Introduction of a Novel Sequential Approach to the Ponte Osteotomy to Minimize Spinal Canal Exposure
Abstract
:1. Introduction
2. Materials and Methods
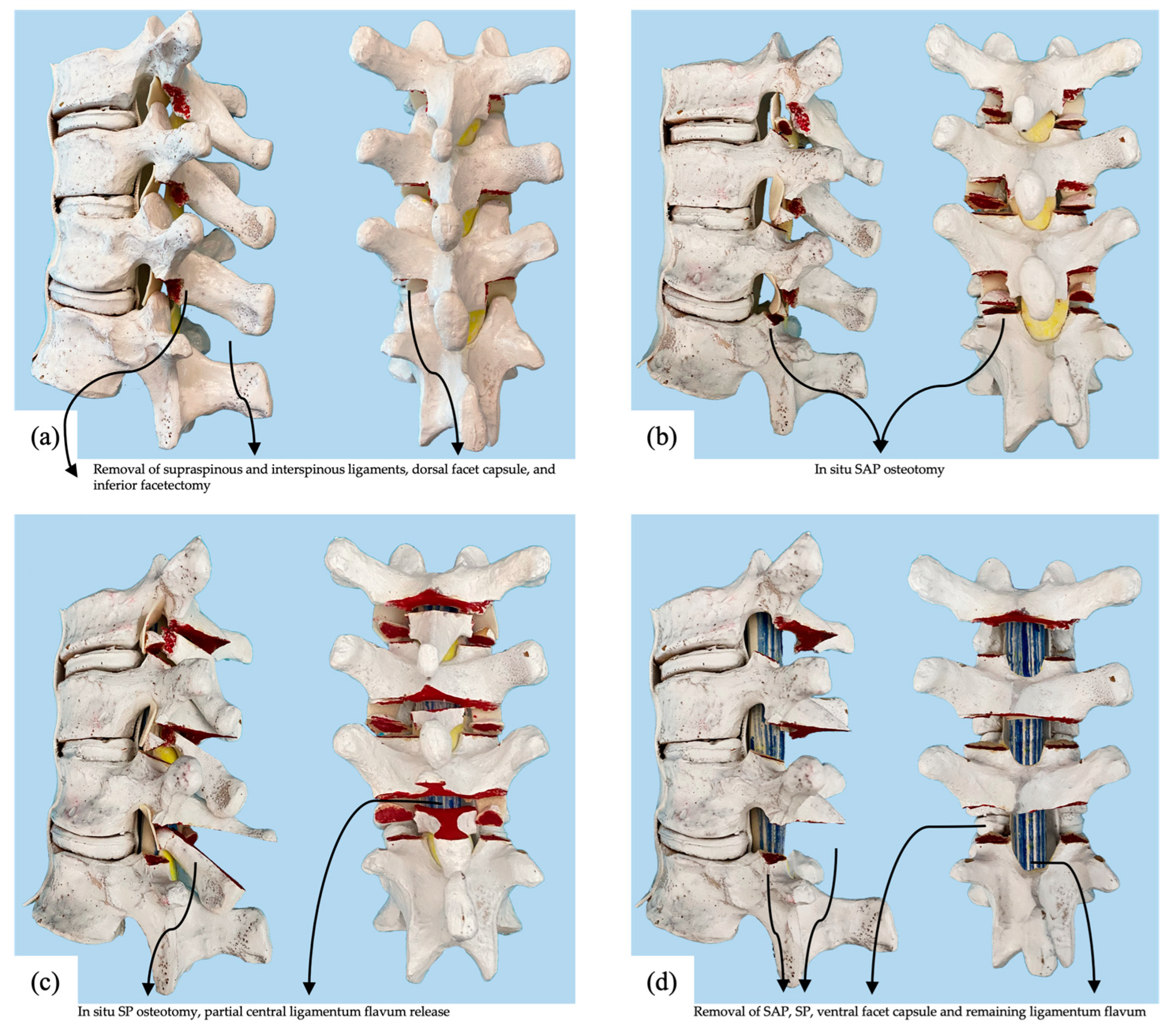
2.1. Surgical Procedure
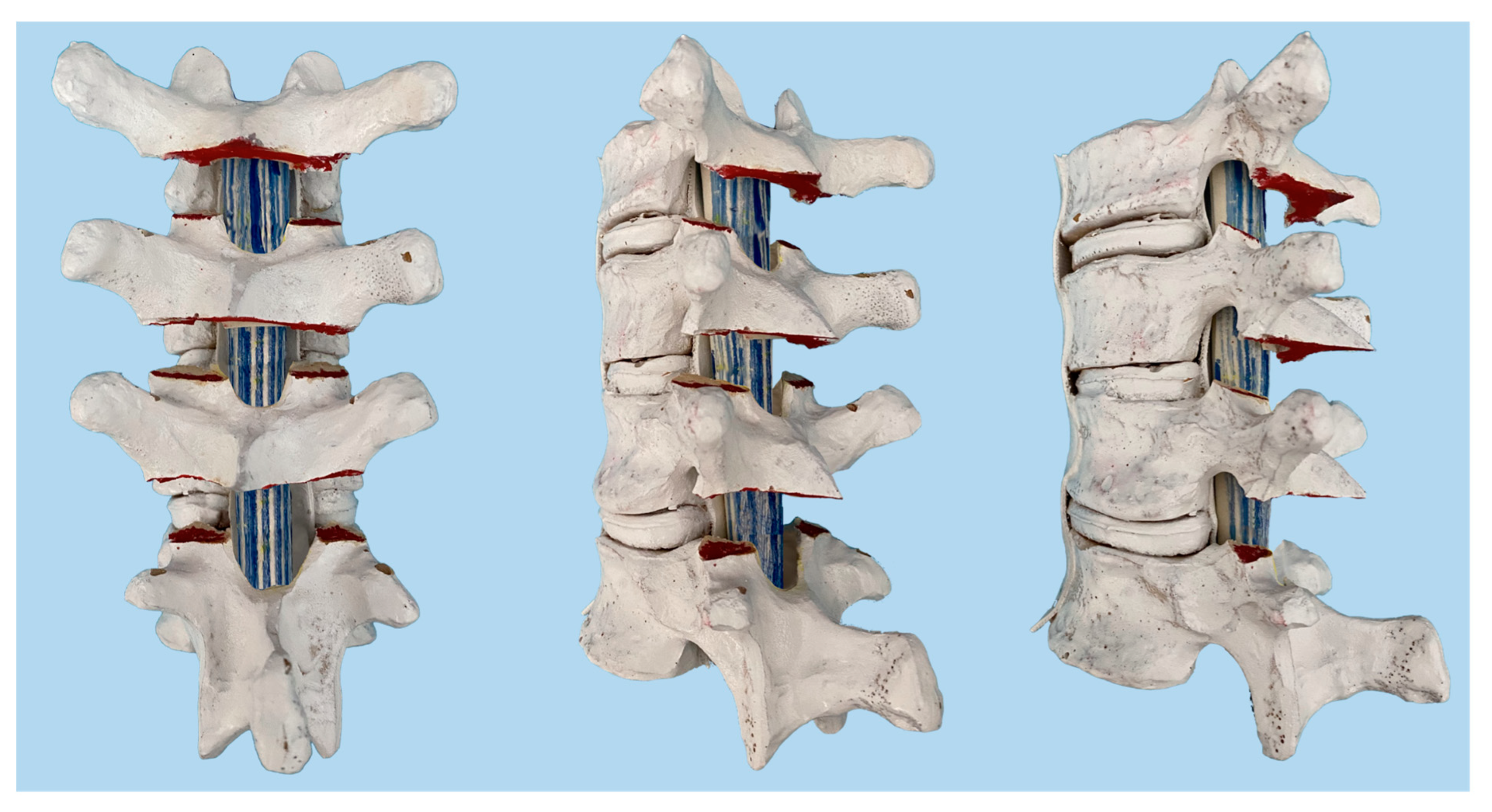
2.2. Specimen Preparation
2.3. Biomechanical Testing and Analysis
3. Results
3.1. Average of the Entire Single Specimen Spine T1–T12
3.2. Upper Thoracic Segment T1–T4 from the Single Thoracic Specimen
3.3. Middle Thoracic Segment T5–T8 from the Single Thoracic Specimen
3.4. Lower Thoracic Segment T9–T12 from the Single Thoracic Specimen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schlösser, T.P.; van Stralen, M.; Brink, R.C.; Chu, W.C.; Lam, T.-P.; Vincken, K.L.; Castelein, R.M.; Cheng, J.C. Three-dimensional characterization of torsion and asymmetry of the intervertebral discs versus vertebral bodies in adolescent idiopathic scoliosis. Spine 2014, 39, E1159–E1166. [Google Scholar] [CrossRef] [PubMed]
- Asher, M.A.; Burton, D.C. Adolescent idiopathic scoliosis: Natural history and long term treatment effects. Scoliosis 2006, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parent, S.; Newton, P.O.; Wenger, D.R. Adolescent idiopathic scoliosis: Etiology, anatomy, natural history, and bracing. Instr. Course Lect. 2005, 54, 529–536. [Google Scholar]
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.; Danielsson, A.; Morcuende, J.A. Adolescent idiopathic scoliosis. Lancet 2008, 371, 1527–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettany-Saltikov, J.; Weiss, H.R.; Chockalingam, N.; Taranu, R.; Srinivas, S.; Hogg, J.; Whittaker, V.; Kalyan, R.V.; Arnell, T. Surgical versus non-surgical interventions in people with adolescent idiopathic scoliosis. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, M.G.; Franzone, J.M.; Vitale, M.G. The role of posterior spinal osteotomies in pediatric spinal deformity surgery: Indications and operative technique. J. Pediatr. Orthop. 2011, 31, S88–S98. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Samdani, A.F.; Marks, M.; Bastrom, T.; Garg, H.; Lonner, B.; Bennett, J.T.; Pahys, J.; Shah, S.; Miyanji, F. Five-year clinical and radiographic outcomes using pedicle screw only constructs in the treatment of adolescent idiopathic scoliosis. Eur. Spine J. 2013, 22, 1292–1299. [Google Scholar] [CrossRef] [Green Version]
- Burton, D.C.; Sama, A.A.; Asher, M.A.; Burke, S.W.; Boachie-Adjei, O.; Huang, R.C.; Green, D.W.; Rawlins, B.A. The treatment of large (>70°) thoracic idiopathic scoliosis curves with posterior instrumentation and arthrodesis: When is anterior release indicated? Spine 2005, 30, 1979–1984. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lenke, L.G.; Kim, J.; Bridwell, K.H.; Cho, S.K.; Cheh, G.; Sides, B. Comparative analysis of pedicle screw versus hybrid instrumentation in posterior spinal fusion of adolescent idiopathic scoliosis. Spine 2006, 31, 291–298. [Google Scholar] [CrossRef]
- Gottlich, C.; Sponseller, P.D. Ponte osteotomy in pediatric spine surgery. JBJS Essent. Surg. Tech. 2020, 10. [Google Scholar] [CrossRef]
- Halanski, M.A.; Cassidy, J.A. Do multilevel Ponte osteotomies in thoracic idiopathic scoliosis surgery improve curve correction and restore thoracic kyphosis? Clin. Spine Surg. 2013, 26, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Pizones, J.; Sánchez-Mariscal, F.; Zúñiga, L.; Izquierdo, E. Ponte osteotomies to treat major thoracic adolescent idiopathic scoliosis curves allow more effective corrective maneuvers. Eur. Spine J. 2015, 24, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Samdani, A.F.; Bennett, J.T.; Singla, A.R.; Marks, M.C.; Pahys, J.M.; Lonner, B.S.; Miyanji, F.; Shah, S.A.; Shufflebarger, H.L.; Newton, P.O. Do Ponte Osteotomies Enhance Correction in Adolescent Idiopathic Scoliosis? An Analysis of 191 Lenke 1A and IB Curves. Spine Deform. 2015, 3, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Dhawale, A.A.; Oda, J.E.; Yorgova, P.; Neiss, G.I.; Holmes, L.; Gabos, P.G. Ponte osteotomies with pedicle screw instrumentation in the treatment of adolescent idiopathic scoliosis. Spine Deform. 2013, 1, 196–204. [Google Scholar] [CrossRef]
- Shufflebarger, H.L.; Geck, M.J.; Clark, C.E. The posterior approach for lumbar and thoracolumbar adolescent idiopathic scoliosis: Posterior shortening and pedicle screws. Spine 2004, 29, 269–276. [Google Scholar] [CrossRef]
- Geck, M.J.; Macagno, A.; Ponte, A.; Shufflebarger, H.L. The Ponte procedure: Posterior only treatment of Scheuermann’s kyphosis using segmental posterior shortening and pedicle screw instrumentation. Clin. Spine Surg. 2007, 20, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Ponte, A.; Orlando, G.; Siccardi, G.L. The true Ponte osteotomy: By the one who developed it. Spine Deform. 2018, 6, 2–11. [Google Scholar] [CrossRef]
- Buckland, A.J.; Moon, J.Y.; Betz, R.R.; Lonner, B.S.; Newton, P.O.; Shufflebarger, H.L.; Errico, T.J.; Group, H.S. Ponte Osteotomies Increase the Risk of Neuromonitoring Alerts in Adolescent Idiopathic Scoliosis Correction Surgery. Spine 2019, 44, E175–E180. [Google Scholar] [CrossRef]
- Harfouch, E.B.; Bunyan, R.F.; Al Faraidy, M.; Alnemari, H.H.; Bashir, S. Ponte osteotomies increase risk of intraoperative neuromonitoring alerts in adolescent idiopathic scoliosis surgery. Surg. Neurol. Int. 2022, 13. [Google Scholar] [CrossRef]
- Floccari, L.V.; Poppino, K.; Greenhill, D.A.; Sucato, D.J. Ponte osteotomies in a matched series of large AIS curves increase surgical risk without improving outcomes. Spine Deform. 2021, 9, 1411–1418. [Google Scholar] [CrossRef]
- Koerner, J.D.; Patel, A.; Zhao, C.; Schoenberg, C.; Mishra, A.; Vives, M.J.; Sabharwal, S. Blood loss during posterior spinal fusion for adolescent idiopathic scoliosis. Spine 2014, 39, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Siegler, S.; Allard, P.; Kirtley, C.; Leardini, A.; Rosenbaum, D.; Whittle, M.; D’Lima, D.D.; Cristofolini, L.; Witte, H.; et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion--part I: Ankle, hip, and spine. International Society of Biomechanics. J. Biomech. 2002, 35, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Mageswaran, P.; Techy, F.; Colbrunn, R.W.; Bonner, T.F.; McLain, R.F. Hybrid dynamic stabilization: A biomechanical assessment of adjacent and supraadjacent levels of the lumbar spine. J. Neurosurg. Spine 2012, 17, 232–242. [Google Scholar] [CrossRef]
- Holewijn, R.M.; Schlösser, T.P.; Bisschop, A.; Van Der Veen, A.J.; Stadhouder, A.; Van Royen, B.J.; Castelein, R.M.; De Kleuver, M. How does spinal release and ponte osteotomy improve spinal flexibility? The law of diminishing returns. Spine Deform. 2015, 3, 489–495. [Google Scholar] [CrossRef]
- Sangiorgio, S.N.; Borkowski, S.L.; Bowen, R.E.; Scaduto, A.A.; Frost, N.L.; Ebramzadeh, E. Quantification of increase in three-dimensional spine flexibility following sequential Ponte osteotomies in a cadaveric model. Spine Deform. 2013, 1, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bell, K.; McClincy, M.; Jacobs, L.; Dede, O.; Roach, J.; Bosch, P. Biomechanical comparison of ponte osteotomy and discectomy. Spine 2015, 40, E141–E145. [Google Scholar] [CrossRef]
- Borkowski, S.L.; Sangiorgio, S.N.; Bowen, R.E.; Scaduto, A.A.; Kwak, J.; Ebramzadeh, E. Flexibility of thoracic spines under simultaneous multi-planar loading. Eur. Spine J. 2017, 26, 173–180. [Google Scholar] [CrossRef]
- Veldhuizen, A.; Wever, D.; Webb, P. The aetiology of idiopathic scoliosis: Biomechanical and neuromuscular factors. Eur. Spine J. 2000, 9, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Wiemann, J.; Durrani, S.; Bosch, P. The effect of posterior spinal releases on axial correction torque: A cadaver study. J. Child. Orthop. 2011, 5, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Cheng, I.; Hay, D.; Iezza, A.; Lindsey, D.; Lenke, L.G. Biomechanical analysis of derotation of the thoracic spine using pedicle screws. Spine 2010, 35, 1039–1043. [Google Scholar] [CrossRef]
- Mannen, E.M.; Arnold, P.M.; Anderson, J.T.; Friis, E.A. Influence of sequential Ponte osteotomies on the human thoracic spine with a rib cage. Spine Deform. 2017, 5, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Oda, I.; Abumi, K.; Cunningham, B.W.; Kaneda, K.; McAfee, P.C. An in vitro human cadaveric study investigating the biomechanical properties of the thoracic spine. Spine 2002, 27, E64–E70. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M.; Hausfeld, J.N.; White, A.A. A biomechanical study of the ligamentous stability of the thoracic spine in man. Acta Orthop. Scand. 1981, 52, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Ghaednia, H.; Fourman, M.S.; Lans, A.; Detels, K.; Dijkstra, H.; Lloyd, S.; Sweeney, A.; Oosterhoff, J.H.; Schwab, J.H. Augmented and virtual reality in spine surgery, current applications and future potentials. Spine J. 2021, 21, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Koltsov, J.C.; Kwong, J.W.; Cheng, I.; Vorhies, J.S. Does navigation make spinal fusion for adolescent idiopathic scoliosis safer? Insights from a national database. Spine 2021, 46, E1049–E1057. [Google Scholar] [CrossRef]
- Kosterhon, M.; Gutenberg, A.; Kantelhardt, S.R.; Archavlis, E.; Giese, A. Navigation and image injection for control of bone removal and osteotomy planes in spine surgery. Oper. Neurosurg. 2017, 13, 297–304. [Google Scholar] [CrossRef] [PubMed]





| T1–T12 | SAP Alone (O1) | SAP + SP (O2) | SAP +SP + PC + LF (O3) |
|---|---|---|---|
| Flexion-Extension | 3.5 ± 2.6 | 15.0 ± 2.0 | 19.6 ± 4.4 |
| Lateral Bending | 7.6 ± 5.9 | 18.3 ± 17.1 | 28.4 ± 31.0 |
| Axial Rotation | 7.2 ± 3.0 | 10.3 ± 3.9 | 12.2 ± 5.3 |
| SAP Alone (O1) | SAP + SP (O2) | SAP + SP + PC + LF (O3) | ||
|---|---|---|---|---|
| T1–T4 | Flexion-Extension | 4.7 | 16.3 | 23.3 |
| Lateral Bending | 6.7 | 10.3 | 13.3 | |
| Axial Rotation | 7.4 | 12.7 | 16.6 | |
| T5–T8 | Flexion-Extension | 0.5 | 16 | 20.9 |
| Lateral Bending | 14 | 38 | 64 | |
| Axial Rotation | 11.1 | 12.4 | 13.7 | |
| T9–T12 | Flexion-Extension | 5.3 | 12.6 | 14.7 |
| Lateral Bending | 2.2 | 6.7 | 7.8 | |
| Axial Rotation | 3.2 | 5.8 | 6.3 |
| Baseline | SAP Alone (O1) | SAP + SP (O2) | SAP + SP + PC + LF (O3) | ||
|---|---|---|---|---|---|
| T1–T4 | Flexion-Extension | 12.9 | 13.5 | 15 | 15.9 |
| Lateral Bending | 16.5 | 17.6 | 18.2 | 18.7 | |
| Axial Rotation | 33.8 | 36.3 | 38.1 | 39.4 | |
| T5–T8 | Flexion-Extension | 18.7 | 18.8 | 21.7 | 22.6 |
| Lateral Bending | 5 | 5.7 | 6.9 | 8.2 | |
| Axial Rotation | 23.4 | 26 | 26.3 | 26.6 | |
| T9–T12 | Flexion-Extension | 9.5 | 10 | 10.7 | 10.9 |
| Lateral Bending | 9 | 9.2 | 9.6 | 9.7 | |
| Axial Rotation | 19 | 19.6 | 20.1 | 20.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollyer, I.; Johnson, T.R.; Kha, S.T.; Foreman, C.; Ho, V.; Klemt, C.; Chan, C.K.; Vorhies, J.S. Introduction of a Novel Sequential Approach to the Ponte Osteotomy to Minimize Spinal Canal Exposure. Children 2023, 10, 470. https://doi.org/10.3390/children10030470
Hollyer I, Johnson TR, Kha ST, Foreman C, Ho V, Klemt C, Chan CK, Vorhies JS. Introduction of a Novel Sequential Approach to the Ponte Osteotomy to Minimize Spinal Canal Exposure. Children. 2023; 10(3):470. https://doi.org/10.3390/children10030470
Chicago/Turabian StyleHollyer, Ian, Taylor Renee Johnson, Stephanie Tieu Kha, Cameron Foreman, Vivian Ho, Christian Klemt, Calvin K. Chan, and John Schoeneman Vorhies. 2023. "Introduction of a Novel Sequential Approach to the Ponte Osteotomy to Minimize Spinal Canal Exposure" Children 10, no. 3: 470. https://doi.org/10.3390/children10030470





