uditivo Neonatale e Percorso Clinico Ed Organizzativo per i Bambini Affetti Da Ipoacusia in Emi-lia-Romagna—Approvazione Linee Guida per le Aziende Sanitarie. Deliberazione della Giunta Regionale 23 Maggio 2011, n. 694. Bologna. Available online: https://bur.regione.emilia-romagna.it (accessed on 23 November 2022).
- Joint Committee on Infant Hearing. Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs. J. Early Hear. Detect. Interv. JEHDI 2021, 4, 1–44. [Google Scholar] [CrossRef]
- Jerger, J. Clinical Experience with Impedance Audiometry. Arch. Otolaryngol. 1970, 92, 311–324. [Google Scholar] [CrossRef] [PubMed]
- WHO. Classification of Hearing Impairment. Available online: http://www.who.int/pbd/deafness/hearing_impairment_grades/,2013 (accessed on 1 December 2022).
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where do we Stand? Arch. Med Res. 2021, 53, 1–8. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Magaziotou, I.; Dedoukou, X.; Eleftheriou, E.; Raftopoulos, V.; Michos, A.; Lourida, A.; Panopoulou, M.; Stamoulis, K.; Papaevangelou, V.; et al. Children and Adolescents With SARS-CoV-2 Infection. Pediatr. Infect. Dis. J. 2020, 39, e388–e392. [Google Scholar] [CrossRef] [PubMed]
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.; Thorburn, F.; von Wissmann, B.; Reynolds, A.; McMenamin, J.; Murcia, P.R. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 2020, 323, 2427. [Google Scholar] [CrossRef] [PubMed]
- Munian, D.; Das, R.; Hazra, A.; Ray, S. Outcome of Neonates Born to COVID-Positive Women at 6 Months of Age. Indian Pediatr. 2021, 58, 853–856. [Google Scholar] [CrossRef]
- Meng, X.; Zhu, K.; Wang, J.; Liu, P. Can SARS-CoV-2 positive pregnant women affect the hearing of their newborns: A systematic review. Am. J. Otolaryngol. 2022, 43, 103523. [Google Scholar] [CrossRef]
- Hobbs, C.V.; Drobeniuc, J.; Kittle, T.; Williams, J.; Byers, P.; Satheshkumar, P.S.; Inagaki, K.; Stephenson, M.; Kim, S.S.; Patel, M.M.; et al. Estimated SARS-CoV-2 Seroprevalence Among Persons Aged <18 Years—Mississippi, May–September 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 312–315. [Google Scholar] [CrossRef]
- Waterfield, T.; Watson, C.; Moore, R.; Ferris, K.; Tonry, C.; Watt, A.; McGinn, C.; Foster, S.; Evans, J.; Christie, S. Seroprevalence of SARS-CoV-2 antibodies in children: A prospective multicentre cohort study. Arch. Dis. Child. 2021, 106, 680–686. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bovo, R.; Trevisi, P.; Ghiselli, S.; Benatti, A.; Martini, A. Is very early hearing assessment always reliable in selecting patients for cochlear im-plants? A case series study. Int. J. Pediatr. Otorhinolaryngol. 2015, 79, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Du, T.; Hong, W.; Chen, L.; Que, H.; Lu, S.; Peng, X. Neurological complications and infection mechanism of SARS-CoV-2. Signal Transduct. Target. Ther. 2021, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gallus, R.; Melis, A.; De Luca, L.M.; Rizzo, D.; Palmas, S.; Degni, E.; Piras, A.; Bussu, F. The Impact of COVID-19 on Universal Newborn Hearing Screening. Ear Hear. 2022, 43, 1917–1919. [Google Scholar] [CrossRef] [PubMed]


| Sample Size (n = 119) | I Trimester (n = 7) 5.8% | II Trimester (n = 45) 37.8% | III Trimester (n = 67) 56.3% | Test Significance | Post-Hoc Analyses | |
|---|---|---|---|---|---|---|
| Female | 61 (51.3%) | 5 (71.4%) | 23 (51.1%) | 33 (49.3%) | 0.543 a | 0.968; 0.810; 1.000 c |
| Male | 58 (48.7%) | 2 (28.6%) | 22 (48.9%) | 34 (50.7%) | ||
| Pregnancy | ||||||
| twin | 3 (2.5%) | - | - | 3 (4.5%) | 0.309 a | 1.000; 1.000; 0.426 c |
| not physiological | 13 (10.9%) | 1 (14.3%) | 5 (11.1%) | 7 (10.4%) | 0.953 a | 1.000; 1.000; 1.000 c |
| complications | 14 (11.8%) | 1 (14.3%) | 1 (2.2%) | 12 (17.9%) | 0.040 a | 1.000; 1.000; 0.035 c |
| Age of the mother | 31.55 (±5.38) | 33.00 (±4.87) | 31.13 (±5.88) | 31.67 (±5.12) | 0.670 b | 1.000; 1.000; 1.000 c |
| Gestational age (weeks) | 38.77 (±2.25) | 40.29 (±1.11) | 39.09 (±1.73) | 38.40 (±2.55) | 0.052 b | 0.559; 0.103; 0.333 c |
| Very preterm (28 to 34 weeks) | 4 (3.4%) | - | 1 (2.2%) | 3 (4.5%) | ||
| Moderate to late preterm (35 to 37 weeks) | 10 (8.4%) | - | 2 (4.4%) | 8 (11.9%) | ||
| Weight at birth (grams) | 3255.6 (±582.6) | 3490.7 (±209.3) | 3278.8 (±422.5) | 3215.0 (±690.0) | 0.469 b | 1.000; 0.707; 1.000 c |
| Apgar at 1 min | 8.65 (±1.65) | 9.00 (±0.58) | 9.04 (±1.13) | 8.36 (±1.95) | 0.095 b | 1.000; 0.971; 0.110 c |
| Apgar at 5 min | 9.66 (±0.83) | 10 (±0.00) | 9.83 (±0.486) | 9.51 (±1.01) | 0.074 b | 1.000; 0.395; 0.143 c |
| Gestational age of first ABR | 50.06 (±7.75) | 50.00 (±4.00) | 48.04 (±4.18) | 51.42 (±9.47) | 0.077 b | 1.000; 1.000; 0.072 c |
| Maternal infection | ||||||
| Symptomatic | 87 (73.1%) | 6 (85.7%) | 33 (73.3%) | 48 (71.6%) | 0.731 a | 1.000; 1.000; 1.000 c |
| less than 7 days | 29 (33.3%) | 1 (14.3%) | 11 (33.3%) | 17 (35.4%) | 0.154 a | 0.167; 0.206; 1.000 c |
| 7–14 days | 30 (34.5%) | 1 (14.3%) | 12 (36.4%) | 17 (35.4%) | ||
| more than 14 days | 28 (32.2%) | 4 (66.7%) | 10 (30.3%) | 14 (29.2%) | ||
| Neonatal infection | 2 (1.7%) | - | - | 2 (3.0%) | 0.461 a | 1.000; 0.701; 1.000 c |
| Symptomatic | 1 (0.8%) | - | - | 1 (1.5%) | 0.157 a | - |
| Id | Birth | Within 3 Months | 1 Month after the Audiological Assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trimester of Maternal Infection | Gestational Age (weeks) | Weight at Birth (Grams) | OAEs Left Ear; Right Ear | ABR V Wave Threshold (dB nHL) Left; Right | OAEs Left; Right | Immittance Test 1 Left; Right | Otoscopy 2 Left; Right | ABR V Wave Threshold (dB nHL) Left; Right | Immittance Test 1 Left; Right | Otoscopy 2 Left; Right | Notes | |
| 1 | I | 41 | 3730 | Pass; pass | 40; 30 | Pass; pass | A; A | D; N | 30; 30 | A; A | N; N | - |
| 2 | II | 39 | 3530 | Pass; pass | 40; 40 | Pass; pass | A; A | N; N | 30; 30 | A; A | N; N | Episode of fever and symptoms of SARS-CoV-2 infection |
| 3 | III | 40 | 3770 | Pass; pass | 50; 30 | Pass; pass | - | N; N | 40; 30 | A; A | N; N | - |
| 4 | III | 38 | 3210 | Pass; pass | 40; 30 | Pass; pass | A; A | D; D | 40; 30 | A; A | D; D | Pharyngitis; Negative oro-nasal swab |
| 5 | III | 38 | 2880 | Pass; pass | 30; 50 | pass; pass | A; A | N; N | 30; 30 | A; A | N; N | - |
| Sample Size (n = 37) | I Trimester (n = 2) | II Trimester (n = 16) | III Trimester (n = 19) | Significance | |
|---|---|---|---|---|---|
| PTA (dB) | 26.39 (±3.53) | 26.25 (±5.30) | 27.19 (±3.15) | 25.72 (±3.80) | 0.227 b |
| Immittance test 1 | |||||
| Type A | 26 (70.3%) | 1 (50.0%) | 11 (68.8%) | 14 (73.7%) | 0.773 a |
| Type B | 5 (13.5%) | 0 (0.0%) | 2 (12.5%) | 3 (15.8%) | |
| Type C | 6 (16.2%) | 1 (50.0%) | 3 (18.7%) | 2 (10.5%) | |
| Acoustic reflexes | |||||
| present | 23 (62.1%) | 1 (50.0%) | 12 (75.0%) | 10 (52.6%) | 0.138 a |
| absent | 14 (38.9%) | 1 (50.0%) | 4 (25.0%) | 9 (47.4%) | |
| Otoscopy | |||||
| normal | 27 (73.0%) | 1 (50.0%) | 12 (75.0%) | 14 (73.7%) | 0.300 a |
| alterations 2 | 10 (27.0%) | 1 (50.0%) | 4 (25.0%) | 5 (26.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apa, E.; Presutti, M.T.; Rossi, C.; Roversi, M.F.; Neri, S.; Gargano, G.; Bianchin, G.; Polizzi, V.; Caragli, V.; Monzani, D.; et al. Monitoring of Auditory Function in Newborns of Women Infected by SARS-CoV-2 during Pregnancy. Children 2023, 10, 194. https://doi.org/10.3390/children10020194
Apa E, Presutti MT, Rossi C, Roversi MF, Neri S, Gargano G, Bianchin G, Polizzi V, Caragli V, Monzani D, et al. Monitoring of Auditory Function in Newborns of Women Infected by SARS-CoV-2 during Pregnancy. Children. 2023; 10(2):194. https://doi.org/10.3390/children10020194
Chicago/Turabian StyleApa, Enrico, Maria Teresa Presutti, Cecilia Rossi, Maria Federica Roversi, Salvatore Neri, Giancarlo Gargano, Giovanni Bianchin, Valeria Polizzi, Valeria Caragli, Daniele Monzani, and et al. 2023. "Monitoring of Auditory Function in Newborns of Women Infected by SARS-CoV-2 during Pregnancy" Children 10, no. 2: 194. https://doi.org/10.3390/children10020194
APA StyleApa, E., Presutti, M. T., Rossi, C., Roversi, M. F., Neri, S., Gargano, G., Bianchin, G., Polizzi, V., Caragli, V., Monzani, D., Berardi, A., Palma, S., & Genovese, E. (2023). Monitoring of Auditory Function in Newborns of Women Infected by SARS-CoV-2 during Pregnancy. Children, 10(2), 194. https://doi.org/10.3390/children10020194





