Long-Term Outcome Following Liver Transplantation for Primary Hepatic Tumors—A Single Centre Observational Study over 40 Years
Abstract
:1. Introduction
2. Patients and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Data Acquisition and Statistical Analysis
2.3. Definitions
2.4. Ethical Considerations
3. Results
3.1. Baseline Characteristics
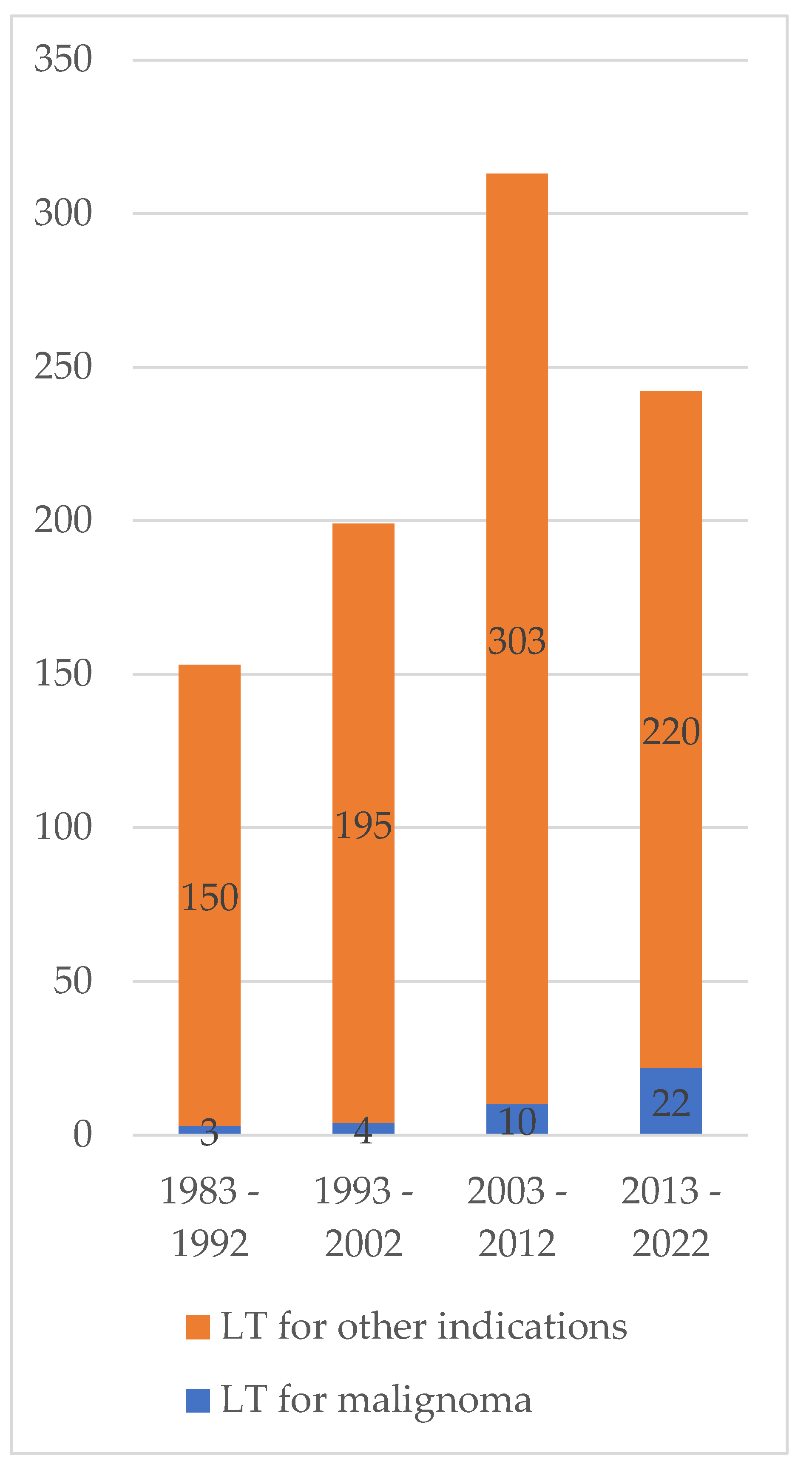
3.2. Incidence of Pediatric Liver Transplantation for Malignoma over Time
3.3. Treatment before Liver Transplantation/Salvage Transplantation
3.4. Perioperative Course/In-Patient Stay for Liver Transplantation
3.5. Outcome after Liver Transplantation
3.5.1. Immunosuppression and Episodes of Rejection
3.5.2. Biliary Complications
3.5.3. Hearing Loss
3.5.4. Secondary Malignoma and Tumor Recurrence
3.5.5. Long-Term Outcome
3.6. Risk Factors for Tumor Recurrence and Mortality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABPR | acute biopsy proven rejection |
| AFP | alpha-feto-protein |
| AFPmax | Highest AFP value measured before liver transplantation |
| AFPprepLTx | AFP value measured prior to liver transplantation |
| CIHL | Cisplatin-induced hearing loss |
| CSA | Cyclosporine A |
| ERCP | Endoscopic retrograde cholangiopancreatography |
| GFR | glomerular filtration rate |
| HCC | hepatocellular carcinoma |
| HR | hazard ration |
| HU | High urgency |
| INR | international normalized ratio |
| ICU | intensive care unit |
| LTotHB | liver tumor other than hepatoblastoma |
| mTOR | mammalian target of Rapamycin |
| OR | odds ratio |
| pLTx | pediatric liver transplantation |
| POSTTEXT | post-treatment extent of tumor |
| PRETEXT | pre-treatment extent of tumor |
| PTCD | percutaneous transhepatic bile duct drainage |
| SD | standard deviation |
| SE | standard error |
| SPLIT | Society of pediatric liver transplantation |
References
- Haeberle, B.; von Schweinitz, D. Treatment of Hepatoblastoma in the German Cooperative Pediatric Liver Tumor Studies. Front. Biosci.-Elite 2012, 4, 493–498. [Google Scholar] [CrossRef]
- Pham, T.H.; Iqbal, C.W.; Grams, J.M.; Zarroug, A.E.; Wall, J.C.H.; Ishitani, M.B.; Nagorney, D.M.; Moir, C. Outcomes of Primary Liver Cancer in Children: An Appraisal of Experience. J. Pediatr. Surg. 2007, 42, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Kahla, J.A.; Siegel, D.A.; Dai, S.; Lupo, P.J.; Foster, J.H.; Scheurer, M.E.; Heczey, A.A. Incidence and 5-Year Survival of Children and Adolescents with Hepatoblastoma in the United States. Pediatr. Blood Cancer 2022, 69, e29763. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Polychronidis, G.; Heger, U.; Frongia, G.; Mehrabi, A.; Hoffmann, K. Incidence Trends and Survival Prediction of Hepatoblastoma in Children: A Population-Based Study. Cancer Commun. 2019, 39, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, U.; Karam, V.; Adam, R.; Fondevila, C.; Dhawan, A.; Sokal, E.; Jacquemin, E.; Kelly, D.A.; Grabhorn, E.; Pawlowska, J.; et al. Prognosis of Children Undergoing Liver Transplantation: A 30-Year European Study. Pediatrics 2022, 150, e2022057424. [Google Scholar] [CrossRef] [PubMed]
- Leiskau, C.; Junge, N.; Pfister, E.D.; Goldschmidt, I.; Mutschler, F.; Laue, T.; Ohlendorf, J.; Nasser, H.; Beneke, J.; Richter, N.; et al. Recipient-Specific Risk Factors Impairing Patient and Graft Outcome after Pediatric Liver Transplantation-Analysis of 858 Transplantations in 38 Years. Children 2021, 8, 641. [Google Scholar] [CrossRef]
- Vinayak, R.; Cruz, R.J.; Ranganathan, S.; Mohanka, R.; Mazariegos, G.; Soltys, K.; Bond, G.; Tadros, S.; Humar, A.; Marsh, J.W.; et al. Pediatric Liver Transplantation for Hepatocellular Cancer and Rare Liver Malignancies: US Multicenter and Single-Center Experience (1981–2015). Liver Transplant. 2017, 23, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Elisofon, S.A.; Magee, J.C.; Ng, V.L.; Horslen, S.P.; Fioravanti, V.; Economides, J.; Erinjeri, J.; Anand, R.; Mazariegos, G.V.; Dunn, S.; et al. Society of Pediatric Liver Transplantation: Current Registry Status 2011–2018. Pediatr. Transpl. 2020, 24, e13605. [Google Scholar] [CrossRef]
- Hamilton, E.C.; Balogh, J.; Nguyen, D.T.; Graviss, E.A.; Heczey, A.A.; Austin, M.T. Liver Transplantation for Primary Hepatic Malignancies of Childhood: The UNOS Experience. J. Pediatr. Surg. 2017, 53, 163–168. [Google Scholar] [CrossRef]
- Ezekian, B.; Mulvihill, M.S.; Schroder, P.M.; Gilmore, B.F.; Leraas, H.J.; Gulack, B.C.; Jane Commander, S.; Mavis, A.M.; Kreissman, S.G.; Knechtle, S.J.; et al. Improved Contemporary Outcomes of Liver Transplantation for Pediatric Hepatoblastoma and Hepatocellular Carcinoma. Pediatr. Transpl. 2018, 22, e13305. [Google Scholar] [CrossRef]
- Boster, J.M.; Superina, R.; Mazariegos, G.V.; Tiao, G.M.; Roach, J.P.; Lovell, M.A.; Greffe, B.S.; Yanni, G.; Leung, D.H.; Elisofon, S.A.; et al. Predictors of Survival Following Liver Transplantation for Pediatric Hepatoblastoma and Hepatocellular Carcinoma: Experience from the Society of Pediatric Liver Transplantation (SPLIT). Am. J. Transpl. 2022, 22, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Baumann, U.; Adam, R.; Duvoux, C.; Mikolajczyk, R.; Karam, V.; D’Antiga, L.; Chardot, C.; Coker, A.; Colledan, M.; Ericzon, B.G.; et al. Survival of Children after Liver Transplantation for Hepatocellular Carcinoma. Liver Transpl. 2018, 24, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roebuck, D.J.; Aronson, D.; Clapuyt, P.; Czauderna, P.; de Ville Goyet, J.; Gauthier, F.; MacKinlay, G.; Maibach, R.; McHugh, K.; Olsen, Ø.E.; et al. 2005 PRETEXT: A Revised Staging System for Primary Malignant Liver Tumours of Childhood Developed by the SIOPEL Group. Pediatr. Radiol. 2007, 37, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trobaugh-Lotrario, A.D.; Meyers, R.L.; Tiao, G.M.; Feusner, J.H. Pediatric Liver Transplantation for Hepatoblastoma. Transl. Gastroenterol. Hepatol. 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Umeda, K.; Okajima, H.; Kawaguchi, K.; Nodomi, S.; Saida, S.; Kato, I.; Hiramatsu, H.; Ogawa, E.; Yoshizawa, A.; Okamoto, S.; et al. Prognostic and Therapeutic Factors Influencing the Clinical Outcome of Hepatoblastoma after Liver Transplantation: A Single-Institute Experience. Pediatr. Transpl. 2018, 22, e13113. [Google Scholar] [CrossRef]
- Ramos-Gonzalez, G.; LaQuaglia, M.; O’Neill, A.F.; Elisofon, S.; Zurakowski, D.; Kim, H.B.; Vakili, K. Long-Term Outcomes of Liver Transplantation for Hepatoblastoma: A Single-Center 14-Year Experience. Pediatr. Transpl. 2018, 22, e13250. [Google Scholar] [CrossRef]
- Kremer, N.; Walther, A.E.; Tiao, G.M. Management of Hepatoblastoma: An Update. Curr. Opin. Pediatr. 2014, 26, 362–369. [Google Scholar] [CrossRef]
- Austin, M.T.; Leys, C.M.; Feurer, I.D.; Lovvorn, H.N.; O’Neill, J.A.; Pinson, C.W.; Pietsch, J.B. Liver Transplantation for Childhood Hepatic Malignancy: A Review of the United Network for Organ Sharing (UNOS) Database. J. Pediatr. Surg. 2006, 41, 182–186. [Google Scholar] [CrossRef]
- Wiseman, J.T.; Guzman-Pruneda, F.; Xourafas, D.; Chun, Y.S.; Ejaz, A.; Tsung, A.; Pawlik, T.M.; Cloyd, J.M. Impact of Neoadjuvant Chemotherapy on the Postoperative Outcomes of Patients Undergoing Liver Resection for Colorectal Liver Metastases: A Population-Based Propensity-Matched Analysis. J. Am. Coll. Surg. 2019, 229, 69–77e2. [Google Scholar] [CrossRef]
- Feier, F.H.; da Fonseca, E.A.; Seda-Neto, J.; Chapchap, P. Biliary Complications after Pediatric Liver Transplantation: Risk Factors, Diagnosis and Management. World J. Hepatol. 2015, 7, 2162. [Google Scholar] [CrossRef]
- Kanneganti, M.; Xu, Y.; Huang, Y.S.; Kitt, E.; Fisher, B.T.; Abt, P.L.; Rand, E.B.; Schaubel, D.E.; Bittermann, T. Center Variability in Acute Rejection and Biliary Complications After Pediatric Liver Transplantation. Liver Transpl. 2022, 28, 454–465. [Google Scholar] [CrossRef]
- Hendrickson, R.J.; Sujka, J.; Fischer, R.; Manalang, M.; Daniel, J.; Andrews, W.S. Indications and Efficacy of Conversion from Tacrolimus- to Sirolimus-Based Immunosuppression in Pediatric Patients Who Underwent Liver Transplantation for Unresectable Hepatoblastoma. Pediatr. Transpl. 2019, 23, e13369. [Google Scholar] [CrossRef] [PubMed]
- Sindhi, R.; Webber, S.; Venkataramanan, R.; McGhee, W.; Phillips, S.; Smith, A.; Baird, C.; Iurlano, K.; Mazariegos, G.; Cooperstone, B.; et al. Sirolimus for Rescue and Primary Immunosuppression in Transplanted Children Receiving Tacrolimus. Transplantation 2001, 72, 851–855. [Google Scholar] [CrossRef]
- Nielsen, D.; Briem-Richter, A.; Sornsakrin, M.; Fischer, L.; Nashan, B.; Ganschow, R. The Use of Everolimus in Pediatric Liver Transplant Recipients: First Experience in a Single Center. Pediatr. Transpl. 2011, 15, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Rivera, C.; Avitzur, Y.; Fecteau, A.H.; Jones, N.; Grant, D.; Ng, V.L. Sirolimus for Pediatric Liver Transplant Recipients with Post-Transplant Lymphoproliferative Disease and Hepatoblastoma. Pediatr. Transpl. 2004, 8, 243–248. [Google Scholar] [CrossRef]
- Moke, D.J.; Luo, C.; Millstein, J.; Knight, K.R.; Rassekh, S.R.; Brooks, B.; Ross, C.J.D.; Wright, M.; Mena, V.; Rushing, T.; et al. Prevalence and Risk Factors for Cisplatin-Induced Hearing Loss in Children, Adolescents, and Young Adults: A Multi-Institutional North American Cohort Study. Lancet Child Adolesc. Health 2021, 5, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Illiano, M.; Colinard, M.; Taque, S.; Mallon, B.; Larue, C.; Laithier, V.; Vérité-Goulard, C.; Sudour-Bonnange, H.; Faure-Conter, C.; Coze, C.; et al. Long-Term Morbidity and Mortality in 2-Year Hepatoblastoma Survivors Treated with SIOPEL Risk-Adapted Strategies. Hepatol. Int. 2022, 16, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Freyer, D.R.; Brock, P.R.; Chang, K.W.; Dupuis, L.L.; Epelman, S.; Knight, K.; Mills, D.; Phillips, R.; Potter, E.; Risby, D.; et al. Prevention of Cisplatin-Induced Ototoxicity in Children and Adolescents with Cancer: A Clinical Practice Guideline. Lancet Child Adolesc. Health 2020, 4, 141–150. [Google Scholar] [CrossRef]
- Brock, P.R.; Maibach, R.; Childs, M.; Rajput, K.; Roebuck, D.; Sullivan, M.J.; Laithier, V.; Ronghe, M.; Dall’Igna, P.; Hiyama, E.; et al. Sodium Thiosulfate for Protection from Cisplatin-Induced Hearing Loss. N. Engl. J. Med. 2018, 378, 2376–2385. [Google Scholar] [CrossRef]
| Categorical Variables | Total (n = 39) | Hepatoblastoma (n = 31) | Liver Tumor Other than Hepatoblastma (n = 8) | p-Value | |||
|---|---|---|---|---|---|---|---|
| N | % | ||||||
| Diagnosis | |||||||
| Hepatoblastoma | 31 | 79.5 | 31 | 100 | 0 | ||
| Hemangioendothelioma | 3 | 7.7 | 0 | 3 | 37.5 | ||
| Sarcoma | 3 | 7.7 | 0 | 3 | 37.5 | ||
| HCC | 1 | 2.6 | 0 | 1 | 12.5 | ||
| Inflammatory myofibroblastic tumor | 1 | 2.6 | 0 | 1 | 12.5 | ||
| Tumor extension surpassing liver | 20 | 51.3 | 19 | 61.2 | 1 | 12.5 | 0.0105 |
| Neoadjuvant chemotherapy | 36 | 92.3 | 30 | 96.8 | 6 | 75 | 0.0393 |
| Chemotherapy after liver transplant | 26 | 66.7 | 23 | 74.1 | 3 | 37.5 | 0.0342 |
| Salvage transplantation | 8 | 20.5 | 6 | 19.4 | 2 | 25 | 0.7244 |
| PRETEXT II | 3 | 7.7 | 3 | 9.7 | |||
| PRETEXT III | 10 | 25.6 | 10 | 32.3 | |||
| PRETEXT IV | 18 | 46.2 | 18 | 58.1 | |||
| Sex female | 16 | 41.0 | 12 | 38.7 | 4 | 50.0 | 0.5627 |
| Era of Transplantation [1,2,3,4] | |||||||
| Era 1 (1983–1992) | 3 | 7.7 | 2 | 6.4 | 1 | 12.5 | 0.0250 |
| Era 2 (1993–2002) | 4 | 10.3 | 1 | 3.2 | 3 | 37.5 | |
| Era 3 (2003–2012) | 10 | 25.6 | 8 | 25.8 | 2 | 25 | |
| Era 4 (2013–2022) | 22 | 56.4 | 20 | 64.5 | 2 | 25 | |
| On high-urgency list [y/n] | 33 | 84.6 | 28 | 90.3 | 5 | 62.5 | 0.0436 |
| Combined pLTx to other organ | 1 | 2.6 | 1 | 3.2 | 0 | 0 | n/a |
| Kidney | 1 | 2.6 | 1 | 3.2 | 0 | 0 | n/a |
| Full size graft | 14 | 39.9 | 10 | 32.3 | 5 | 62.5 | 0.1170 |
| Hepaticojejunostomy as biliary anastomosis | 22 | 56.4 | 19 | 61.3 | 3 | 37.5 | 0.2263 |
| Bile flow impairment requiring intervention | 19 | 48.7 | 18 | 58.1 | 1 | 12.5 | 0.0215 |
| Initial immunosuppression | n/a | ||||||
| CSA/Prednisolone | 37 | 94.9 | 30 | 96.8 | 7 | 87.5 | |
| Tacrolimus | 1 | 2.6 | 0 | 0 | 1 | 12.5 | |
| CSA/MMF | 1 | 2.6 | 1 | 3.2 | 0 | 0 | |
| Last documented immunosuppression | 0.3124 | ||||||
| CSA | 13 | 33.3 | 10 | 32.3 | 3 | 37.5 | |
| Sirolimus | 16 | 41.0 | 14 | 45.2 | 2 | 25 | |
| Tacrolimus | 6 | 15.4 | 4 | 12.9 | 2 | 25 | |
| MMF | 2 | 5.1 | 2 | 6.4 | 0 | 0 | |
| MMF + Predni | 1 | 2.6 | 0 | 0 | 1 | 12.5 | |
| CSA + Everolimus | 1 | 2.6 | 1 | 3.2 | 0 | 0 | |
| Acute biopsy-proven rejection episode | 20 | 51.3 | 14 | 45.2 | 6 | 75 | 0.1322 |
| Hearing loss | 15 | 38.4 | 15 | 48.4 | 0 | 0 | 0.0083 |
| Renal function impairment [GFR < 60] | 6 | 15.4 | 4 | 16.1 | 2 | 25 | 0.5020 |
| Secondary malignoma | 9 | 23.1 | 8 | 25.9 | 1 | 12.5 | 0.4258 |
| Graft loss due to death or re-pLTx | 9 | 23.1 | 7 | 22.6 | 2 | 25 | 0.8849 |
| Subsequent re-Tx | 4 | 10.3 | 2 | 6.5 | 2 | 25 | 0.1231 |
| Mortality after transplantation | 6 | 15.3 | 6 | 19.4 | 0 | 0 | 0.0820 |
| Variable | pLTx for All Liver Tumours (n = 39) | pLTx for Hepatoblastoma (n = 31) | pLTx for Liver Tumours Other than Hepatoblastoma (n = 8) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Mean (Median) | SD (Range) | Mean | SE | Mean | SE | ||
| Age at diagnosis | 3.35 (1.69) | 3.45 (0.28–12.12) | 2.73 | 3.05 | 5.74 | 4.07 | 0.0129 |
| Age at pLTx [years] | 4.05 (2.59) | 3.69 (0.56–14.34) | 3.35 | 3.05 | 6.77 | 4.86 | 0.0088 |
| Time between diagnosis and pLTx [years] | 0.72 (0.47) | 0.76 (0.05–4.00) | 0.62 | 0.27 | 1.03 | 0.27 | 0.1912 |
| Weight at pLTx [kg] | 17.01 (12.15) | 11.65 (7.3–60.0) | 14.70 | 1.92 | 27.25 | 4.04 | 0.0081 |
| Height at pLTx [cm] | 96.4 (86.5) | 25.7 (63–169) | 92.6 | 4.44 | 113.0 | 9.35 | 0.0282 |
| BMI [kg/m2] | 16.75 (16.51) | 2.17 (13.0–23.5) | 16.25 | 0.34 | 19.01 | 0.72 | 0.0014 |
| AFP maximum [IU/L] | n/a | 541,945 (338,065) | 717,927 (526–3,114,000) | n/a | |||
| AFP at pLTx [IU/L] | 48,458 (108) | 251,033 (2–1,400,000) | |||||
| AFP max/AFP LTx | 9270 (719.9) | 17,034 (1.33–60,000) | |||||
| Creatinine at pLTx [µmol/L] | 47.3 (27) | 112.9 (10–692) | 34.2 | 46.7 | 50.0 | 21.2 | 0.7594 |
| Bilirubine at pLTx [µmol/L] | 21 (5) | 68 (3–390) | 23.1 | 12.8 | 9.0 | 30.8 | 0.6757 |
| Albumine at pLTx (g/L) | 39.4 (40) | 5.6 (25–48) | 39.5 | 1.08 | 39.0 | 2.86 | 0.8742 |
| INR at pLTx | 1.12 (1.13) | 0.14 (0.9–1.69) | 1.12 | 0.03 | 1.10 | 0.07 | 0.8063 |
| Waiting time for pLTx all status [days] | 41.2 (37) | 16.7 (2–127) | 36.9 | 9.59 | 55.5 | 35.9 | 0.3613 |
| Waiting time for pLTx on high urgency (HU) list (only HU patients) [days] | 9.72 (9) | 7.1 (0–23) | 10.17 | 1.35 | 8.12 | 2.53 | 0.4795 |
| ICU stay post pLtx [days] | 9.07 (6) | 12.3 (1–65) | 9.20 | 2.79 | 8.71 | 4.72 | 0.9301 |
| NON-ICU stay post Ltx [days] | 26.44 (22) | 16.23 (0–68) | 25.70 | 3.69 | 28.57 | 6.24 | 0.6953 |
| Follow-up after pLTx [years] | 9.49 (7.97) | 9.14 (0.84–36.76) | 7.59 | 1.46 | 16.87 | 2.86 | 0.0064 |
| Follow-up after diagnosis [years] | 10.20 (9.49) | 8.84 (0.28–34.08) | 8.21 | 1.50 | 17.89 | 2.95 | 0.0059 |
| Variable | HR for Mortality | HR for Tumor Recurrence | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-Value | OR | 95%CI | p | |
| Hepatoblastoma | 3.024 | 0.842–16.352 | 0.0820 | 1.346 | 0.134–13.474 | 0.7961 |
| Tumor extension beyond liver | 5.667 | 0.593–54.114 | 0.1319 | 4.620 | 0.510–41.887 | 0.1736 |
| Previous surgery before pLTx | 6.786 | 0.711–64.723 | 0.0961 | 2.400 | 0.385–14.968 | 0.3356 |
| Waiting time for pLTx | 1.002 | 0.994–1.010 | 0.6118 | 0.999 | 0.989–1.009 | 0.8977 |
| Age at transplantation | 1.007 | 0.796–1.274 | 0.9536 | 1.039 | 0.831–1.299 | 0.7381 |
| Time span between diagnosis and transplantation | 1.723 | 0.699–4.242 | 0.2370 | 1.822 | 0.717–4.631 | 0.2072 |
| Current Era (2013–2022) | 0.736 | 0.129–4.210 | 0.7313 | 1.667 | 0.267–10.394 | 0.5844 |
| Salvage transplant | 4.283 | 0.864–21.241 | 0.0749 | 6.116 | 1.020–36.683 | 0.0476 |
| Neo-adjuvant chemotherapy | 0.483 | 0.038–6.111 | 0.5739 | 0.323 | 0.024–4.255 | 0.4179 |
| Log (AFP max.) | 1.229 | 0.475–3.182 | 0.6612 | 1.370 | 0.465–4.035 | 0.5482 |
| Log (AFP pre-pLTx) | 1.854 | 0.941–3.651 | 0.0587 | 2.327 | 1.049–5.168 | 0.0378 |
| Log (AFP max/AFP pre-pLTx) | 0.498 | 0.219–1.132 | 0.0729 | 0.371 | 0.136–0.926 | 0.0254 |
| Full size graft | 1.818 | 0.379–8.731 | 0.4552 | 1.750 | 0.304–10.075 | 0.5309 |
| Biliodigestive anastomosis | 1.666 | 0.267–10.394 | 0.5777 | 1.372 | 0.278–6.775 | 0.6975 |
| Biliary complication requiring intervention | 0.563 | 0.114–2.773 | 0.4796 | 0.471 | 0.076–2.932 | 0.4194 |
| Post-operative chemotherapy | 0.909 | 0.142–5.809 | 0.9198 | 0.900 | 0.183–4.429 | 0.8972 |
| Maintenance immunosuppression CSA | 4.600 | 0.721–29.332 | 0.1064 | 2.000 | 0.346–11.583 | 0.4392 |
| Maintenance immunosuppression Sirolimus | 0.240 | 0.025–2.286 | 0.2146 | 0.152 | 0.017–1.369 | 0.0953 |
| Episodes of ABPR | 0.938 | 0.198–4.437 | 0.9352 | 0.941 | 0.165–5.361 | 0.9456 |
| Renal function impairment | 0.628 | 0.062–6.329 | 0.6935 | 0.960 | 0.091–10.099 | 0.9729 |
| Recurrence of tumor | 15.896 | 2.886–87.567 | 0.0015 | n/a | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiskau, C.; Junge, N.; Mutschler, F.E.; Laue, T.; Ohlendorf, J.; Richter, N.; Vondran, F.W.R.; Pfister, E.-D.; Baumann, U. Long-Term Outcome Following Liver Transplantation for Primary Hepatic Tumors—A Single Centre Observational Study over 40 Years. Children 2023, 10, 202. https://doi.org/10.3390/children10020202
Leiskau C, Junge N, Mutschler FE, Laue T, Ohlendorf J, Richter N, Vondran FWR, Pfister E-D, Baumann U. Long-Term Outcome Following Liver Transplantation for Primary Hepatic Tumors—A Single Centre Observational Study over 40 Years. Children. 2023; 10(2):202. https://doi.org/10.3390/children10020202
Chicago/Turabian StyleLeiskau, Christoph, Norman Junge, Frauke E. Mutschler, Tobias Laue, Johanna Ohlendorf, Nicolas Richter, Florian W. R. Vondran, Eva-Doreen Pfister, and Ulrich Baumann. 2023. "Long-Term Outcome Following Liver Transplantation for Primary Hepatic Tumors—A Single Centre Observational Study over 40 Years" Children 10, no. 2: 202. https://doi.org/10.3390/children10020202





