Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside
Abstract
1. Introduction
- Respiratory management of the preterm infant in the delivery room;
- Noninvasive respiratory support of the preterm infants in the neonatal intensive care unit;
- Mechanical ventilation of the preterm infants in the neonatal intensive care unit;
- Exogenous surfactant therapy in preterm infants.
2. Methods
3. Respiratory Management of the Preterm Infant in the Delivery Room
3.1. Ventilation in the Delivery Room
3.2. Oxygen Supplementation in the Delivery Room
4. Noninvasive Respiratory Support of the Preterm Infants in the NICU
4.1. Nasal Continuous Positive Airway Pressure (nCPAP)
Two Main Categories of CPAP Devices Should Be Described
- CPAP delivered through a double tube circuit, the so-called “continuous-flow system”. This system uses a flow resistance placed at the end of the circuit to produce a pressure greater than atmospheric pressure. The resistance can be provided from the expiratory valve of a conventional ventilator or by placing the end-expiratory portion of a breathing circuit in water (Bubble-CPAP).
- CPAP delivered through a single tube circuit, the so-called “variable-flow system”. In this system, two injector jets generate a positive pressure near the interface (Infant Flow Driver). The expiration is facilitated by the Coanda effect [24].
4.2. High-Flow Nasal Cannula (HFNC) Oxygen Therapy
4.3. Nasal Intermittent Positive Pressure Ventilation (NIPPV)
4.4. Practical Tools of Noninvasive Respiratory Support in the NICU
5. Mechanical Ventilation of Preterm Infants in the NICU
5.1. Indications for Mechanical Ventilation in Preterm Infants
5.2. Goals of Mechanical Ventilation in Preterm Infants
5.2.1. Oxygenation and Use of Supplemental Oxygen
5.2.2. Ventilation (CO2 Elimination)
5.3. Practical Suggestions and Monitoring during Mechanical Ventilation in Preterm Infants
5.4. Physiologic Concepts of Respiratory Failure, Ventilator-Induced Lung Injury and Lung-Protective Ventilation in Preterm Infants
5.4.1. Physiological Concepts of Respiratory Failure in Preterm Infants
5.4.2. Ventilator-Induced Lung Injury (VILI) and Its Consequence
- Volutrauma is associated to alveolar/saccular overdistension, often caused by high tidal volume ventilation. Sometimes, it may be induced by low tidal volumes provided at the airway opening, which result in regional overdistention in atelectatic lungs or by low tidal volumes superimposed on a high EELV that can still exceed total lung capacity [88];
- Atelectrauma is associated with repetitive collapse and reopening of alveoli/sacculi as a result of surfactant deficiency or inhibition [89];
- Oxygen toxicity in preterm infants has been shown in several studies demonstrating the inability of preterm infants to increase antioxidant enzymes in response to hyperoxia, resulting in a major vulnerability to oxidative stress [76].
5.4.3. Lung-Protective Ventilation and Optimal Lung Volume Strategy
- Minimize atelectrauma by optimizing EELV. This is achieved by reversing atelectasis using recruitment maneuvers and stabilizing lung units during the ventilatory cycle (by applying sufficient airway pressure at the end of expiration);
5.4.4. Practical Tools for Lung-Protective Ventilation in CMV
5.4.5. Practical Tools for Lung-Protective Ventilation in HFOV
5.5. Volume-Targeted Ventilation (VTV) in Preterm Infants
5.6. Weaning and Extubation from Mechanical Ventilation in Preterm Infants
6. Exogenous Surfactant Therapy in Preterm Infants
6.1. Surfactant Therapy in Preterm Infants with RDS
6.1.1. Indications for Surfactant Administration in Preterm Infants with RDS
- Surfactant derived from animal sources;
- Synthetic surfactant without protein components;
- Synthetic surfactant with protein components.
6.1.2. Strategy of Surfactant Administration in Preterm Infants with RDS
6.2. Surfactant Therapy in Preterm Infants with Other Respiratory Disorders
7. Results
8. Discussion
9. Conclusions
- –
- In delivery room: nCPAP initiated in the delivery room compared with intubation reduces death or BPD in very preterm infants. Current European guidelines advise using CPAP of at least 6 cm H2O. Endotracheal intubation should be considered only for infants who do not develop adequate respiratory effort and/or who remain bradycardic and/or hypoxic despite adequate mask or nasal prongs PPV.
- –
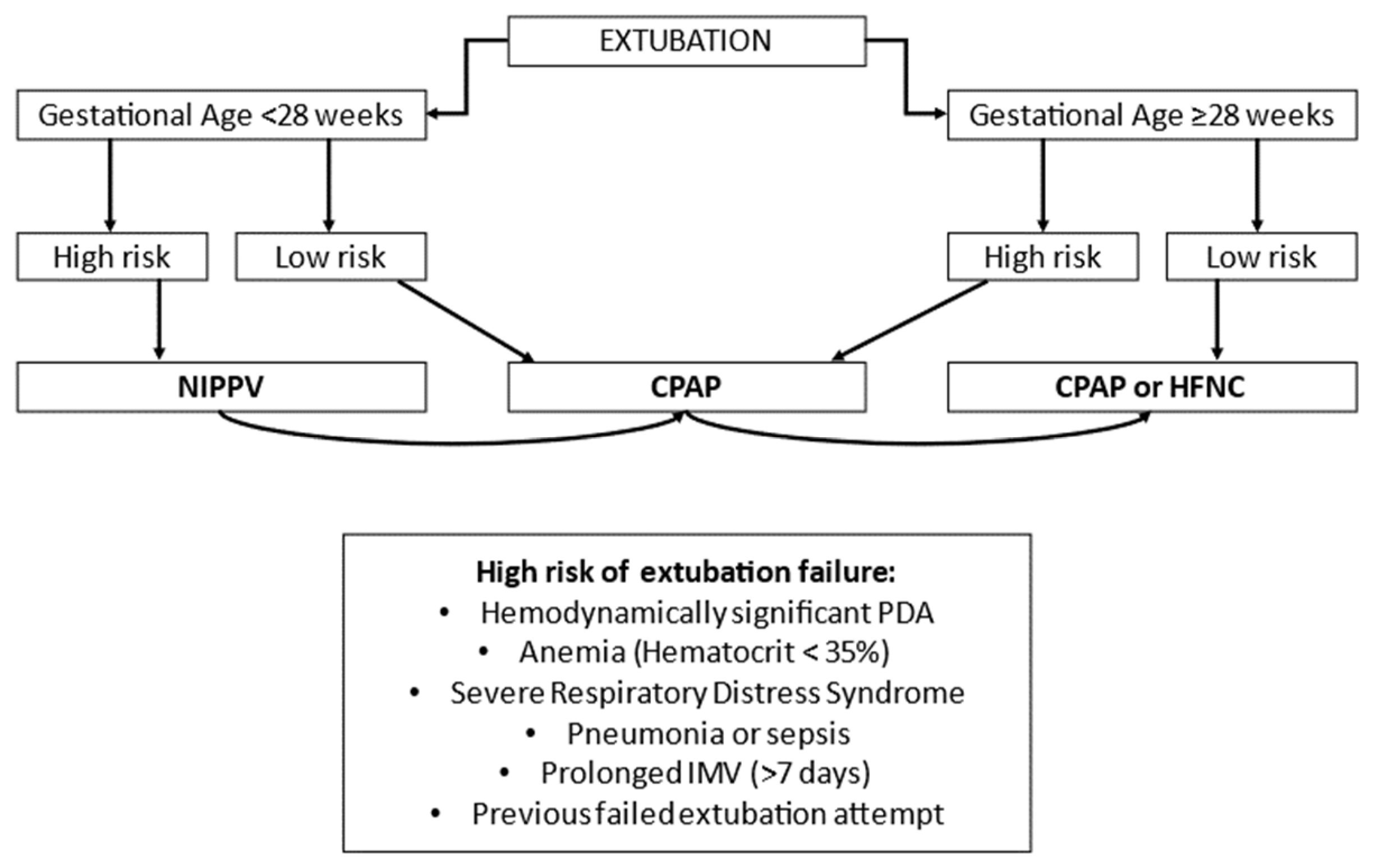
- In the NICU, the most effective noninvasive respiratory support as primary mode is NIPPV, especially sNIPPV. As an alternative primary support, it seems appropriate to choose nCPAP in infants born <28 weeks of GA. As post-extubation respiratory support, it seems reasonable to choose NIPPV in preterm neonates at high risk of extubation failure, especially in ELGANs. In case of low risk of extubation failure, it is possible to choose nCPAP.
- –
- MV is associated with increased mortality and pulmonary and systemic morbidities. Preterm infants needing MV should be ventilated using volume-target ventilation (VTV), resulting in a reduction in the risk of BPD, hypocapnia, and other morbidities. The selection of VT or VThf may be individualized for the specific patient, considering her/his lung mechanics, lung dimension and the phase of the lung disease being treated (acute/chronic/weaning). Weaning preterm infants from MV as soon as possible represents an imperative for all neonatologists.
- –
- Nowadays, guidelines recommended administering surfactant if FiO2 is >0.30 on CPAP pressure of at least 6 cm H2O for all preterm infants with a clinical diagnosis of RDS. The need to find new criteria for surfactant administration is emerging in the literature. Although the most recent European consensus guidelines on the management of RDS recommend LISA as the preferred mode of surfactant administration, the level of evidence is weak, and more studies are required before firm conclusions can be drawn about the optimal method of administration of surfactant.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Donnell, C.P.; Kamlin, C.O.F.; Davis, P.G.; Morley, C.J. Crying and Breathing by Extremely Preterm Infants Immediately After Birth. J. Pediatr. 2010, 156, 846–847. [Google Scholar] [CrossRef]
- Saugstad, O.D. Delivery Room Management of Term and Preterm Newly Born Infants. Neonatology 2015, 107, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Schmölzer, G.M.; Kumar, M.; Pichler, G.; Aziz, K.; O’Reilly, M.; Cheung, P.-Y. Non-invasive versus invasive respiratory support in preterm infants at birth: Systematic review and meta-analysis. BMJ 2013, 347, f5980. [Google Scholar] [CrossRef]
- Leone, T.A.; Rich, W.; Finer, N.N. A Survey of Delivery Room Resuscitation Practices in the United States. Pediatrics 2006, 117, e164–e175. [Google Scholar] [CrossRef] [PubMed]
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome–2019 Update. Neonatology 2019, 115, 432–450. [Google Scholar] [CrossRef]
- Kanaan, Z.; Bloch-Queyrat, C.; Boubaya, M.; Lévy, V.; Bolot, P.; Waszak, P. Feasibility of combining two individualized lung recruitment maneuvers at birth for very low gestational age infants: A retrospective cohort study. BMC Pediatr. 2020, 20, 144. [Google Scholar] [CrossRef]
- Positive End-Expiratory Pressure (PEEP) Levels during Resuscitation of Preterm Infants at Birth (The POLAR Trial). Clinical-trials.gov: NCT04372953. Available online: https://clinicaltrials.gov/ct2/show/NCT04372953 (accessed on 4 May 2020).
- O’Donnell, C.P.F.; Bruschettini, M.; Davis, P.G.; Morley, C.J.; Moja, L.; Calevo, M.G.; Zappettini, S. Sustained versus Standard Inflations during Neonatal Resuscitation to Prevent Mortality and Improve Respiratory Outcomes. Cochrane Database Syst. Rev. 2015, 3, 1858. [Google Scholar]
- Wyckoff, M.H.; Singletary, E.M.; Soar, J.; Olasveengen, T.M.; Greif, R.; Liley, H.G.; Zideman, D.; Bhanji, F.; Andersen, L.W.; Avis, S.R.; et al. 2021 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations: Summary from the Basic Life Support; Advanced Life Support; Neonatal Life Support; Education, Implementation, and Teams; First Aid Task Forces; and the COVID-19 Working Group. Circulation 2022, 169, 229–311. [Google Scholar] [CrossRef]
- Szyld, E.; Aguilar, A.; Musante, G.A.; Vain, N.; Prudent, L.; Fabres, J.; Carlo, W.A. Delivery Room Ventilation Devices Trial Group. Comparison of Devices for Newborn Ventilation in the Delivery Room. J. Pediatr. 2014, 165, 234–239.e3. [Google Scholar] [CrossRef]
- Welsford, M.; Nishiyama, C.; Shortt, C.; Weiner, G.; Roehr, C.C.; Isayama, T.; Dawson, J.A.; Wyckoff, M.H.; Rabi, Y. Initial Oxygen Use for Preterm Newborn Resuscitation: A Systematic Review with Meta-analysis. Pediatrics 2018, 143, e20181828. [Google Scholar] [CrossRef] [PubMed]
- Saugstad, O.D.; Oei, J.-L.; Lakshminrusimha, S.; Vento, M. Oxygen therapy of the newborn from molecular understanding to clinical practice. Pediatr. Res. 2018, 85, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Martherus, T.; Lopriore, E.; Giera, M.; McGillick, E.V.; Hutten, J.; van Leuteren, R.W.; van Kaam, A.H.; Hooper, S.B.; Pas, A.B.T. The Effect of Initial High vs. Low FiO2 on Breathing Effort in Preterm Infants at Birth: A Randomized Controlled Trial. Front. Pediatr. 2019, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.L.; Finer, N.N.; Saugstad, O.D.; Wright, I.M.; Rabi, Y.; Tarnow-Mordi, W.; Rich, W.; Kapadia, V.; Rook, D.; Smyth, J.P.; et al. Outcomes of oxygen saturation targeting during delivery room stabilisation of preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 103, F446–F454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jobe, A.H. Mechanisms of Lung Injury and Bronchopulmonary Dysplasia. Am. J. Perinatol. 2016, 33, 1076–1078. [Google Scholar] [CrossRef]
- Govindaswami, B.; Nudelman, M.; Narasimhan, S.R.; Huang, A.; Misra, S.; Urquidez, G.; Kifle, A.; Stemmle, M.; Angell, C.; Patel, R.; et al. Eliminating Risk of Intubation in Very Preterm Infants with Noninvasive Cardiorespiratory Support in the Delivery Room and Neonatal Intensive Care Unit. BioMed Res. Int. 2019, 3, 5984305. [Google Scholar] [CrossRef]
- Boel, L.; Banerjee, S.; Clark, M.; Greenwood, A.; Sharma, A.; Goel, N.; Bagga, G.; Poon, C.; Odd, D.; Chakraborty, M. Temporal trends of care practices, morbidity, and mortality of extremely preterm infants over 10-years in South Wales, UK. Sci. Rep. 2020, 10, 18738. [Google Scholar] [CrossRef]
- Owen, L.S.; Manley, B.J.; Davis, P.G.; Doyle, L.W. The evolution of modern respiratory care for preterm infants. Lancet 2017, 389, 1649–1659. [Google Scholar] [CrossRef]
- Boel, L.; Broad, K.; Chakraborty, M. Non-invasive respiratory support in newborn infants. Paediatr. Child Health 2017, 28, 6–12. [Google Scholar] [CrossRef]
- Diblasi, R.M. Nasal continuous positive airway pressure (CPAP) for the respiratory care of the newborn infant. Respir. Care 2009, 54, 1209–1235. [Google Scholar] [PubMed]
- Subramaniam, P.; Ho, J.J.; Davis, P.G. Prophylactic or very early initiation of continuous positive airway pressure (CPAP) for preterm infants. Cochrane Database Syst. Rev. 2021, 10, CD001243. [Google Scholar] [CrossRef]
- Davis, P.G.; Henderson-Smart, D.J. Nasal continuous positive airway pressure immediately after extubation for preventing morbidity in preterm infants. Cochrane Database Syst. Rev. 2003, 10, CD000143. [Google Scholar] [CrossRef]
- Ho, J.J.; Subramaniam, P.; Davis, P.G. Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants. Cochrane Database Syst. Rev. 2020, 10, CD002271. [Google Scholar]
- Pillow, J.J. Which continuous positive airway pressure system is best for the preterm infant with respiratory distress syndrome? Clin Perinatol. 2012, 39, 483–496. [Google Scholar] [CrossRef]
- Jasani, B.; Ismail, A.; Rao, S.; Patole, S. Effectiveness and safety of nasal mask versus binasal prongs for providing continuous positive airway pressure in preterm infants-A systematic review and meta-analysis. Pediatr. Pulmonol. 2018, 53, 987–992. [Google Scholar] [CrossRef]
- Gupta, S.; Donn, S.M. Continuous positive airway pressure: Physiology and comparison of devices. Semin. Fetal Neonatal Med. 2016, 21, 204–211. [Google Scholar] [CrossRef]
- Klausner, J.F.; Lee, A.Y.; Hutchison, A.A. Decreased imposed work with a new nasal continuous positive airway pressure device. Pediatr Pulmonol. 1996, 22, 188–194. [Google Scholar] [CrossRef]
- Bamat, N.; Fierro, J.; Mukerji, A.; Wright, C.J.; Millar, D.; Kirpalani, H. Nasal continuous positive airway pressure levels for the prevention of morbidity and mortality in preterm infants. Cochrane Database Syst. Rev. 2021, 11, Cd012778. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.S.; Bührer, C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: A meta-analysis. Pediatrics 2013, 132, e1351–e1361. [Google Scholar] [CrossRef] [PubMed]
- Jeon, G.W. Respiratory support with heated humidified high flow nasal cannula in preterm infants. Korean J. Pediatr. 2016, 59, 389–394. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chou, A.-K.; Chen, Y.-L.; Chou, H.-C.; Tsao, P.-N.; Hsieh, W.-S. Quality Improvement of Nasal Continuous Positive Airway Pressure Therapy in Neonatal Intensive Care Unit. Pediatr. Neonatol. 2017, 58, 229–235. [Google Scholar] [CrossRef]
- Shalshin, A. Nasal CPAP or intubation at birth for preterm infants. Thorax 2008, 63, 577. [Google Scholar]
- Roberts, C.T.; Owen, L.S.; Manley, B.J.; Frøisland, D.H.; Donath, S.M.; Dalziel, K.M.; Pritchard, M.A.; Cartwright, D.W.; Collins, C.L.; Malhotra, A.; et al. Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants. N. Engl. J. Med. 2016, 375, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Murki, S.; Singh, J.; Khant, C.; Dash, S.K.; Oleti, T.P.; Joy, P.; Kabra, N.S. High-Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure for Primary Respiratory Support in Preterm Infants with Respiratory Distress: A Randomized Controlled Trial. Neonatology 2018, 113, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Manley, B.J.; Owen, L.S.; Doyle, L.W.; Andersen, C.C.; Cartwright, D.W.; Pritchard, M.A.; Donath, S.M.; Davis, P.G. High-Flow Nasal Cannulae in Very Preterm Infants after Extubation. N. Engl. J. Med. 2013, 369, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Lavizzari, A.; Colnaghi, M.; Ciuffini, F.; Veneroni, C.; Musumeci, S.; Cortinovis, I.; Mosca, F. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: A randomized clinical noninferiority trial. JAMA Pediatr. 2016, 170, 1228. [Google Scholar] [CrossRef]
- Badiee, Z.; Eshghi, A.; Mohammadizadeh, M. High flow nasal cannula as a method for rapid weaning from nasal continuous positive airway pressure. Int. J. Prev. Med. 2015, 6, 33. [Google Scholar] [CrossRef]
- Vento, G.; Tirone, C.; Paladini, A.; Aurilia, C.; Lio, A.; Tana, M. Weaning from the Ventilator in Bronchopulmonary Dysplasia. Clin. Perinatol. 2021, 48, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hady, H.; Shouman, B.; Aly, H. Early weaning from CPAP to high flow nasal cannula in preterm infants is associated with prolonged oxygen requirement: A randomized controlled trial. Early Hum. Dev. 2011, 87, 205–208. [Google Scholar] [CrossRef]
- Kotecha, S.J.; Adappa, R.; Gupta, N.; Watkins, W.J.; Kotecha, S.; Chakraborty, M. Safety and Efficacy of High-Flow Nasal Cannula Therapy in Preterm Infants: A Meta-analysis. Pediatrics 2015, 136, 542–553. [Google Scholar] [CrossRef]
- Wilkinson, D.; Andersen, C.; O’Donnell, C.P.; De Paoli, A.G.; Manley, B.J. High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2016, 2, Cd006405. [Google Scholar] [CrossRef]
- Fleeman, N.; Dundar, Y.; Shah, P.S.; Shaw, B.N. Heated Humidified High-Flow Nasal Cannula for Preterm Infants: An Updated Systematic Review and Meta-analysis. Int. J. Technol. Assess. Health Care 2019, 35, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Li, X.-X.; Li, J.; Zhang, Z.-Q. High-flow nasal cannula versus nasal continuous positive airway pressure for respiratory support in preterm infants: A meta-analysis of randomized controlled trials. J. Matern. Neonatal Med. 2021, 34, 259–266. [Google Scholar] [CrossRef]
- Conte, F.; Orfeo, L.; Gizzi, C.; Massenzi, L.; Fasola, S. Rapid systematic review shows that using a high-flow nasal cannula is inferior to nasal continuous positive airway pressure as first-line support in preterm neonates. Acta Paediatr. 2018, 107, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Bruet, S.; Butin, M.; Dutheil, F. Systematic review of high-flow nasal cannula versus continuous positive airway pressure for primary support in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Huang, Y.; Xiong, T.; Tang, J. High-flow nasal cannula versus continuous positive airway pressure in primary res-piratory support for preterm infants: A systematic review and meta-analysis. Front. Pediatr. 2022, 10, 980024. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Park, K.; Lee, E.H.; Choi, B.M. Humidified High Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure as an Initial Respiratory Support in Preterm Infants with Respiratory Distress: A Randomized, Controlled Non-Inferiority Trial. J. Korean Med. Sci. 2017, 32, 650–655. [Google Scholar] [CrossRef]
- Armanian, A.M.; Iranpour, R.; Parvaneh, M.; Salehimehr, N.; Feizi, A.; Hajirezaei, M. Heated humidified high flow nasal cannula (HHHFNC) is not an effective method for initial treatment of respiratory distress syndrome (RDS) versus nasal intermittent mandatory ventilation (NIMV) and nasal continuous positive airway pressure (NCPAP). J. Res. Med. Sci. 2019, 24, 73. [Google Scholar]
- Demirel, G.; Vatansever, B.; Tastekin, A. High Flow Nasal Cannula versus Nasal Continuous Positive Airway Pressure for Primary Respiratory Support in Preterm Infants: A Prospective Randomized Study. Am. J. Perinatol. 2019, 38, 237–241. [Google Scholar] [CrossRef]
- Öktem, A.; Yiğit, Ş.; Çelik, H.T.; Yurdakök, M. Comparison of four different non-invasive respiratory support techniques as primary respiratory support in preterm infants. Turk. J. Pediatr. 2021, 63, 23–30. [Google Scholar] [CrossRef]
- Poonia, A.K.; Sharma, P.K.; Bansal, R.K. Comparison of efficacy of nasal continuous positive airway pressure and heated humidified high-flow nasal cannula as a primary mode of respiratory support in preterm infants. J. Clin. Neonatol. 2019, 8, 102. [Google Scholar] [CrossRef]
- Wright, M.F.A.; Wallis, C. Investigation and management of the long-term ventilated premature infant. Early Hum Dev. 2018, 126, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Muniraman, H.; Biniwale, M.; Ramanathan, R. A Review on Non-invasive Respiratory Support for Management of Respiratory Distress in Extremely Preterm Infants. Front. Pediatr. 2020, 8, 270. [Google Scholar] [CrossRef]
- Roberts, C.T.; Davis, P.G.; Owen, L.S. Neonatal non-invasive respiratory support: Synchronised NIPPV, non-synchronised NIPPV or bi-level CPAP: What is the evidence in 2013? Neonatology 2013, 104, 203–209. [Google Scholar] [CrossRef]
- Owen, L.S.; Morley, C.J.; Dawson, J.A.; Davis, P.G. Effects of non-synchronised nasal intermittent positive pressure ventilation on spontaneous breathing in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F422–F428. [Google Scholar] [CrossRef] [PubMed]
- Lemyre, B.; Laughon, M.; Bose, C.; Davis, P.G. Early nasal intermittent positive pressure ventilation (NIPPV) versus early nasal continuous positive airway pressure (NCPAP) for preterm infants. Cochrane Database Syst. Rev. 2016, 12, CD005384. [Google Scholar] [CrossRef] [PubMed]
- Rüegger, C.M.; Owen, L.S.; Davis, P.G. Nasal Intermittent Positive Pressure Ventilation for Neonatal Respiratory Distress Syndrome. Clin. Perinatol. 2021, 48, 725–744. [Google Scholar] [CrossRef]
- Lemyre, B.; Davis, P.G.; De Paoli, A.G.; Kirpalani, H. Nasal intermittent positive pressure ventilation (NIPPV) versus nasal continuous positive airway pressure (NCPAP) for preterm neonates after extubation. Cochrane Database Syst. Rev. 2017, 2, CD003212. [Google Scholar] [CrossRef]
- Moretti, C.; Giannini, L.; Fassi, C.; Gizzi, C.; Papoff, P.; Colarizi, P. Nasal flow-synchronized intermittent positive pressure ventilation to facilitate weaning in very low-birthweight infants: Unmasked randomized controlled trial. Pediatr. Int. 2008, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Aghai, Z.H.; Saslow, J.G.; Nakhla, T.; Milcarek, B.; Hart, J.; Lawrysh-Plunkett, R.; Stahl, G.; Habib, R.H.; Pyon, K.H. Synchronized nasal intermittent positive pressure ventilation (SNIPPV) decreases work of breathing (WOB) in premature infants with respiratory distress syndrome (RDS) compared to nasal continuous positive airway pressure (NCPAP). Pediatr Pulmonol. 2006, 41, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Moretti, C.; Gizzi, C.; Papoff, P.; Lampariello, S.; Capoferri, M.; Calcagnini, G.; Bucci, G. Comparing the effects of nasal syn-chronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Hum Dev. 1999, 56, 167–177. [Google Scholar] [CrossRef]
- Gizzi, C.; Montecchia, F.; Panetta, V.; Castellano, C.; Mariani, C.; Campelli, M.; Papoff, P.; Moretti, C.; Agostino, R. Is syn-chronised NIPPV more effective than NIPPV and NCPAP in treating apnoea of prematurity (AOP)? A randomised cross-over trial. Arch. Dis. Child Fetal Neonatal Ed. 2015, 100, F17–F23. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Claure, N.; D’Ugard, C.; Torres, J.; Nwajei, P.; Bancalari, E. Effects of Synchronization During Nasal Ventilation in Clinically Stable Preterm Infants. Pediatr. Res. 2011, 69, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Dumpa, V.; Katz, K.; Northrup, V.; Bhandari, V. SNIPPV vs NIPPV: Does synchronization matter? J. Perinatol. 2012, 32, 438–442. [Google Scholar] [CrossRef]
- Ramaswamy, V.V.; Bandyopadhyay, T.; Nanda, D.; Bandiya, P.; More, K.; Oommen, V.I.; Gupta, A. Efficacy of noninvasive respiratory support modes as postextubation respiratory support in preterm neonates: A systematic review and network meta-analysis. Pediatr. Pulmonol. 2020, 55, 2924–2939. [Google Scholar] [CrossRef] [PubMed]
- Shehadeh, A.M. Non-invasive respiratory support for preterm infants following extubation from mechanical ventilation. A narrative review and guideline suggestion. Pediatr. Neonatol. 2020, 61, 142–147. [Google Scholar] [CrossRef]
- Hermeto, F.; Martins, B.M.; Ramos, J.R.; Bhering, C.A.; Sant’Anna, G.M. Incidence and main risk factors associated with ex-tubation failure in newborns with birth weight < 1250 grams. J. Pediatr. 2009, 85, 397–402. [Google Scholar]
- Al Mandhari, H.K.; Al Riyami, B.; Khan, A.; Nonoyama, M.; Rizvi, S.G. Risk Factors of Extubation Failure in Intubated Preterm Infants at a Tertiary Care Hospital in Oman. Sultan Qaboos Univ. Med. J. SQUMJ 2022, 22, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, G.; Mukhopadhyay, K.; Narang, A. Clinical risk factors associated with extubation failure in ventilated neonates. Indian Pediatr. 2009, 46, 887–890. [Google Scholar] [PubMed]
- Wheeler, C.R.; Smallwood, C.D. Neonatal Respiratory Support: 2019 Year in Review. Respir. Care 2020, 65, 693–704. [Google Scholar] [CrossRef]
- Keszler, M. Overview of assisted ventilation. In Goldsmith’s Assisted Ventilation of the Neonate, 7th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2022; pp. 221–231. [Google Scholar]
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus Surfactant in Extremely Preterm Infants. N. Engl. J. Med. 2010, 362, 1970–1979. [Google Scholar] [CrossRef]
- Gilfillan, M.; Bhandari, A.; Bhandari, V. Diagnosis and management of bronchopulmonary dysplasia. BMJ 2021, 375, n1974. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; DeMauro, S.B.; Kornhauser, M.; Aghai, Z.H.; Greenspan, J.S.; Dysart, K.C. Effects of Multiple Ventilation Courses and Duration of Mechanical Ventilation on Respiratory Outcomes in Extremely Low-Birth-Weight Infants. JAMA Pediatr. 2015, 169, 1011–1017. [Google Scholar] [CrossRef]
- Robbins, M.; Trittmann, J.; Martin, E.; Reber, K.M.; Nelin, L.; Shepherd, E. Early extubation attempts reduce length of stay in extremely preterm infants even if re-intubation is necessary. J. Neonatal-Perinat. Med. 2015, 8, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Georgeson, G.D.; Szőny, B.J.; Streitman, K.; Varga, I.S.; Kovács, A.; Kovács, L.; László, A. Antioxidant enzyme activities are decreased in preterm infants and in neonates born via caesarean section. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 103, 136–139. [Google Scholar] [CrossRef]
- Vento, M. Oxygen therapy. In Goldsmith’s Assisted Ventilation of the Neonate, 7th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2022; pp. 185–195. [Google Scholar]
- Askie, L.M.; Darlow, B.A.; Davis, P.G.; Finer, N.; Stenson, B.; Vento, M.; Whyte, R. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst. Rev. 2017, 2018, CD011190. [Google Scholar] [CrossRef]
- Manja, V.; Lakshminrusimha, S.; Cook, D.J. Oxygen Saturation Target Range for Extremely Preterm Infants. JAMA Pediatr. 2015, 169, 332–340. [Google Scholar] [CrossRef] [PubMed]
- van Kaam, A.H. Principles of Lung-protective ventilation. In Goldsmith’s Assisted Ventilation of the Neonate, 7th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2022; pp. 241–248. [Google Scholar]
- Woodgate, P.G.; Davies, M.W. Permissive Hypercapnia for the Prevention of Morbidity and Mortality in Mechanically Ven-tilated Newborn Infants. Cochrane Database Syst. Rev. 2001, 2001, CD002061. [Google Scholar]
- Thome, U.H.; Genzel-Boroviczeny, O.; Bohnhorst, B.; Schmid, M.; Fuchs, H.; Rohde, O.; Avenarius, S.; Topf, H.-G.; Zimmermann, A.; Faas, D.; et al. Permissive Hypercapnia in Extremely Low Birthweight Infants (PHELBI): A Randomised Con-trolled Multicentre Trial. Lancet Respir. Med. 2015, 3, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Snijders, C.; van Lingen, R.A.; van der Schaaf, T.W.; Fetter, W.P.F.; Molendijk, H.A.; on behalf of the NEOSAFE study group. Incidents associated with mechanical ventilation and intravascular catheters in neonatal intensive care: Exploration of the causes, severity and methods for prevention. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 96, F121–F126. [Google Scholar] [CrossRef]
- Sant’Anna, G.; Keszler, M. Developing a neonatal unit ventilation protocol for the preterm baby. Early Hum. Dev. 2012, 88, 925–929. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Saumon, G. Ventilator-Induced Lung Injury. Am. J. Respir. Crit. Care Med. 1998, 157, 294–323. [Google Scholar] [CrossRef]
- Ikegami, M.; Kallapur, S.G.; Jobe, A.H. Initial Responses to Ventilation of Premature Lambs Exposed to Intra-Amniotic En-dotoxin 4 Days before Delivery. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L573–L579. [Google Scholar] [CrossRef]
- van Kaam, A.H.; Rimensberger, P.C.; Borensztajn, D.; De Jaegere, A.P. Ventilation Practices in the Neonatal Intensive Care Unit: A Cross-Sectional Study. J. Pediatr. 2010, 157, 767–771.e3. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, D.; Saumon, G. Role of Tidal Volume, FRC, and End-inspiratory Volume in the Development of Pulmonary Edema following Mechanical Ventilation. Am. Rev. Respir. Dis. 1993, 148, 1194–1203. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Engelberts, D.; Peltekova, V.; Hopkins, N.; Frndova, H.; Babyn, P.; McKerlie, C.; Post, M.; McLoughlin, P.; Kavanagh, B.P. Atelectasis Causes Alveolar Injury in Nonatelectatic Lung Regions. Am. J. Respir. Crit. Care Med. 2006, 174, 279–289. [Google Scholar] [CrossRef]
- Quinn, D.A.; Moufarrej, R.K.; Volokhov, A.; Hales, C.A. Interactions of lung stretch, hyperoxia, and MIP-2 production in ventilator-induced lung injury. J. Appl. Physiol. 2002, 93, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Suter, P.M.; Tortorella, C.; De Tullio, R.; Dayer, J.M.; Brienza, A.; Bruno, F.; Slutsky, A.S. Effect of Mechanical Ventilation on Inflammatory Mediators in Patients with Acute Respiratory Distress Syndrome. JAMA 1999, 282, 54–61. [Google Scholar] [CrossRef]
- Griese, M.; Westerburg, B.; Potz, C.; Dietrich, P. Respiratory Support, Surface Activity and Protein Content during Nosocomial Infection in Preterm Neonates. Neonatology 1996, 70, 271–279. [Google Scholar] [CrossRef]
- Coalson, J.J.; Winter, V.; Delemos, R.A. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 1995, 152, 640–646. [Google Scholar] [CrossRef]
- van Kaam, A.H.; Rimensberger, P.C. Lung-Protective Ventilation Strategies in Neonatology: What Do We Know—What Do We Need to Know? Crit. Care Med. 2007, 35, 925–931. [Google Scholar] [CrossRef]
- Miedema, M.; de Jongh, F.H.; Frerichs, I.; van Veenendaal, M.B.; van Kaam, A.H. Changes in Lung Volume and Ventilation during Lung Recruitment in High-Frequency Ventilated Preterm Infants with Respiratory Distress Syndrome. J. Pediatr. 2011, 159, 199–205.e2. [Google Scholar] [CrossRef] [PubMed]
- Tingay, D.G.; Mills, J.F.; Morley, C.J.; Pellicano, A.; Dargaville, P.A. The Deflation Limb of the Pressure–Volume Relationship in Infants during High-Frequency Ventilation. Am. J. Respir. Crit. Care Med. 2006, 173, 414–420. [Google Scholar] [CrossRef]
- Klingenberg, C.; Wheeler, K.I.; McCallion, N.; Morley, C.J.; Davis, P.G. Volume-Targeted versus Pressure-Limited Ventilation in Neonates. Cochrane Database Syst. Rev. 2017, 10, CD003666. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, F.; Daniele, I.; Fontana, P.; Cavigioli, F.; Lupo, E.; Lista, G. Lung Recruitment Maneuver during Volume Guarantee Ventilation of Preterm Infants with Acute Respiratory Distress Syndrome. Am. J. Perinatol. 2011, 28, 521–528. [Google Scholar] [CrossRef]
- Mcculloch, P.R.; Forkert, P.G.; Froese, A.B. Lung Volume Maintenance Prevents Lung Injury during High Frequency Oscil-latory Ventilation in Surfactant-Deficient Rabbits. Am. Rev. Respir. Dis. 1988, 137, 1185–1192. [Google Scholar] [CrossRef]
- Solis-Garcia, G.; González-Pacheco, N.; Ramos-Navarro, C.; Vigil-Vázquez, S.; Gutiérrez-Vélez, A.; Merino-Hernández, A.; De la Blanca, A.R.S.; Sánchez-Luna, M. Lung Recruitment in Neonatal High-Frequency Oscillatory Ventilation with Volume-Guarantee. Pediatr. Pulmonol. 2022, 57, 3000–3008. [Google Scholar] [CrossRef]
- Tana, M.; Paladini, A.; Tirone, C.; Aurilia, C.; Lio, A.; Bottoni, A.; Costa, S.; Tiberi, E.; Pastorino, R.; Vento, G. Effects of High-Frequency Oscillatory Ventilation with Volume Guarantee During Surfactant Treatment in Extremely Low Gestational Age Newborns with Respiratory Distress Syndrome: An Observational Study. Front. Pediatr. 2022, 9, 1683. [Google Scholar] [CrossRef]
- Dreyfuss, D.; Soler, P.; Basset, G.; Saumon, G. High Inflation Pressure Pulmonary Edema: Respective Effects of High Airway Pressure, High Tidal Volume, and Positive End-expiratory Pressure. Am. Rev. Respir. Dis. 1988, 137, 1159–1164. [Google Scholar] [CrossRef]
- Hernandez, L.A.; Peevy, K.J.; Moise, A.A.; Parker, J.C. Chest wall restriction limits high airway pressure-induced lung injury in young rabbits. J. Appl. Physiol. 1989, 66, 2364–2368. [Google Scholar] [CrossRef] [PubMed]
- Wiswell, T.E.; Graziani, L.J.; Kornhauser, M.S.; Stanley, C.; Merton, D.A.; Spitzer, A.R. Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 1996, 98, 918–924. [Google Scholar] [CrossRef]
- Fabres, J.; Carlo, W.A.; Phillips, V.; Howard, G.; Ambalavanan, N. Both Extremes of Arterial Carbon Dioxide Pressure and the Magnitude of Fluctuations in Arterial Carbon Dioxide Pressure Are Associated with Severe Intraventricular Hemorrhage in Preterm Infants. Pediatrics 2007, 119, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Zhu, H.; Shi, H.; Liu, E. Volume-targeted ventilation is more suitable than pressure-limited ventilation for preterm infants: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F158–F165. [Google Scholar] [CrossRef]
- Keszler, M. Volume-targeted ventilation: One size does not fit all. Evidence-based recommendations for successful use. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F108–F112. [Google Scholar] [CrossRef] [PubMed]
- Nassabeh-Montazami, S.; Abubakar, K.M.; Keszler, M. The impact of instrumental dead-space in volume-targeted ventilation of the extremely low birth weight (ELBW) infant. Pediatr. Pulmonol. 2008, 44, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Keszler, M. Mechanical ventilation strategies. Semin. Fetal Neonatal Med. 2017, 22, 267–274. [Google Scholar] [CrossRef]
- van Kaam, A.H.; de Jaegere, A.; Haitsma, J.J.; Van Aalderen, W.M.; Kok, J.H.; Lachmann, B. Positive pressure ventilation with the Open Lung Concept optimizes gas exchange and reduces ventilator-induced lung injury in newborn piglets. Pediatr. Res. 2003, 53, 245–253. [Google Scholar] [CrossRef]
- Enomoto, M.; Keszler, M.; Sakuma, M.; Kikuchi, S.; Katayama, Y.; Takei, A.; Ikegami, H.; Minami, H. Effect of volume guar-antee in preterm infants on high-frequency oscillatory ventilation: A pilot study. Am. J. Perinatol. 2017, 34, 26–30. [Google Scholar]
- Iscan, B.; Duman, N.; Tuzun, F.; Kumral, A.; Ozkan, H. Impact of Volume Guarantee on High-Frequency Oscillatory Venti-lation in Preterm Infants: A Randomized Crossover Clinical Trial. Neonatology 2015, 108, 277–282. [Google Scholar] [CrossRef]
- Tana, M.; Lio, A.; Tirone, C.; Aurilia, C.; Tiberi, E.; Serrao, F.; Purcaro, V.; Corsello, M.; Catenazzi, P.; D’Andrea, V.; et al. Extubation from high-frequency oscillatory ventilation in extremely low birth weight infants: A prospective observational study. BMJ Paediatr. Open 2018, 2, e000350. [Google Scholar] [CrossRef]
- Bhat, P.; Peacock, J.L.; Rafferty, G.F.; Hannam, S.; Greenough, A. Prediction of Infant Extubation Outcomes Using the Tension-Time Index. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F444–F447. [Google Scholar] [CrossRef]
- Teixeira, R.F.; Carvalho, A.C.A.; De Araujo, R.D.; Veloso, F.C.S.; Kassar, S.B.; Medeiros, A.M.C. Spontaneous Breathing Trials in Preterm Infants: Systematic Review and Meta-Analysis. Respir. Care 2020, 66, 129–137. [Google Scholar] [CrossRef]
- Shalish, W.; Kanbar, L.; Kovacs, L.; Chawla, S.; Keszler, M.; Rao, S.; Latremouille, S.; Precup, D.; Brown, K.; Kearney, R.E.; et al. Assessment of Extubation Readiness Using Spontaneous Breathing Trials in Extremely Preterm Neonates. JAMA Pediatr. 2020, 174, 178. [Google Scholar] [CrossRef]
- Nakato, A.M.; de Ribeiro, D.F.; Simão, A.C.; Da Silva, R.P.; Nohama, P. Impact of Spontaneous Breathing Trials in Cardi-orespiratory Stability of Preterm Infants. Respir. Care 2020, 66, 286–291. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Chawla, S.; Marchica, C.; Dwaihy, M.; Grundy, L.; Sant’Anna, G.M. Heart Rate Variability and Extubation Readiness in Extremely Preterm Infants. Neonatology 2013, 104, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.; Kamlin, C.O.F.; Morley, C.J.; Davis, P.G.; Sant’Anna, G.M. Variability of Respiratory Parameters and Extubation Readiness in Ventilated Neonates. Arch. Dis. Child. Fetal Neonatal Ed. 2012, 98, F70–F73. [Google Scholar] [CrossRef]
- Williams, E.E.; Thodika, F.M.S.A.; Chappelow, I.; Chapman-Hatchett, N.; Dassios, T.; Greenough, A. Diaphragmatic electromyography during a spontaneous breathing trial to predict extubation failure in preterm infants. Pediatr. Res. 2022, 92, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Ventol, G.; Tortorolo, L.; Zecca, E.; Rosano, A.; Matassa, P.G.; Papacci, P.; Romagnoli, C. Spontaneous minute ventilation is a predictor of extubation failure in extremely-low-birth-weight infants. J. Matern. Neonatal Med. 2004, 15, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mammel, M.C.; Donn, S.M. Real-Time Pulmonary Graphics. Semin. Fetal Neonatal Med. 2015, 20, 181–191. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Respiratory Support in Preterm Infants at Birth. Pediatrics 2013, 133, 171–174. [Google Scholar]
- Raimondi, F.; Migliaro, F.; Corsini, I.; Meneghin, F.; Pierri, L.; Salomè, S.; Perri, A.; Aversa, S.; Nobile, S.; Lama, S.; et al. Neonatal Lung Ultrasound and Surfactant Administration. Chest 2021, 160, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Daniel, I.W.B.S.; Fiori, H.H.; Piva, J.P.; Munhoz, T.P.; Nectoux, A.V.; Fiori, R.M. Lamellar Body Count and Stable Microbubble Test on Gastric Aspirates from Preterm Infants for the Diagnosis of Respiratory Distress Syndrome. Neonatology 2010, 98, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Osborn, D.A.; Jeffery, H.E.; Bredemeyer, S.L.; Polverino, J.M.; Reid, S. Targeted Early Rescue Surfactant in Ventilated Preterm Infants Using the Click Test. Pediatrics 2000, 106, e30. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Halliday, H.L.; Stevens, T.P.; Suresh, G.; Soll, R.; Rojas-Reyes, M.X. Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants. Cochrane Database Syst. Rev. 2015, 12, CD010249. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.P.; Blennow, M.; Myers, E.H.; Soll, R. Early Surfactant Administration with Brief Ventilation vs. Selective Surfactant and Continued Mechanical Ventilation for Preterm Infants with or at Risk for Respiratory Distress Syndrome. Cochrane Database Syst. Rev. 2007, 4, CD003063. [Google Scholar] [CrossRef]
- Brix, N.; Sellmer, A.; Jensen, M.S.; Pedersen, L.V.; Henriksen, T.B. Predictors for an Unsuccessful INtuba-tion-SURfactant-Extubation Procedure: A Cohort Study. BMC Pediatr. 2014, 14, 155. [Google Scholar] [CrossRef]
- Dani, C.; Corsini, I.; Bertini, G.; Fontanelli, G.; Pratesi, S.; Rubaltelli, F.F. The INSURE method in preterm infants of less than 30 weeks’ gestation. J. Matern. Neonatal Med. 2010, 23, 1024–1029. [Google Scholar] [CrossRef]
- Vento, G.; Ventura, M.L.; Pastorino, R.; van Kaam, A.H.; Carnielli, V.; Cools, F.; Dani, C.; Mosca, F.; Polglase, G.; Tagliabue, P.; et al. Lung recruitment before surfactant administration in extremely preterm neonates with respiratory distress syndrome (IN-REC-SUR-E): A randomised, unblinded, controlled trial. Lancet Respir. Med. 2021, 9, 159–166. [Google Scholar] [CrossRef]
- Kribs, A. Minimally Invasive Surfactant Therapy and Noninvasive Respiratory Support. Clin. Perinatol. 2016, 43, 755–771. [Google Scholar] [CrossRef]
- Kribs, A.; Roll, C.; Göpel, W.; Wieg, C.; Groneck, P.; Laux, R.; Teig, N.; Hoehn, T.; Böhm, W.; Welzing, L.; et al. Nonintubated Surfactant Application vs Conventional Therapy in Extremely Preterm Infants: A Randomized Clinical Trial. JAMA Pediatr. 2015, 169, 723–730. [Google Scholar] [CrossRef]
- Göpel, W.; Kribs, A.; Ziegler, A.; Laux, R.; Hoehn, T.; Wieg, C.; Siegel, J.; Avenarius, S.; von der Wense, A.; Vochem, M.; et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): An open-label, randomised, controlled trial. Lancet 2011, 378, 1627–1634. [Google Scholar] [CrossRef]
- Dargaville, P.A.; Kamlin, C.O.F.; Orsini, F.; Wang, X.; De Paoli, A.G.; Kutman, H.G.K.; Cetinkaya, M.; Kornhauser-Cerar, L.; Derrick, M.; Özkan, H.; et al. Effect of Minimally Invasive Surfactant Therapy vs Sham Treatment on Death or Bronchopulmonary Dysplasia in Preterm Infants With Respiratory Distress Syndrome. JAMA 2021, 326, 2478. [Google Scholar] [CrossRef] [PubMed]
- Bellos, I.; Fitrou, G.; Panza, R.; Pandita, A. Comparative Efficacy of Methods for Surfactant Administration: A Network Me-ta-Analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Nobile, S.; Bottoni, A.; Giordano, L.; Paladini, A.; Vento, G. Critical appraisal of the evidence underpinning the efficacy of less invasive surfactant administration. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 108, 90–91. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.I.; Abdel-Latif, M.E.; Davis, P.G.; De Paoli, A.G.; Dargaville, P.A. Surfactant Therapy via Brief Tracheal Catheterization in Preterm Infants with or at Risk of Respiratory Distress Syndrome. Cochrane Database Syst. Rev. 2021, 5, CD011672. [Google Scholar] [CrossRef]
- Reigstad, H.; Hufthammer, K.O.; Rønnestad, A.E.; Klingenberg, C.; Stensvold, H.J.; Markestad, T. Early surfactant and non-invasive ventilation versus intubation and surfactant: A propensity score-matched national study. BMJ Paediatr. Open 2022, 6, e001527. [Google Scholar] [CrossRef] [PubMed]
- De Luca, D.; Shankar-Aguilera, S.; Bancalari, E. LISA/MIST: Complex Clinical Problems Almost Never Have Easy Solutions. Semin. Fetal Neonatal Med. 2021, 26, 101230. [Google Scholar] [CrossRef]
- Janssen, L.C.; Van Der Spil, J.; van Kaam, A.H.; Dieleman, J.P.; Andriessen, P.; Onland, W.; Niemarkt, H.J. Minimally Invasive Surfactant Therapy Failure: Risk Factors and Outcome. Arch. Dis. Child. Fetal Neonatal Ed. 2019, 104, F636–F642. [Google Scholar] [CrossRef]
- Bancalari, E. The Newborn Lung; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Minocchieri, S.; Berry, C.A.; Pillow, J.J. Nebulised surfactant to reduce severity of respiratory distress: A blinded, parallel, randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 104, F313–F319. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.D.; Brown, R.; Lampland, A.L.; Leone, T.A.; Rudser, K.D.; Finer, N.N.; Rich, W.D.; Merritt, T.A.; Czynski, A.J.; Kessel, J.M.; et al. Laryngeal Mask Airway for Surfactant Administration in Neonates: A Randomized, Controlled Trial. J. Pediatr. 2018, 193, 40–46.e1. [Google Scholar] [CrossRef]
- Stewart, C.; Stukenborg, G.; Kattwinkel, J.; Attridge, J. Administration of Rescue Surfactant by Laryngeal Mask Airway: Les-sons from a Pilot Trial. Am. J. Perinatol. 2012, 30, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Keiser, A.; Bhandari, V. The Role of Surfactant Therapy in Nonrespiratory Distress Syndrome Conditions in Neonates. Am. J. Perinatol. 2015, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rüegger, C.M.; Bassler, D. Alternatives to systemic postnatal corticosteroids: Inhaled, nebulized and intratracheal. Semin. Fetal Neonatal Med. 2019, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, R.; Kamaluddeen, M.; Hasan, S.U.; Robertson, H.L.; Lodha, A. Intratracheal Administration of Budesonide-Surfactant in Prevention of Bronchopulmonary Dysplasia in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Pediatr. Pulmonol. 2017, 52, 968–975. [Google Scholar] [CrossRef] [PubMed]

| Criteria | Description |
|---|---|
| Excessive work of breathing | Dyspnea with Silverman score > 6 and/or severe tachypnea (>100 breaths/min), despite optimized noninvasive respiratory support |
| Absent or inadequate respiratory effort | Apnea > 4 events/hour or >2 events/hour requiring positive pressure ventilation with mask, despite optimized noninvasive respiratory support and adequate caffeine therapy |
| Severe respiratory acidosis | Arterial/Capillary blood: pH < 7.20 and pCO2 > 60 mmHg at 0–72 h of life, pCO2 > 65 mmHg beyond 72 h of life, despite optimized noninvasive respiratory support |
| High oxygen requirement | FiO2 > 0.50 for ELGANs or FiO2 > 0.60 for newborns between 28 and 32 weeks of GA, to maintain adequate range value of paO2 > 50–60 mmHg (6.7–8 kPa) and adequate range of SpO2 (90–95%) despite optimized noninvasive respiratory support and surfactant treatment for RDS |
| Moderate or severe respiratory distress and contraindications for noninvasive ventilatory support | Intestinal perforation, Intestinal obstruction, esophageal atresia Recent gastrointestinal surgery |
| Postoperative period | Recent abdominal incision Recent tracheostomy Residual effects of anesthetic agents Need for muscle relaxant drugs |
| Value | Preterm Infants | Infants with BPD | Infants with BPD and PPHN |
|---|---|---|---|
| pH (arterial) | 7.25–7.35 | ≥7.25 | ≥7.25 |
| PaO2 (mmHg) | 45–65 | 45–65 | 55–75 |
PaCO2 (mmHg):
| 45–55 <60 <65 | 55–65 <70 | 45–60 <70 |
| SpO2 (%) | 90–95 | 92–95 | 97–98 |
| Phase | Respiratory Management |
|---|---|
| Delivery room stabilization | Early initiation of CPAP Noninvasive respiratory support in spontaneously breathing infants to avoid intubation |
| Use of T-piece resuscitators Use of oxygen blender Preductal SpO2 > 80% by 5 min of life Target SpO2: 90–95% Avoid prolonged period of hypoxia (SpO2 < 80%) and fluctuation in SpO2 | |
| Noninvasive respiratory support in Neonatal Intensive Care Unit | Encourage noninvasive respiratory support (CPAP, NIPPV, SNIPPV) avoiding endotracheal intubation and mechanical ventilation (see Figure 1 and Figure 2) Avoid prolonged period of hypoxia (SpO2 < 80%) and fluctuation in SpO2 |
| Mechanical ventilation in Neonatal Intensive Care Unit | Refer to specific indications for intubation and mechanical ventilation (see Table 1) |
| Refer to specific goals of MV Avoid prolonged period of hypoxia (SpO2 < 80%) and fluctuation in SpO2 Choose lung protective ventilation both during CMV and HFOV Consider volume target ventilation strategy both during CMV and HFOV Refer to specific weaning and extubation criteria (i.e., clinical stability, MAP < 8 cm H2O and FiO2 < 30%) Trial of extubation to CPAP/NIPPV/SNIPPV prior to 7 days of life or as early as possible | |
| Surfactant administration | Administer surfactant as early as possible in preterm infants with RDS that require FiO2 > 30% in CPAP ≥ 6 cm H2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tana, M.; Tirone, C.; Aurilia, C.; Lio, A.; Paladini, A.; Fattore, S.; Esposito, A.; De Tomaso, D.; Vento, G. Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside. Children 2023, 10, 535. https://doi.org/10.3390/children10030535
Tana M, Tirone C, Aurilia C, Lio A, Paladini A, Fattore S, Esposito A, De Tomaso D, Vento G. Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside. Children. 2023; 10(3):535. https://doi.org/10.3390/children10030535
Chicago/Turabian StyleTana, Milena, Chiara Tirone, Claudia Aurilia, Alessandra Lio, Angela Paladini, Simona Fattore, Alice Esposito, Davide De Tomaso, and Giovanni Vento. 2023. "Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside" Children 10, no. 3: 535. https://doi.org/10.3390/children10030535
APA StyleTana, M., Tirone, C., Aurilia, C., Lio, A., Paladini, A., Fattore, S., Esposito, A., De Tomaso, D., & Vento, G. (2023). Respiratory Management of the Preterm Infant: Supporting Evidence-Based Practice at the Bedside. Children, 10(3), 535. https://doi.org/10.3390/children10030535






