Body Adiposity Partially Mediates the Association between FTO rs9939609 and Lower Adiponectin Levels in Chilean Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Plasma Determinations
2.3. Anthropometric Measurements
2.4. Identification of Allelic Variants of FTO rs9939609 Polymorphism
2.5. Ethics
2.6. Statistical Analyses
3. Results
3.1. Sample Description
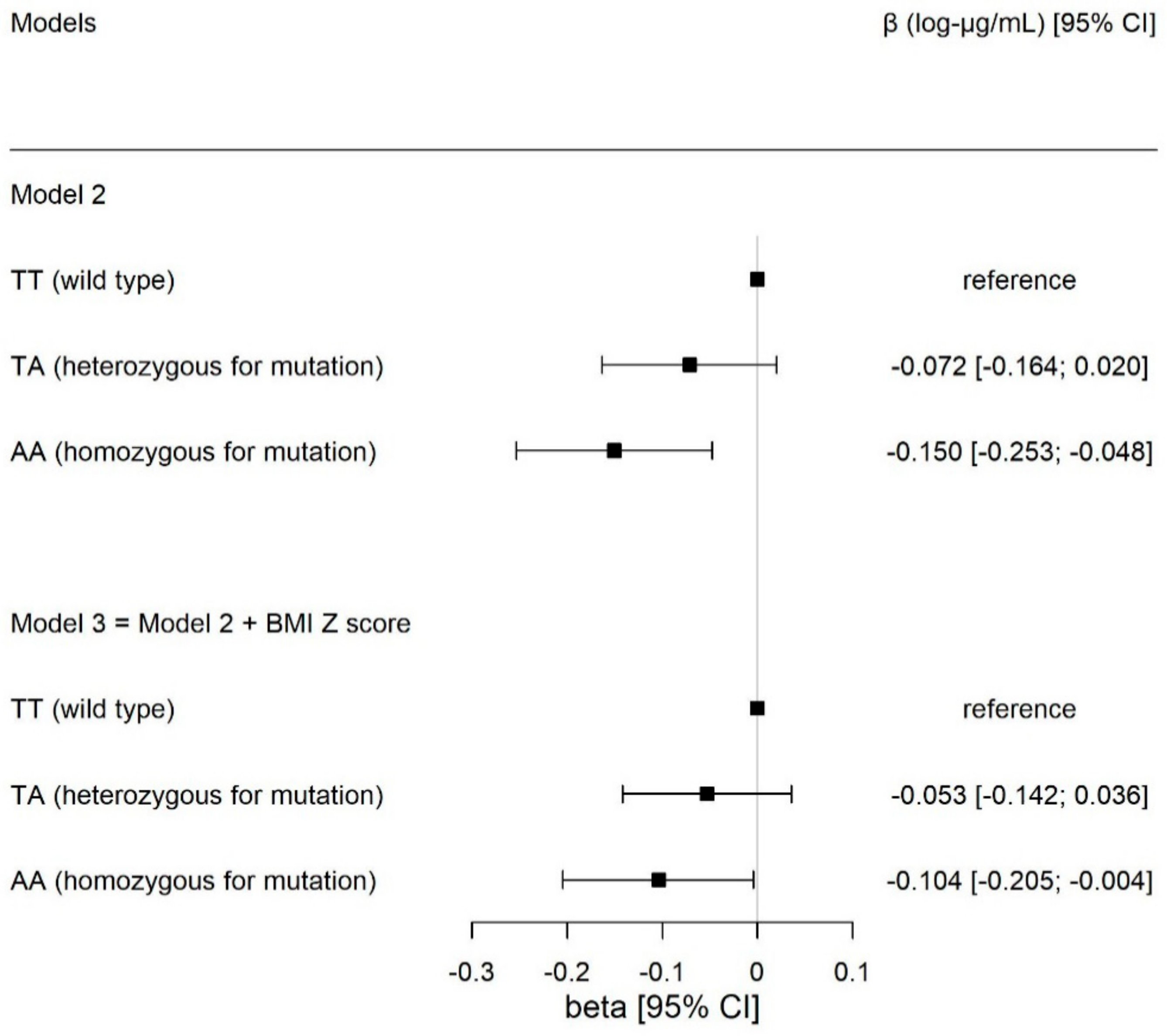
3.2. Associations between Circulating Adiponectin and FTO rs9939609
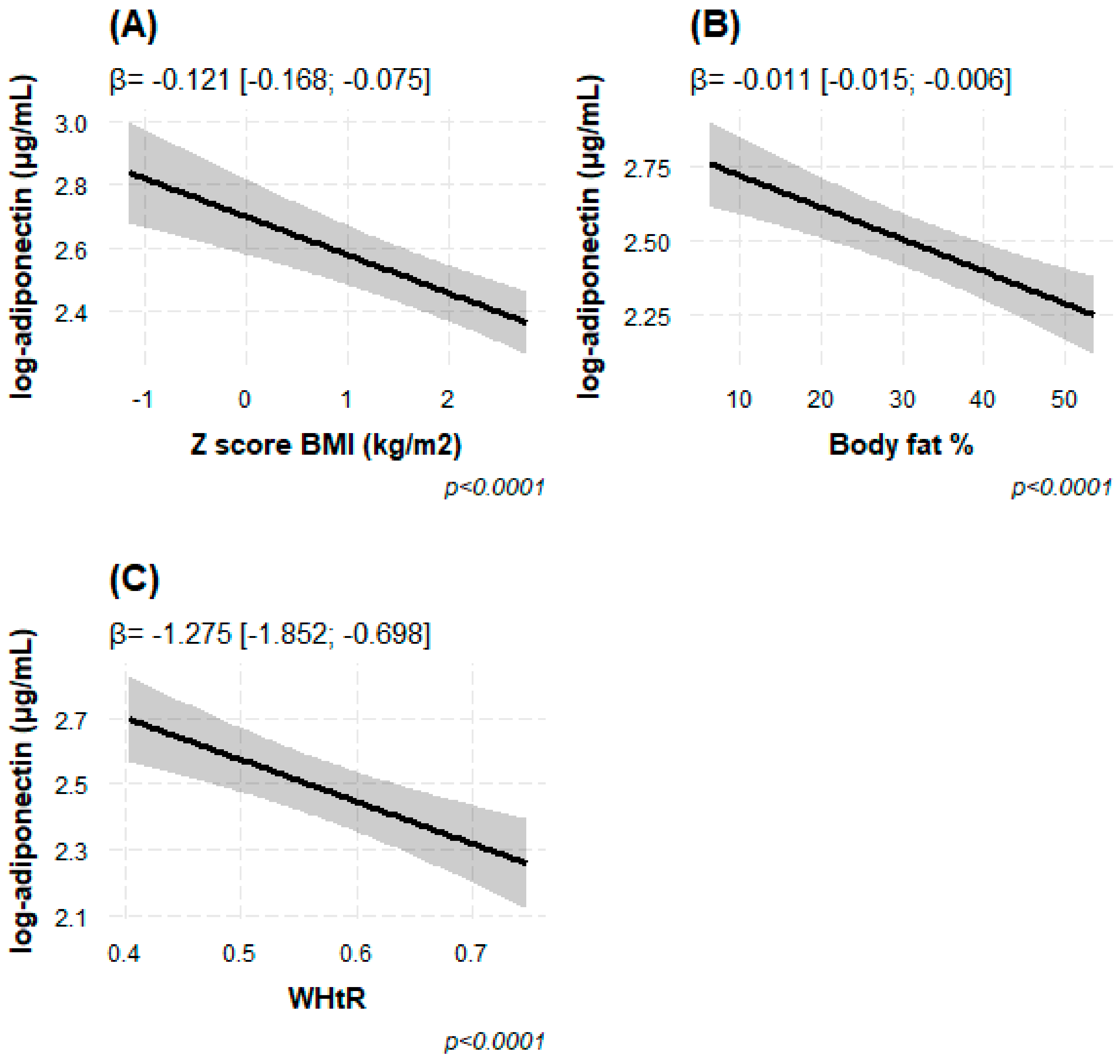
3.3. Link between Circulating Adiponectin and Body Adiposity
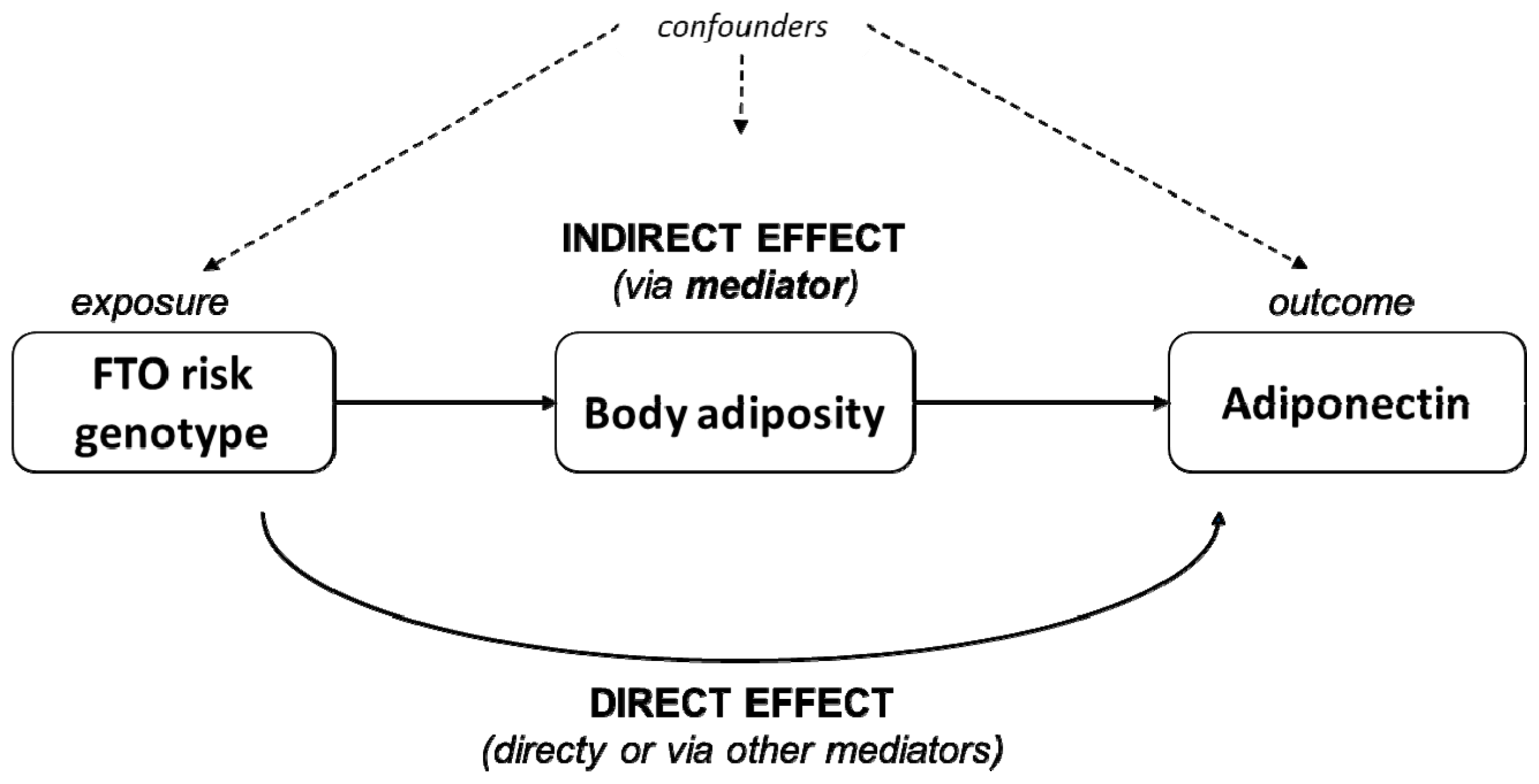
3.4. Body Adiposity as Partial Mediator of the Adiponectin–FTO rs9939609 Association
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, T.; Yang, W.; Chen, C.-S.; Reynolds, K.; He, J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Kain, J.; Uauy, R.; Lera, L.; Taibo, M.; Espejo, F.; Albala, C. Evolution of the nutritional status of six years old Chilean children (1987–2003). Rev. Med. Chil. 2005, 133, 1013–1020. [Google Scholar] [PubMed]
- Herrera, J.C.; Lira, M.; Kain, J. Socioeconomic vulnerability and obesity in Chilean schoolchildren attending first grade: Comparison between 2009 and 2013. Rev. Chil. Pediatr. 2017, 88, 736–743. [Google Scholar] [CrossRef] [PubMed]
- JUNAEB. Mapa Nutricional 2019; Ministerio de Educación, Gobierno de Chile: Santiago, Chile, 2020.
- Allman-Farinelli, M.A.; King, L.; Bauman, A.E. Overweight and obesity from childhood to adulthood: A follow-up of participants in the 1985 Australian Schools Health and Fitness Survey. Med. J. Aust. 2007, 187, 314–315. [Google Scholar] [CrossRef]
- Eckel, R.H. Obesity and heart disease: A statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation 1997, 96, 3248–3250. [Google Scholar] [CrossRef]
- Freedman, D.S.; Dietz, W.H.; Tang, R.; Mensah, G.; Bond, M.G.; Urbina, E.M.; Srinivasan, S.; Berenson, G.S. The relation of obesity throughout life to carotid intima-media thickness in adulthood: The Bogalusa Heart Study. Int. J. Obes. Relat. Metab. Disord 2004, 28, 159–166. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Roy, B.; Palaniyandi, S.S. Tissue-specific role and associated downstream signaling pathways of adiponectin. Cell Biosci. 2021, 11, 77. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Ohashi, K.; Ouchi, N.; Matsuzawa, Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie 2012, 94, 2137–2142. [Google Scholar] [CrossRef]
- Berner, H.S.; Lyngstadaas, S.P.; Spahr, A.; Monjo, M.; Thommesen, L.; Drevon, C.A.; Syversen, U.; Reseland, J.E. Adiponectin and its receptors are expressed in bone-forming cells. Bone 2004, 35, 842–849. [Google Scholar] [CrossRef]
- Chen, J.; Du, B. Novel positioning from obesity to cancer: FTO, an m(6)A RNA demethylase, regulates tumour progression. J. Cancer Res. Clin. Oncol. 2019, 145, 19–29. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Hawkins, M.; Combs, T.P.; Rajala, M.W.; Doebber, T.; Berger, J.P.; Wagner, J.A.; Wu, M.; Knopps, A.; Xiang, A.H.; et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 2004, 279, 12152–12162. [Google Scholar] [CrossRef]
- Hakim, O.; Bello, O.; Ladwa, M.; Peacock, J.L.; Umpleby, A.M.; Charles-Edwards, G.; Amiel, S.A.; Goff, L.M. The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity. Nutrients 2020, 12, 3796. [Google Scholar] [CrossRef]
- Yasuda, Y.; Miyake, N.; Matsuoka, H.; Sugihara, S. Adiponectin, ALT and family history as critical markers for the development of type 2 diabetes in obese Japanese children. Endocrinol. Diabetes Metab. 2021, 4, e00178. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The genetics of obesity: From discovery to biology. Nat. Rev. Genet. 2022, 23, 120–133. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The bigger picture of FTO: The first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.-F.; Zhao, Y.-L.; Yang, Y.-G. FTO and obesity: Mechanisms of association. Curr. Diab. Rep. 2014, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, N.; Villagrán, M.; Riffo, B.; Gleisner, A.; Petermann-Rocha, F.; Mardones, L.; Leiva, A.M.; Martínez-Sanguinetti, M.A.; Celis-Morales, C. Association between FTO gene rs9939609 and adiposity markers in Chilean children. Rev. Chil. Pediatr. 2020, 91, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Isgin-Atici, K.; Alsulami, S.; Turan-Demirci, B.; Surendran, S.; Sendur, S.N.; Lay, I.; Karabulut, E.; Ellahi, B.; Lovegrove, J.A.; Alikasifoglu, M.; et al. FTO gene-lifestyle interactions on serum adiponectin concentrations and central obesity in a Turkish population. Int. J. Food Sci. Nutr. 2021, 72, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Al-Serri, A.; Alroughani, R.; Al-Temaimi, R.A. The FTO gene polymorphism rs9939609 is associated with obesity and disability in multiple sclerosis patients. Sci. Rep. 2019, 9, 19071. [Google Scholar] [CrossRef]
- Andreasen, C.H.; Stender-Petersen, K.L.; Mogensen, M.S.; Torekov, S.S.; Wegner, L.; Andersen, G.; Nielsen, A.L.; Albrechtsen, A.; Borch-Johnsen, K.; Rasmussen, S.S.; et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008, 57, 95–101. [Google Scholar] [CrossRef]
- Fang, H.; Li, Y.; Du, S.; Hu, X.; Zhang, Q.; Liu, A.; Ma, G. Variant rs9939609 in the FTO gene is associated with body mass index among Chinese children. BMC Med. Genet. 2010, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Mittal, B.; Srivastava, A.; Awasthi, S.; Srivastava, N. Association of FTO rs9939609 SNP with Obesity and Obesity- Associated Phenotypes in a North Indian Population. Oman Med. J. 2016, 31, 99–106. [Google Scholar] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.D.; Islam, M.; Hydrie, M.Z.I.; Chaudhary, B.; Bellary, S.; Hashmi, S.; O’Hare, J.P.; Kumar, S.; Sanghera, D.K.; Chaturvedi, N.; et al. An FTO variant is associated with Type 2 diabetes in South Asian populations after accounting for body mass index and waist circumference. Diabet. Med. 2011, 28, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mehrdad, M.; Doaei, S.; Gholamalizadeh, M.; Fardaei, M.; Fararouei, M.; Eftekhari, M.H. Association of FTO rs9939609 polymorphism with serum leptin, insulin, adiponectin, and lipid profile in overweight adults. Adipocyte 2020, 9, 51–56. [Google Scholar] [CrossRef]
- Qi, L.; Kang, K.; Zhang, C.; van Dam, R.M.; Kraft, P.; Hunter, D.; Lee, C.H.; Hu, F.B. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: Longitudinal analyses in two cohort studies and functional test. Diabetes 2008, 57, 3145–3151. [Google Scholar] [CrossRef]
- Callaway, C.; Chumlea, W.; Bouchard, C.; Himes, J.; Lohman, T.; Martin, A.; Mitchell, C.D.; Mueller, W.H. Circumferences. In Anthropometric Standardization Reference Manual; Lohman, T.G., Roche, A., Martorell, R., Eds.; Human Kinetics Books: Champaign, IL, USA, 1991; pp. 44–45. [Google Scholar]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child. 1976, 51, 170–179. [Google Scholar] [CrossRef]
- WHO. Growth Reference Data for 5–19 Years. 2006. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years (accessed on 22 April 2021).
- Riffo, B.; Asenjo, S.; Sáez, K.; Aguayo, C.; Muñoz, I.; Bustos, P.; Celis-Morales, C.; Lagos, J.; Sapunar, J.; Ulloa, N. FTO gene is related to obesity in Chilean Amerindian children and impairs HOMA-IR in prepubertal girls. Pediatr. Diabetes 2012, 13, 384–391. [Google Scholar] [CrossRef]
- López-Bermejo, A.; Petry, C.J.; Díaz, M.; Sebastiani, G.; de Zegher, F.; Dunger, D.B.; Ibáñez, L. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J. Clin. Endocrinol. Metab. 2008, 93, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Erali, M.; Voelkerding, K.V.; Wittwer, C.T. High resolution melting applications for clinical laboratory medicine. Exp. Mol. Pathol. 2008, 85, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Keele, L.; Imai, K. Mediation: R Package for Causal Mediation Analysis. J. Stat. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar]
- Elghazy, A.M.; Elsaeid, A.M.; Refaat, M.; Youssef, M.M. Biochemical studies of adiponectin gene polymorphism in patients with obesity in Egyptians. Arch. Physiol. Biochem. 2022, 128, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Kim, J.W.; Son, Y.-J.; Ma, M.J.; Um, J.-H.; Kim, N.D.; Min, S.H.; Kim, D.I.; Kim, B.B. Discovery of a novel potent peptide agonist to adiponectin receptor 1. PLoS ONE 2018, 13, e0199256. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. The ‘Fat Mass and Obesity Related’ (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance. Curr. Obes. Rep. 2015, 4, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Ben Halima, M.; Kallel, A.; Baara, A.; Ben Wafi, S.; Sanhagi, H.; Slimane, H.; Jemaa, R.; Feki, M. The rs9939609 polymorphism in the fat mass and obesity associated (FTO) gene is associated with obesity in Tunisian population. Biomarkers 2018, 23, 787–792. [Google Scholar] [CrossRef]
- Duicu, C.; Mărginean, C.O.; Voidăzan, S.; Tripon, F.; Bănescu, C. FTO rs 9939609 SNP Is Associated With Adiponectin and Leptin Levels and the Risk of Obesity in a Cohort of Romanian Children Population. Medicine 2016, 95, e3709. [Google Scholar] [CrossRef]
- Dorling, J.L.; Belsky, D.W.; Racette, S.B.; Das, S.K.; Ravussin, E.; Redman, L.M.; Höchsmann, C.; Huffman, K.M.; Kraus, W.E.; Kobor, M.S.; et al. Association between the FTO rs9939609 single nucleotide polymorphism and dietary adherence during a 2-year caloric restriction intervention: Exploratory analyses from CALERIE phase 2. Exp. Gerontol. 2021, 155, 111555. [Google Scholar] [CrossRef]
- de Luis, D.A.; Aller, R.; Izaola, O.; de la Fuente, B.; Conde, R.; Sagrado, M.G.; Primo, D. Evaluation of weight loss and adipocytokines levels after two hypocaloric diets with different macronutrient distribution in obese subjects with rs9939609 gene variant. Diabetes/Metab. Res. Rev. 2012, 28, 663–668. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, T.; Zhang, X.; Rood, J.; Bray, G.A.; Sacks, F.M.; Qi, L. Dietary Fat Modifies the Effects of FTO Genotype on Changes in Insulin Sensitivity. J. Nutr. 2015, 145, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Cannon, C.P.; White, W.B.; Jarolim, P.; Liu, Y.; Bonaca, M.P.; Zannad, F.; Morrow, D.A. Baseline adiponectin concentration and clinical outcomes among patients with diabetes and recent acute coronary syndrome in the EXAMINE trial. Diabetes Obes. Metab. 2017, 19, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.W.; Lee, W.Y.; Rhee, E.J.; Baek, K.H.; Yoon, K.H.; Kang, M.I.; Yun, E.J.; Park, C.Y.; Ihm, S.H.; Choi, M.G.; et al. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle-aged men. Clin. Endocrinol. 2005, 63, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Krauel, M.; Leal-Witt, M.J.; Osorio-Conles, Ó.; Amat-Bou, M.; Lerin, C.; Selva, D.M. Relationship between adiponectin, TNFα, and SHBG in prepubertal children with obesity. Mol. Cell Pediatr. 2021, 8, 3. [Google Scholar] [CrossRef]
- Weiss, R.; Dufour, S.; Groszmann, A.; Petersen, K.; Dziura, J.; Taksali, S.E.; Shulman, G.; Caprio, S. Low adiponectin levels in adolescent obesity: A marker of increased intramyocellular lipid accumulation. J. Clin. Endocrinol. Metab. 2003, 88, 2014–2018. [Google Scholar] [CrossRef]
- Molina-Luque, R.; Ulloa, N.; Romero-Saldaña, M.; Zilic, M.; Gleisner, A.; Lanuza, F.; Molina-Recio, G. Association between the FTO SNP rs9939609 and Metabolic Syndrome in Chilean Children. Nutrients 2021, 13, 2014. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1321. [Google Scholar] [CrossRef]
- Evans, C.; Curtis, J.; Antonio, J. FTO and Anthropometrics: The Role of Modifiable Factors. J. Funct. Morphol. Kinesiol. 2022, 7, 90. [Google Scholar] [CrossRef]
- Goodarzi, G.; Hosseini-Esfahani, F.; Ataie-Jafari, A.; Haji-Hosseini-Gazestani, N.; Daneshpour, M.S.; Keshavarz, S.-A.; Mirmiran, P. Dietary diversity modifies the association between FTO polymorphisms and obesity phenotypes. Int. J. Food Sci. Nutr. 2021, 72, 997–1007. [Google Scholar] [CrossRef]
- Doaei, S.; Kalantari, N.; Izadi, P.; Salonurmi, T.; Jarrahi, A.M.; Rafieifar, S.; Tabesh, G.A.; Rahimzadeh, G.; Gholamalizadeh, M.; Goodarzi, M.O. Interactions between macro-nutrients’ intake, FTO and IRX3 gene expression, and FTO genotype in obese and overweight male adolescents. Adipocyte 2019, 8, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Noormohammadi, M.; Ghorbani, Z.; Löber, U.; Mahdavi-Roshan, M.; Bartolomaeus, T.U.; Kazemi, A.; Shoaibinobarian, N.; Forslund, S.K. The effect of probiotic and synbiotic supplementation on appetite-regulating hormones and desire to eat: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2023, 187, 106614. [Google Scholar] [CrossRef]
- Sánchez-Rosales, A.I.; Guadarrama-López, A.L.; Gaona-Valle, L.S.; Martínez-Carrillo, B.E.; Valdés-Ramos, R. The Effect of Dietary Patterns on Inflammatory Biomarkers in Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 4577. [Google Scholar] [CrossRef] [PubMed]
- García-Hermoso, A.; Ramírez-Vélez, R.; Díez, J.; González, A.; Izquierdo, M. Exercise training-induced changes in exerkine concentrations may be relevant to the metabolic control of type 2 diabetes mellitus patients: A systematic review and meta-analysis of randomized controlled trials. J. Sport Health Sci. 2022; in press. [Google Scholar]
- Drolet, R.; Bélanger, C.; Fortier, M.; Huot, C.; Mailloux, J.; Légaré, D.; Tchernof, A. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity 2009, 17, 424–430. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall Study Population | TT | TA | AA |
|---|---|---|---|---|
| Total (n) | 323 | 167 | 91 | 65 |
| Age, years, mean (SD) | 8.83 (1.28) | 8.82 (1.29) | 8.59 (1.22) | 8.91 (1.32) |
| Females, n (%) | 164 (50.8) | 88 (52.7) | 43 (47.3) | 33 (50.8) |
| Tanner’s stage | ||||
| Pre-pubertal, n (%) | 250 (77.4) | 127 (76.0) | 75 (82.4) | 48 (73.8) |
| Pubertal, n (%) | 73 (22.6) | 40 (24.0) | 16 (17.6) | 17 (26.2) |
| Height, cm, median [IQR] | 133 [126, 140] | 133 [126, 140] | 132 [127, 139] | 137 [128, 143] |
| Weight, kg, median [IQR] | 37.9 [30.9, 47.4] | 37.2 [29.8, 45.5] | 38.5 [32.5, 44.7] | 43.8 [34.1, 52.8] |
| Waist circumference, cm, median [IQR] | 73.5 [64.6, 81.0] | 70.8 [63.8, 80.3] | 74.0 [66.0, 80.6] | 77.0 [70.0, 83.3] |
| Body mass index, kg/m2 median [IQR] | 22.2 [17.9, 24.5] | 21.3 [17.6, 23.9] | 22.3 [17.7, 24.2] | 23.8 [20.5, 26.1] |
| z-score BMI, median [IQR] | 1.83 [0.69, 2.12] | 1.78 [0.62, 1.99] | 1.86 [0.64, 2.17] | 2.01 [1.75, 2.23] |
| Waist circumference to height ratio, median [IQR] | 0.56 [0.49, 0.60] | 0.55 [0.48, 0.59] | 0.57 [0.49, 0.60] | 0.57 [0.53, 0.61] |
| Body fat mass, %, mean [IQR] | 30.2 [21.6, 36.2] | 28.4 [21.3, 34.9] | 29.5 [21.3, 36.1] | 34.8 [24.8, 37.8] |
| Adiponectin, µg/mL, median [IQR] | 14.2 [10.58, 17.12] | 14.2 [10.9, 18.4] | 13.0 [10.6, 16.8] | 12.4 [9.7, 15.3] |
| Model 1 | Model 2 | Model 3 a,b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Independent Variables | β | 95% CI | p | β | 95% CI | p | Β | 95% CI | p |
| FTO genotypes a | |||||||||
| TT (wild type) | Ref. | Ref. | Ref. | ||||||
| TA | −0.069 | (−0.161; 0.022) | 0.137 | −0.072 | (−0.164; 0.020) | 0.127 | −0.053 | (−0.142; 0.036) | 0.243 |
| AA (mutant) | −0.150 | (−0.253; −0.048) | 0.004 | −0.150 | (−0.253; −0.048) | 0.004 | −0.104 | (−0.205; −0.004) | 0.041 |
| Additive | −0.074 | (−0.124; −0.025) | 0.003 | −0.075 | (−0.124; −0.025) | 0.003 | −0.052 | (−0.101; −0.004) | 0.035 |
| Adiposity phenotypes b,c | |||||||||
| BMI Z score | −0.130 | (−0.176; −0.085) | 4.63 × 10−8 | −0.130 | (−0.176; −0.084) | 5.10 × 10−8 | −0.121 | (−1.677; −0.075) | 4.50 × 10−7 |
| WHtR | −1.393 | (−1.957; −0.828) | 1.90 × 10−6 | −1.386 | (−1.959; −0.814) | 2.91 × 10−6 | −1.275 | (−1.852; −0.698) | 1.85 × 10−5 |
| BF% | −0.011 | (−0.015; −0.007) | 2.66 × 10−7 | −0.012 | (−0.016; −0.007) | 2.38 × 10−7 | −0.011 | (−0.015; −0.006) | 2.00 × 10−6 |
| Potential | Average Mediation Effect | Total Effect | Proportion Mediated | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mediators | β | 95% CI | p | β | 95% CI | p | % | 95% CI | p |
| BMI Z-score | −0.022 | (−0.041; −0.010) | 0.001 | −0.074 | (−0.125; −0.030) | 0.002 | 29.8 | (10.4; 79.0) | 0.003 |
| WHtR | −0.018 | (−0.035; 0.000) | 0.003 | −0.074 | (−0.125; −0.030) | 0.003 | 23.9 | (6.8; 68.0) | 0.006 |
| BF% | −0.022 | (−0.040; −0.010) | 0.003 | −0.074 | (−0.125; −0.030) | 0.002 | 28.6 | (9.3; 77.0) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochoa-Rosales, C.; Mardones, L.; Villagrán, M.; Aguayo, C.; Martorell, M.; Celis-Morales, C.; Ulloa, N. Body Adiposity Partially Mediates the Association between FTO rs9939609 and Lower Adiponectin Levels in Chilean Children. Children 2023, 10, 426. https://doi.org/10.3390/children10030426
Ochoa-Rosales C, Mardones L, Villagrán M, Aguayo C, Martorell M, Celis-Morales C, Ulloa N. Body Adiposity Partially Mediates the Association between FTO rs9939609 and Lower Adiponectin Levels in Chilean Children. Children. 2023; 10(3):426. https://doi.org/10.3390/children10030426
Chicago/Turabian StyleOchoa-Rosales, Carolina, Lorena Mardones, Marcelo Villagrán, Claudio Aguayo, Miquel Martorell, Carlos Celis-Morales, and Natalia Ulloa. 2023. "Body Adiposity Partially Mediates the Association between FTO rs9939609 and Lower Adiponectin Levels in Chilean Children" Children 10, no. 3: 426. https://doi.org/10.3390/children10030426
APA StyleOchoa-Rosales, C., Mardones, L., Villagrán, M., Aguayo, C., Martorell, M., Celis-Morales, C., & Ulloa, N. (2023). Body Adiposity Partially Mediates the Association between FTO rs9939609 and Lower Adiponectin Levels in Chilean Children. Children, 10(3), 426. https://doi.org/10.3390/children10030426








