The Mediating Role of Brain Structural Imaging Markers in Connecting Adverse Childhood Experiences and Psychological Resilience
Abstract
1. Introduction
1.1. Childhood Adversity on Young Adult Development
1.2. Role of Psychological Resilience Related to Childhood Adversity
1.3. Childhood Adversity and Brain Imaging Markers
1.4. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Resilience Score Measurement
2.3. Adverse Childhood Experiences
2.4. Image Acquisitions
2.5. Image Preprocessing
2.5.1. Structural MRI (sMRI)
2.5.2. Resting-State Functional MRI (rfMRI)
2.5.3. Diffusion MRI (dMRI)
2.5.4. Joint ICA Analysis
2.6. Statistics
2.6.1. Correlation Analysis
2.6.2. Mediation Analysis
3. Results
3.1. Demographic Data
3.2. Correlation between Adverse Childhood Experiences and Resilience for Adults
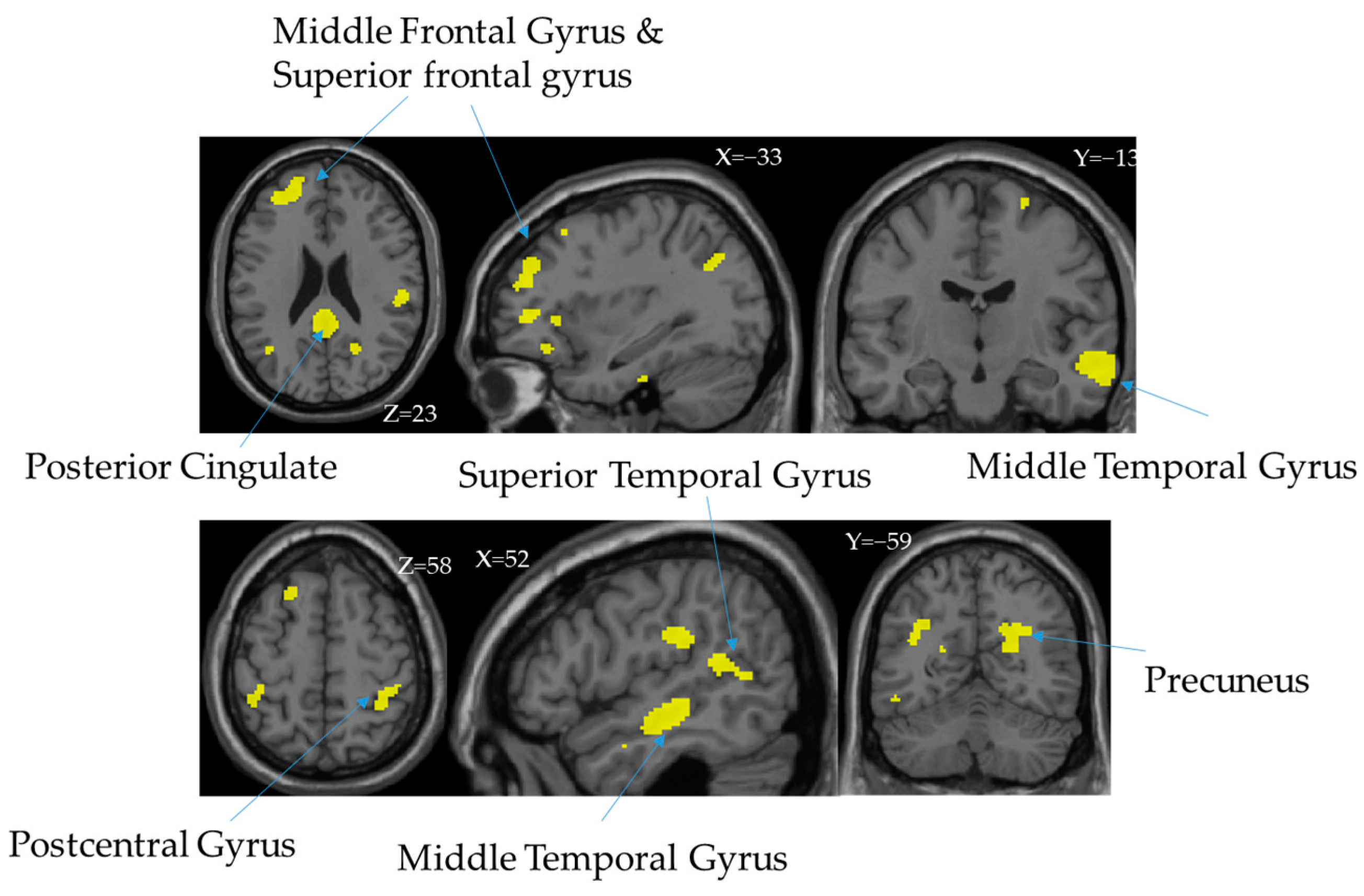
3.3. Independent Components (ICs)
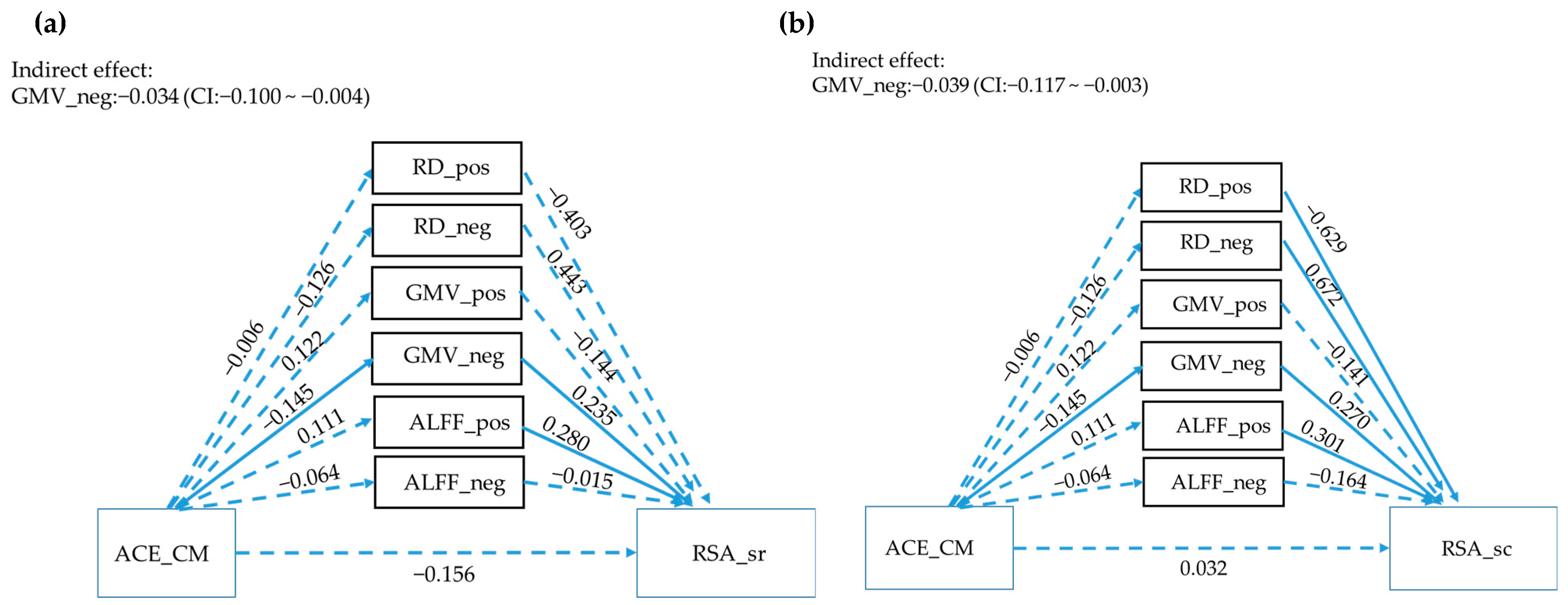
3.4. Mediation Analysis
3.5. The Mediator between RSA Subscales and ACE_CM
4. Discussion
Limitations and Future Directions
5. Implications and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veroude, K.; Jolles, J.; Croiset, G.; Krabbendam, L. Changes in neural mechanisms of cognitive control during the transition from late adolescence to young adulthood. Dev. Cogn. Neurosci. 2013, 5, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Brent, D.A.; Silverstein, M. Shedding light on the long shadow of childhood adversity. JAMA 2013, 309, 1777–1778. [Google Scholar] [CrossRef] [PubMed]
- Shonkoff, J.P.; Garner, A.S.; Child Committee on Psychosocial Aspects of, Health Family; Adoption Committee on Early Childhood, Care Dependent; Developmental Section on, and Pediatrics Behavioral. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 2012, 129, e232–e246. [Google Scholar] [CrossRef]
- Blodgett, C.; Lanigan, J. The association between adverse childhood experience (ACE) and school success in elementary school children. Sch. Psychol. Q. 2018, 33, 137–146. [Google Scholar] [CrossRef]
- Deater-Deckard, K.; Mullineaux, P.Y.; Beekman, C.; Petrill, S.A.; Schatschneider, C.; Thompson, L.A. Conduct problems, IQ, and household chaos: A longitudinal multi-informant study. J. Child Psychol. Psychiatry Allied Discip. 2009, 50, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Evans, G.W.; Angstadt, M.; Ho, S.S.; Sripada, C.S.; Swain, J.E.; Liberzon, I.; Phan, K.L. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. USA 2013, 110, 18442–18447. [Google Scholar] [CrossRef]
- Briggs, E.C.; Amaya-Jackson, L.; Putnam, K.T.; Putnam, F.W. All adverse childhood experiences are not equal: The contribution of synergy to adverse childhood experience scores. Am. Psychol. 2021, 76, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, K.; Davis, J.; Berman, T. Adverse childhood experiences and associated health outcomes: A systematic review and meta-analysis. Child Abuse Negl. 2019, 97, 104127. [Google Scholar] [CrossRef]
- Windle, M.; Haardörfer, R.; Getachew, B.; Shah, J.; Payne, J.; Pillai, D.; Berg, C.J. A multivariate analysis of adverse childhood experiences and health behaviors and outcomes among college students. J. Am. Coll. Health J ACH 2018, 66, 246–251. [Google Scholar] [CrossRef]
- Shonkoff, J.P.; Boyce, W.T.; McEwen, B.S. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA 2009, 301, 2252–2259. [Google Scholar] [CrossRef]
- Hochberg, Z.E.; Konner, M. Emerging Adulthood, a Pre-adult Life-History Stage. Front. Endocrinol. 2019, 10, 918. [Google Scholar] [CrossRef]
- Werner, E.E. The children of Kauai: Resiliency and recovery in adolescence and adulthood. J. Adolesc. Health 1992, 13, 262–268. [Google Scholar] [CrossRef]
- Luthar, S.S.; Cicchetti, D.; Becker, B. The Construct of Resilience: A Critical Evaluation and Guidelines for Future Work. Child Dev. 2000, 71, 543–562. [Google Scholar] [CrossRef]
- Masten, A.S. Ordinary magic. Resilience processes in development. Am. Psychol. 2001, 56, 227–238. [Google Scholar] [CrossRef]
- Rutter, M. Resilience in the Face of Adversity: Protective Factors and Resistance to Psychiatric Disorder. Br. J. Psychiatry 2018, 147, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, G.A. Loss, trauma, and human resilience: Have we underestimated the human capacity to thrive after extremely aversive events? Am. Psychol. 2004, 59, 20–28. [Google Scholar] [CrossRef]
- Davydov, D.M.; Stewart, R.; Ritchie, K.; Chaudieu, I. Resilience and mental health. Clin. Psychol. Rev. 2010, 30, 479–495. [Google Scholar] [CrossRef]
- Hu, T.; Zhang, D.; Wang, J. A meta-analysis of the trait resilience and mental health. Personal. Individ. Differ. 2015, 76, 18–27. [Google Scholar] [CrossRef]
- Wilks, S.E.; Spivey, C.A. Resilience in Undergraduate Social Work Students: Social Support and Adjustment to Academic Stress. Soc. Work. Educ. 2010, 29, 276–288. [Google Scholar] [CrossRef]
- Bethell Christina, D.; Newacheck, P.; Hawes, E.; Halfon, N. Adverse Childhood Experiences: Assessing The Impact On Health And School Engagement And The Mitigating Role Of Resilience. Health Aff. 2014, 33, 2106–2115. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.A.; Ramirez, A.N. Adverse Childhood Experience and Adolescent Well-being: Do Protective Factors Matter? Child Indic. Res. 2016, 9, 299–316. [Google Scholar] [CrossRef]
- Heard-Garris, N.; Davis, M.M.; Szilagyi, M.; Kan, K. Childhood adversity and parent perceptions of child resilience. BMC Pediatr. 2018, 18, 204. [Google Scholar] [CrossRef] [PubMed]
- Young-Wolff, K.C.; Alabaster, A.; McCaw, B.; Stoller, N.; Watson, C.; Sterling, S.; Ridout, K.K.; Flanagan, T. Adverse Childhood Experiences and Mental and Behavioral Health Conditions During Pregnancy: The Role of Resilience. J. Women’s Health 2019, 28, 452–461. [Google Scholar] [CrossRef]
- Morgan, C.A.; Chang, Y.H.; Choy, O.; Tsai, M.C.; Hsieh, S. Adverse Childhood Experiences Are Associated with Reduced Psychological Resilience in Youth: A Systematic Review and Meta-Analysis. Children 2021, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ming, Q.; Dong, D.; Sun, X.; Cheng, C.; Xiong, G.; Li, C.; Zhang, X.; Yao, S. Childhood Maltreatment Experience Influences Neural Response to Psychosocial Stress in Adults: An fMRI Study. Front. Psychol. 2019, 10, 2961. [Google Scholar] [CrossRef]
- Cohen, R.A.; Grieve, S.; Hoth, K.F.; Paul, R.H.; Sweet, L.; Tate, D.; Gunstad, J.; Stroud, L.; McCaffery, J.; Hitsman, B. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol. Psychiatry 2006, 59, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Dannlowski, U.; Stuhrmann, A.; Beutelmann, V.; Zwanzger, P.; Lenzen, T.; Grotegerd, D.; Domschke, K.; Hohoff, C.; Ohrmann, P.; Bauer, J. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry 2012, 71, 286–293. [Google Scholar] [CrossRef]
- van Harmelen, A.-L.; van Tol, M.-J.; van der Wee, N.J.; Veltman, D.J.; Aleman, A.; Spinhoven, P.; van Buchem, M.A.; Zitman, F.G.; Penninx, B.W.; Elzinga, B.M. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol. Psychiatry 2010, 68, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Oshri, A.; Gray, J.C.; Owens, M.M.; Liu, S.; Duprey, E.B.; Sweet, L.H.; MacKillop, J. Adverse Childhood Experiences and Amygdalar Reduction: High-Resolution Segmentation Reveals Associations With Subnuclei and Psychiatric Outcomes. Child Maltreat 2019, 24, 400–410. [Google Scholar] [CrossRef]
- McLaughlin, K.A.; Sheridan, M.A.; Nelson, C.A. Adverse childhood experiences and brain development: Neurobiological mechanisms linking the social environment to psychiatric disorders. In A Life Course Approach to Mental Disorders; Koenen, K.C., Rudenstine, S., Susser, E., Galea, S., Eds.; Oxford University Press: Oxford, UK, 2013; p. 249. [Google Scholar]
- Lawson, G.M.; Duda, J.T.; Avants, B.B.; Wu, J.; Farah, M.J. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev. Sci. 2013, 16, 641–652. [Google Scholar] [CrossRef]
- Shaked, D.; Katzel, L.I.; Seliger, S.L.; Gullapalli, R.P.; Davatzikos, C.; Erus, G.; Evans, M.K.; Zonderman, A.B.; Waldstein, S.R. Dorsolateral prefrontal cortex volume as a mediator between socioeconomic status and executive function. Neuropsychology 2018, 32, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, F.; Radaelli, D.; Poletti, S.; Falini, A.; Cavallaro, R.; Dallaspezia, S.; Riccaboni, R.; Scotti, G.; Smeraldi, E. Emotional reactivity in chronic schizophrenia: Structural and functional brain correlates and the influence of adverse childhood experiences. Psychol. Med. 2011, 41, 509–519. [Google Scholar] [CrossRef]
- Buimer, E.E.L.; Brouwer, R.M.; Mandl, R.C.W.; Pas, P.; Schnack, H.G.; Hulshoff Pol, H.E. Adverse childhood experiences and fronto-subcortical structures in the developing brain. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Fujiwara, T.; Murayama, H.; Machida, M.; Inoue, S.; Shobugawa, Y. Association between adverse childhood experiences and brain volumes among Japanese community-dwelling older people: Findings from the NEIGE study. Child Abus. Negl. 2022, 124, 105456. [Google Scholar] [CrossRef]
- van der Werff, S.J.; Pannekoek, J.N.; Veer, I.M.; van Tol, M.J.; Aleman, A.; Veltman, D.J.; Zitman, F.G.; Rombouts, S.A.; Elzinga, B.M.; van der Wee, N.J. Resting-state functional connectivity in adults with childhood emotional maltreatment. Psychol. Med. 2013, 43, 1825–1836. [Google Scholar] [CrossRef]
- Goetschius, L.G.; Hein, T.C.; McLanahan, S.S.; Brooks-Gunn, J.; McLoyd, V.C.; Dotterer, H.L.; Lopez-Duran, N.; Mitchell, C.; Hyde, L.W.; Monk, C.S.; et al. Association of Childhood Violence Exposure With Adolescent Neural Network Density. JAMA Netw. Open 2020, 3, e2017850. [Google Scholar] [CrossRef]
- Choi, J.; Jeong, B.; Polcari, A.; Rohan, M.L.; Teicher, M.H. Reduced fractional anisotropy in the visual limbic pathway of young adults witnessing domestic violence in childhood. NeuroImage 2012, 59, 1071–1079. [Google Scholar] [CrossRef]
- Ugwu, I.D.; Amico, F.; Carballedo, A.; Fagan, A.J.; Frodl, T. Childhood adversity, depression, age and gender effects on white matter microstructure: A DTI study. Brain Struct. Funct. 2015, 220, 1997–2009. [Google Scholar] [CrossRef]
- Lu, S.; Gao, W.; Wei, Z.; Wang, D.; Hu, S.; Huang, M.; Xu, Y.; Li, L. Intrinsic brain abnormalities in young healthy adults with childhood trauma: A resting-state functional magnetic resonance imaging study of regional homogeneity and functional connectivity. Aust. N. Z. J. Psychiatry 2017, 51, 614–623. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Y.; Du, M.; Hussein, N.M.; Li, L.; Wang, Y.; Mao, C.; Chen, T.; Chen, F.; Liu, X.; et al. Altered Spontaneous Brain Activity in Left-Behind Children: A Resting-State Functional MRI Study. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Kitamura, S.; Matsuoka, K.; Takahashi, M.; Ishida, R.; Kishimoto, N.; Yasuno, F.; Yasuda, Y.; Hashimoto, R.; Miyasaka, T.; et al. Adverse Childhood Experience Is Associated With Disrupted White Matter Integrity in Autism Spectrum Disorder: A Diffusion Tensor Imaging Study. Front. Psychiatry 2021, 12, 823260. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Liu, J.; Adali, T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. Neuroimage 2009, 45, S163–S172. [Google Scholar] [CrossRef] [PubMed]
- Park, A.T.; Tooley, U.A.; Leonard, J.A.; Boroshok, A.L.; McDermott, C.L.; Tisdall, M.D.; Mackey, A.P. Early childhood stress is associated with blunted development of ventral tegmental area functional connectivity. Dev. Cogn. Neurosci. 2021, 47, 100909. [Google Scholar] [CrossRef] [PubMed]
- Friborg, O.; Hjemdal, O.; Rosenvinge, J.H.; Martinussen, M. A new rating scale for adult resilience: What are the central protective resources behind healthy adjustment? Int. J. Methods Psychiatr. Res. 2003, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y. Associations of Sense of Self, Resilience and Posttraumatic Stress Symptoms among Burn Patients. Master’s Thesis, National Taiwan University, Taipei, Taiwan, 2007. [Google Scholar]
- Wang, Y.C.; Moya Guerola, M.; Lin, Y.C.; Hsieh, Y.P.; Strong, C.; Tsai, M.C.; Lin, C.Y. Effects of childhood adversity and resilience on Taiwanese youth health behaviors. Pediatr. Neonatol. 2019, 60, 368–376. [Google Scholar] [CrossRef]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. NeuroImage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in Functional and Structural Mr Image Analysis and Implementation as Fsl. NeuroImage 2004, 23, S208–S219. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of Brain Mr Images through a Hidden Markov Random Field Model and the Expectation-Maximization Algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Mazziotta, J.C.; Toga, A.W.; Evans, A.; Fox, P.; Lancaster, J. A Probabilistic Atlas of the Human Brain: Theory and Rationale for Its Development: The International Consortium for Brain Mapping (Icbm). NeuroImage 1995, 2, 89–101. [Google Scholar] [CrossRef]
- Andersson, J.L.; Jenkinson, M.; Smith, S. Non-Linear Registration Aka Spatial Normalisation; Fmrib Technial Report Tr07ja2; ScienceOpen Inc.: Berlin, Germany, 2007. [Google Scholar]
- Hsieh, S.; Yao, Z.F.; Yang, M.H. Multimodal Imaging Analysis Reveals Frontal-Associated Networks in Relation to Individual Resilience Strength. Int. J. Environ. Res. Public Health 2021, 18, 1123. [Google Scholar] [CrossRef]
- Zou, Q.H.; Zhu, C.Z.; Yang, Y.; Zuo, X.N.; Long, X.Y.; Cao, Q.J.; Wang, Y.F.; Zang, Y.F. An Improved Approach to Detection of Amplitude of Low-Frequency Fluctuation (Alff) for Resting-State Fmri: Fractional Alff. J. Neurosci. Methods 2008, 172, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Mattiello, J.; LeBihan, D. Mr Diffusion Tensor Spectroscopy and Imaging. Biophys. J. 1994, 66, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-Based Spatial Statistics: Voxelwise Analysis of Multi-Subject Diffusion Data. NeuroImage 2006, 31, 1487–1505. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Johansen-Berg, H.; Jenkinson, M.; Rueckert, D.; Nichols, T.E.; Miller, K.L.; Robson, M.D.; Jones, D.K.; Klein, J.C.; Bartsch, A.J.; et al. Acquisition and Voxelwise Analysis of Multi-Subject Diffusion Data with Tract-Based Spatial Statistics. Nat. Protoc. 2007, 2, 499–503. [Google Scholar] [CrossRef]
- Yang, M.H.; Yao, Z.F.; Hsieh, S. Multimodal Neuroimaging Analysis Reveals Age-Associated Common and Discrete Cognitive Control Constructs. Hum. Brain Mapp. 2019, 40, 2639–2661. [Google Scholar] [CrossRef]
- Luby, J.L.; Tillman, R.; Barch, D.M. Association of Timing of Adverse Childhood Experiences and Caregiver Support With Regionally Specific Brain Development in Adolescents. JAMA Netw. Open 2019, 2, e1911426. [Google Scholar] [CrossRef]
- Ramanoël, S.; Hoyau, E.; Kauffmann, L.; Renard, F.; Pichat, C.; Boudiaf, N.; Krainik, A.; Jaillard, A.; Baciu, M. Gray Matter Volume and Cognitive Performance During Normal Aging. A Voxel-Based Morphometry Study. Front. Aging Neurosci. 2018, 10, 235. [Google Scholar] [CrossRef]
- Moreno-López, L.; Ioannidis, K.; Askelund, A.D.; Smith, A.J.; Schueler, K.; van Harmelen, A.-L. The Resilient Emotional Brain: A Scoping Review of the Medial Prefrontal Cortex and Limbic Structure and Function in Resilient Adults With a History of Childhood Maltreatment. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2020, 5, 392–402. [Google Scholar] [CrossRef]
- Tendolkar, I.; Mårtensson, J.; Kühn, S.; Klumpers, F.; Fernández, G. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum. Brain Mapp. 2018, 39, 1283–1290. [Google Scholar] [CrossRef]
- Corbo, V.; Amick, M.A.; Milberg, W.P.; McGlinchey, R.E.; Salat, D.H. Early life trauma is associated with altered white matter integrity and affective control. J. Psychiatr. Res. 2016, 79, 70–77. [Google Scholar] [CrossRef]
- Pechtel, P.; Pizzagalli, D.A. Effects of early life stress on cognitive and affective function: An integrated review of human literature. Psychopharmacology 2011, 214, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Wessing, I.; Fürniss, T.; Zwitserlood, P.; Dobel, C.; Junghöfer, M. Early emotion discrimination in 8- to 10-year-old children: Magnetoencephalographic correlates. Biol. Psychol. 2011, 88, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.M.; Williams, L.M.; Korgaonkar, M.S.; Cohen, R.A.; Heaps, J.M.; Paul, R.H. Impact of early vs. late childhood early life stress on brain morphometrics. Brain Imaging Behav. 2013, 7, 196–203. [Google Scholar] [CrossRef]
- Ozbay, F.; Johnson, D.C.; Dimoulas, E.; Morgan, C.A.; Charney, D.; Southwick, S. Social support and resilience to stress: From neurobiology to clinical practice. Psychiatry (Edgmont) 2007, 4, 35–40. [Google Scholar] [PubMed]
| ACE_CM | ACE_PV | ACE_FF | ACE_ES | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| RSA_total | −0.356 | 0.000 | −0.319 | 0.001 | −0.109 | 0.265 | 0.013 | 0.897 |
| RSA_ps | −0.256 | 0.008 | −0.213 | 0.029 | −0.013 | 0.897 | −0.039 | 0.689 |
| RSA_fc | −0.501 | 0.000 | −0.276 | 0.004 | −0.323 | 0.001 | −0.013 | 0.896 |
| RSA_sr | −0.240 | 0.013 | −0.341 | 0.000 | −0.019 | 0.843 | 0.047 | 0.629 |
| RSA_sc | −0.064 | 0.516 | −0.239 | 0.013 | −0.077 | 0.431 | 0.007 | 0.946 |
| RSA_fss | −0.132 | 0.178 | −0.002 | 0.986 | 0.087 | 0.375 | 0.043 | 0.659 |
| Component No. | ACE_CM | ACE_PV | ACE_FF | ACE_ES | RSA total | RSA_ps | RSA_fc | RSA_sr | RSA_sc | RSA_fss | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| 1 | −0.036 | 0.709 | −0.106 | 0.278 | 0.041 | 0.675 | 0.070 | 0.476 | −0.008 | 0.938 | 0.029 | 0.767 | −0.089 | 0.364 | 0.018 | 0.852 | −0.033 | 0.736 | 0.057 | 0.559 |
| 4 | −0.121 | 0.216 | 0.041 | 0.679 | −0.028 | 0.776 | 0.125 | 0.201 | 0.067 | 0.492 | 0.059 | 0.544 | 0.095 | 0.331 | 0.108 | 0.268 | −0.068 | 0.490 | −0.017 | 0.859 |
| 7 | −0.003 | 0.975 | 0.127 | 0.194 | −0.02 | 0.838 | −0.021 | 0.832 | 0.109 | 0.262 | 0.111 | 0.255 | 0.108 | 0.268 | 0.002 | 0.982 | 0.098 | 0.313 | 0.104 | 0.288 |
| 10 | −0.137 | 0.158 | 0.059 | 0.544 | −0.03 | 0.758 | 0.008 | 0.934 | −0.02 | 0.838 | −0.105 | 0.281 | 0.062 | 0.529 | 0.046 | 0.639 | −0.06 | 0.538 | −0.062 | 0.530 |
| 12 | −0.007 | 0.942 | 0.090 | 0.359 | −0.117 | 0.232 | 0.175 | 0.071 | 0.029 | 0.766 | −0.039 | 0.689 | 0.092 | 0.344 | 0.018 | 0.851 | 0.073 | 0.455 | −0.051 | 0.605 |
| 14 | −0.009 | 0.926 | −0.052 | 0.593 | −0.067 | 0.492 | 0.047 | 0.627 | 0.11 | 0.261 | 0.102 | 0.297 | 0.062 | 0.525 | 0.053 | 0.591 | 0.145 | 0.137 | 0.054 | 0.582 |
| 15 | −0.046 | 0.637 | −0.086 | 0.379 | −0.036 | 0.715 | 0.06 | 0.536 | 0.036 | 0.71 | 0.104 | 0.284 | −0.006 | 0.949 | 0.034 | 0.729 | −0.029 | 0.769 | 0.013 | 0.895 |
| 16 | −0.026 | 0.793 | 0.047 | 0.630 | −0.118 | 0.227 | −0.343 | 0.000 | −0.028 | 0.774 | −0.062 | 0.524 | −0.005 | 0.959 | −0.03 | 0.758 | 0.048 | 0.621 | −0.039 | 0.693 |
| 17 | −0.088 | 0.367 | −0.018 | 0.855 | 0.013 | 0.893 | 0.052 | 0.592 | 0.108 | 0.267 | 0.137 | 0.161 | 0.067 | 0.494 | 0.069 | 0.479 | −0.003 | 0.978 | 0.114 | 0.243 |
| 19 | 0.134 | 0.169 | 0.065 | 0.506 | 0.121 | 0.215 | −0.016 | 0.871 | 0.023 | 0.813 | 0.034 | 0.726 | 0.012 | 0.903 | −0.01 | 0.922 | 0.006 | 0.952 | 0.055 | 0.574 |
| 20 | −0.174 | 0.072 | 0.110 | 0.258 | −0.074 | 0.448 | −0.116 | 0.235 | 0.113 | 0.246 | 0.064 | 0.512 | 0.196 | 0.043 | 0.002 | 0.981 | −0.014 | 0.886 | 0.169 | 0.083 |
| 21 | −0.053 | 0.591 | −0.091 | 0.353 | 0.015 | 0.875 | 0.048 | 0.622 | 0.200 | 0.039 | 0.112 | 0.251 | 0.054 | 0.582 | 0.271 | 0.005 | 0.142 | 0.144 | 0.111 | 0.259 |
| 22 | 0.083 | 0.393 | 0.042 | 0.665 | 0.099 | 0.308 | 0.021 | 0.829 | 0.001 | 0.990 | 0.006 | 0.949 | −0.065 | 0.504 | 0.043 | 0.662 | 0.048 | 0.627 | −0.026 | 0.794 |
| 23 | 0.043 | 0.662 | 0.139 | 0.154 | −0.112 | 0.252 | 0.012 | 0.905 | 0.010 | 0.921 | −0.059 | 0.545 | 0.138 | 0.155 | −0.028 | 0.772 | −0.022 | 0.818 | −0.009 | 0.924 |
| 24 | −0.096 | 0.325 | −0.044 | 0.653 | −0.086 | 0.381 | −0.003 | 0.972 | 0.043 | 0.662 | 0.024 | 0.808 | 0.091 | 0.351 | 0.047 | 0.631 | −0.024 | 0.807 | −0.020 | 0.841 |
| 25 | −0.041 | 0.676 | 0.018 | 0.857 | −0.019 | 0.85 | 0.063 | 0.522 | −0.014 | 0.883 | −0.071 | 0.468 | 0.243 | 0.012 | −0.091 | 0.351 | −0.118 | 0.226 | −0.058 | 0.556 |
| 26 | −0.063 | 0.518 | −0.049 | 0.613 | 0.031 | 0.752 | 0.104 | 0.286 | 0.023 | 0.811 | 0.031 | 0.753 | 0.009 | 0.923 | 0.019 | 0.845 | 0.145 | 0.137 | −0.119 | 0.224 |
| 27 | 0.213 | 0.027 | 0.071 | 0.469 | 0.10 | 0.303 | −0.040 | 0.686 | −0.112 | 0.251 | −0.089 | 0.359 | −0.118 | 0.225 | −0.128 | 0.189 | −0.006 | 0.949 | −0.011 | 0.913 |
| 28 | 0.019 | 0.844 | −0.100 | 0.305 | −0.019 | 0.849 | −0.090 | 0.356 | −0.043 | 0.658 | −0.051 | 0.602 | −0.11 | 0.259 | 0.072 | 0.460 | 0.009 | 0.930 | −0.104 | 0.290 |
| 31 | 0.179 | 0.066 | 0.024 | 0.807 | 0.079 | 0.419 | −0.019 | 0.843 | −0.172 | 0.076 | −0.031 | 0.749 | −0.055 | 0.575 | −0.230 | 0.017 | −0.191 | 0.049 | −0.107 | 0.277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Yang, M.-H.; Yao, Z.-F.; Tsai, M.-C.; Hsieh, S. The Mediating Role of Brain Structural Imaging Markers in Connecting Adverse Childhood Experiences and Psychological Resilience. Children 2023, 10, 365. https://doi.org/10.3390/children10020365
Chang Y-H, Yang M-H, Yao Z-F, Tsai M-C, Hsieh S. The Mediating Role of Brain Structural Imaging Markers in Connecting Adverse Childhood Experiences and Psychological Resilience. Children. 2023; 10(2):365. https://doi.org/10.3390/children10020365
Chicago/Turabian StyleChang, Yun-Hsuan, Meng-Heng Yang, Zai-Fu Yao, Meng-Che Tsai, and Shulan Hsieh. 2023. "The Mediating Role of Brain Structural Imaging Markers in Connecting Adverse Childhood Experiences and Psychological Resilience" Children 10, no. 2: 365. https://doi.org/10.3390/children10020365
APA StyleChang, Y.-H., Yang, M.-H., Yao, Z.-F., Tsai, M.-C., & Hsieh, S. (2023). The Mediating Role of Brain Structural Imaging Markers in Connecting Adverse Childhood Experiences and Psychological Resilience. Children, 10(2), 365. https://doi.org/10.3390/children10020365






