Development and Disorders of the Airway in Bronchopulmonary Dysplasia
Abstract
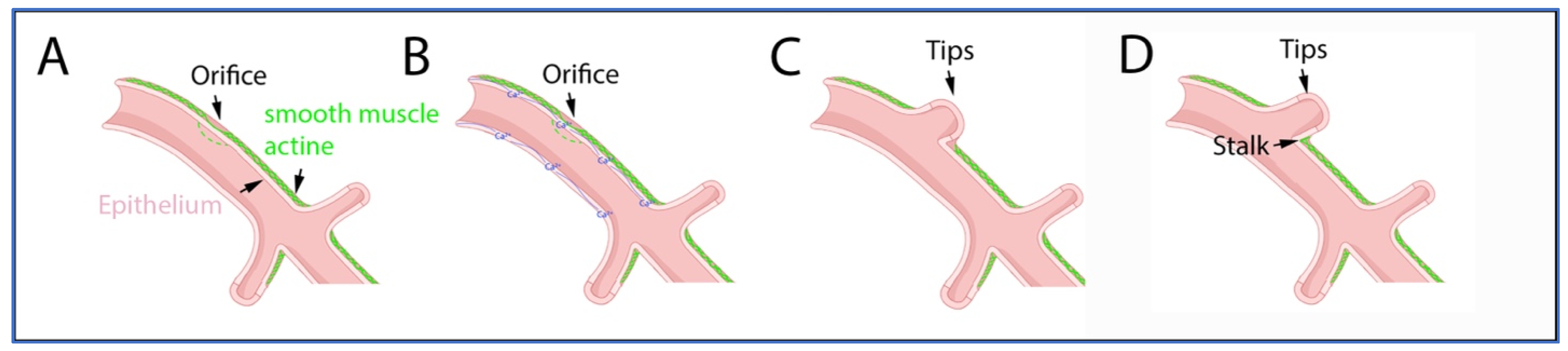
1. Overview of Airway Development
2. The Large Airways
2.1. Subglottic Stenosis
2.2. Tracheomalacia and Bronchomalacia
3. The Small Airways
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tracy, M.C.; Cornfield, D.N. Bronchopulmonary Dysplasia: Then, Now, and Next. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.Z.; Sun, D.; Rawlins, E.L. Human lung development: Recent progress and new challenges. Development 2018, 145, dev163485. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; Schwarz, M.; Tefft, D.; Flores-Delgado, G.; Anderson, K.D.; Cardoso, W.V. The molecular basis of lung morphogenesis. Mech. Dev. 2000, 92, 55–81. [Google Scholar] [CrossRef] [PubMed]
- Ardini-Poleske, M.E.; Clark, R.F.; Ansong, C.; Carson, J.P.; Corley, R.A.; Deutsch, G.H.; Hagood, J.S.; Kaminski, N.; Mariani, T.J.; Potter, S.S.; et al. LungMAP: The Molecular Atlas of Lung Development Program. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L733–L740. [Google Scholar] [CrossRef]
- Zepp, J.A.; Morrisey, E.E. Cellular crosstalk in the development and regeneration of the respiratory system. Nat. Rev. Mol. Cell Biol. 2019, 20, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Minoo, P.; Hamdan, H.; Bu, D.; Warburton, D.; Stepanik, P.; deLemos, R. TTF-1 regulates lung epithelial morphogenesis. Dev. Biol. 1995, 172, 694–698. [Google Scholar] [CrossRef]
- Warburton, D.; El-Hashash, A.; Carraro, G.; Tiozzo, C.; Sala, F.; Rogers, O.; De Langhe, S.; Kemp, P.J.; Riccardi, D.; Torday, J.; et al. Lung organogenesis. Curr. Top. Dev. Biol. 2010, 90, 73–158. [Google Scholar]
- Que, J.; Luo, X.; Schwartz, R.J.; Hogan, B.L. Multiple roles for Sox2 in the developing and adult mouse trachea. Development 2009, 136, 1899–1907. [Google Scholar] [CrossRef]
- Lungova, V.; Thibeault, S.L. Mechanisms of larynx and vocal fold development and pathogenesis. Cell. Mol. Life Sci. 2020, 77, 3781–3795. [Google Scholar] [CrossRef]
- Jones, M.R.; Chong, L.; Bellusci, S. Fgf10/Fgfr2b Signaling Orchestrates the Symphony of Molecular, Cellular, and Physical Processes Required for Harmonious Airway Branching Morphogenesis. Front. Cell Dev. Biol. 2020, 8, 620667. [Google Scholar] [CrossRef]
- Metzger, R.J.; Klein, O.D.; Martin, G.R.; Krasnow, M.A. The branching programme of mouse lung development. Nature 2008, 453, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.D.; Bordoni, B. Embryology, Aortic Arch; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Schittny, J.C. Development of the lung. Cell Tissue Res. 2017, 367, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, S.; Alonso, I.; Thornton, M.E.; Grubbs, B.H.; Bellusci, S.; Warburton, D.; Al Alam, D. Human lung branching morphogenesis is orchestrated by the spatiotemporal distribution of ACTA2, SOX2, and SOX9. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L144–L149. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, P.K. The development of large and small airways. Am. J. Respir. Crit. Care Med. 1998, 157, S174–S180. [Google Scholar] [CrossRef]
- Mullassery, D.; Smith, N.P. Lung development. Semin. Pediatr. Surg. 2015, 24, 152–155. [Google Scholar] [CrossRef]
- Davis, R.P.; Mychaliska, G.B. Neonatal pulmonary physiology. Semin. Pediatr. Surg. 2013, 22, 179–184. [Google Scholar] [CrossRef]
- Sala, F.G.; Del Moral, P.M.; Tiozzo, C.; Alam, D.A.; Warburton, D.; Grikscheit, T.; Veltmaat, J.M.; Bellusci, S. FGF10 controls the patterning of the tracheal cartilage rings via Shh. Development 2011, 138, 273–282. [Google Scholar] [CrossRef]
- Tiozzo, C.; De Langhe, S.; Carraro, G.; Alam, D.A.; Nagy, A.; Wigfall, C.; Hajihosseini, M.K.; Warburton, D.; Minoo, P.; Bellusci, S. Fibroblast growth factor 10 plays a causative role in the tracheal cartilage defects in a mouse model of Apert syndrome. Pediatr. Res. 2009, 66, 386–390. [Google Scholar] [CrossRef]
- Elluru, R.G.; Thompson, F.; Reece, A. Fibroblast growth factor 18 gives growth and directional cues to airway cartilage. Laryngoscope 2009, 119, 1153–1165. [Google Scholar] [CrossRef]
- Kina, Y.P.; Khadim, A.; Seeger, W.; El Agha, E. The Lung Vasculature: A Driver or Passenger in Lung Branching Morphogenesis? Front. Cell Dev. Biol. 2020, 8, 623868. [Google Scholar] [CrossRef]
- Belgacemi, R.; Danopoulos, S.; Deutsch, G.; Glass, I.; Dormoy, V.; Bellusci, S.; Al Alam, D. Hedgehog Signaling Pathway Orchestrates Human Lung Branching Morphogenesis. Int. J. Mol. Sci. 2022, 23, 5265. [Google Scholar] [CrossRef]
- Aros, C.J.; Pantoja, C.J.; Gomperts, B.N. Wnt signaling in lung development, regeneration, and disease progression. Commun. Biol. 2021, 4, 601. [Google Scholar] [CrossRef]
- Noe, N.; Shim, A.; Millette, K.; Luo, Y.; Azhar, M.; Shi, W.; Warburton, D.; Turcatel, G. Mesenchyme-specific deletion of Tgf-beta1 in the embryonic lung disrupts branching morphogenesis and induces lung hypoplasia. Lab. Invest. 2019, 99, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D. Conserved Mechanisms in the Formation of the Airways and Alveoli of the Lung. Front. Cell Dev. Biol. 2021, 9, 662059. [Google Scholar] [CrossRef] [PubMed]
- Copland, I.; Post, M. Lung development and fetal lung growth. Paediatr. Respir. Rev. 2004, 5 (Suppl. A), S259–S264. [Google Scholar] [CrossRef]
- Hislop, A.A. Airway and blood vessel interaction during lung development. J. Anat. 2002, 201, 325–334. [Google Scholar] [CrossRef]
- Burri, P.H. Structural aspects of postnatal lung development—Alveolar formation and growth. Neonatology 2006, 89, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Thebaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef]
- Northway, W.H., Jr.; Rosan, R.C.; Porter, D.Y. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N. Engl. J. Med. 1967, 276, 357–368. [Google Scholar] [CrossRef]
- West, J.B. Mechanics of Breathing: How the lung is supported and moved. In Respiratory Physiology: The Essentials, 8th ed.; Duff, N., Ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2008; pp. 95–122. [Google Scholar]
- Lumb, A. Nunn’s Applied Respiratory Physiology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Bush, D.; Juliano, C.; Laitman, B.M.; Londino, A.; Spencer, C. A comprehensive, multidisciplinary approach to the evaluation of the neonatal airway. Curr. Pediatr. Rep. 2019, 7, 107–115. [Google Scholar] [CrossRef]
- Hysinger, E.B. Central airway issues in bronchopulmonary dysplasia. Pediatr. Pulmonol. 2021, 56, 3518–3526. [Google Scholar] [CrossRef]
- Deoras, K.S.; Wolfson, M.R.; Searls, R.L.; Hilfer, S.R.; Shaffer, T.H. Developmental changes in tracheal structure. Pediatr. Res. 1991, 30, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Croteau, J.R.; Cook, C.D. Volume-pressure and length-tension measurements in human tracheal and bronchial segments. J. Appl. Physiol. 1961, 16, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Panitch, H.B.; Allen, J.L.; Alpert, B.E.; Schidlow, D.V. Effects of CPAP on lung mechanics in infants with acquired tracheobronchomalacia. Am. J. Respir. Crit. Care Med. 1994, 150, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, M.; Wakai, Y.; Osborne, S.; Pare, P.D.; Road, J.D. Effect of vagal stimulation and parenteral acetylcholine on canine trachealis muscle shortening. J. Appl. Physiol. 1992, 72, 2463–2468. [Google Scholar] [CrossRef]
- Panitch, H.B.; Deoras, K.S.; Wolfson, M.R.; Shaffer, T.H. Maturational changes in airway smooth muscle structure-function relationships. Pediatr. Res. 1992, 31, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Panitch, H.B.; Allen, J.L.; Ryan, J.P.; Wolfson, M.R.; Shaffer, T.H. A comparison of preterm and adult airway smooth muscle mechanics. J. Appl. Physiol. 1989, 66, 1760–1765. [Google Scholar] [CrossRef]
- Gunatilaka, C.C.; Higano, N.S.; Hysinger, E.B.; Gandhi, D.B.; Fleck, R.J.; Hahn, A.D.; Fain, S.B.; Woods, J.C.; Bates, A.J. Increased Work of Breathing due to Tracheomalacia in Neonates. Ann. Am. Thorac. Soc. 2020, 17, 1247–1256. [Google Scholar] [CrossRef]
- Snijders, D.; Barbato, A. An Update on Diagnosis of Tracheomalacia in Children. Eur. J. Pediatr. Surg. 2015, 25, 333–335. [Google Scholar]
- Vijayasekaran, S.; Lioy, J.; Maschhoff, K. Airway disorders of the fetus and neonate: An overview. Semin. Fetal Neonatal Med. 2016, 21, 220–229. [Google Scholar] [CrossRef]
- Allen, J.L.; Greenspan, J.S.; Deoras, K.S.; Keklikian, E.; Wolfson, M.R.; Shaffer, T.H. Interaction between chest wall motion and lung mechanics in normal infants and infants with bronchopulmonary dysplasia. Pediatr. Pulmonol. 1991, 11, 37–43. [Google Scholar] [CrossRef]
- Deoras, K.S.; Wolfson, M.R.; Bhutani, V.K.; Shaffer, T.H. Structural changes in the tracheae of preterm lambs induced by ventilation. Pediatr. Res. 1989, 26, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Penn, R.B.; Wolfson, M.R.; Shaffer, T.H. Effect of ventilation on mechanical properties and pressure-flow relationships of immature airways. Pediatr. Res. 1988, 23, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Rao, S.C.; Minutillo, C.; Vijayasekaran, S.; Nathan, E.A. Severe acquired subglottic stenosis in neonatal intensive care graduates: A case-control study. Arch. Dis. Child Fetal Neonatal Ed. 2018, 103, F349–F354. [Google Scholar] [CrossRef]
- Walner, D.L.; Loewen, M.S.; Kimura, R.E. Neonatal subglottic stenosis--incidence and trends. Laryngoscope 2001, 111, 48–51. [Google Scholar] [CrossRef]
- Holzki, J.; Brown, K.A.; Carroll, R.G.; Cote, C.J. The anatomy of the pediatric airway: Has our knowledge changed in 120 years? A review of historic and recent investigations of the anatomy of the pediatric larynx. Paediatr. Anaesth. 2018, 28, 13–22. [Google Scholar] [CrossRef]
- Rutter, M.; Kuo, I.C. Predicting and managing the development of subglottic stenosis following intubation in children. J. Pediatr. 2020, 96, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Higano, N.S.; Bates, A.J.; Gunatilaka, C.C.; Hysinger, E.B.; Critser, P.J.; Hirsch, R.; Woods, J.C.; Fleck, R.J. Bronchopulmonary dysplasia from chest radiographs to magnetic resonance imaging and computed tomography: Adding value. Pediatr. Radiol. 2022, 52, 643–660. [Google Scholar] [CrossRef]
- Upadhyay, K.; Vallarino, D.A.; Talati, A.J. Outcomes of neonates with tracheostomy secondary to bronchopulmonary dysplasia. BMC Pediatr. 2020, 20, 414. [Google Scholar] [CrossRef]
- Amin, R.S.; Rutter, M.J. Airway Disease and Management in Bronchopulmonary Dysplasia. Clin. Perinatol. 2015, 42, 857–870. [Google Scholar] [CrossRef]
- Eichenwald, E.C.; Committee On Fetus And Newborn; Cummings, J.J.; Aucott, S.W.; Goldsmith, J.P.; Hand, I.L.; Juul, S.E.; Poindexter, B.B.; Puopolo, K.M.; Stewart, D.L. Diagnosis and Management of Gastroesophageal Reflux in Preterm Infants. Pediatrics 2018, 142, e20181061. [Google Scholar] [CrossRef] [PubMed]
- Maresh, A.; Preciado, D.A.; O’Connell, A.P.; Zalzal, G.H. A comparative analysis of open surgery vs endoscopic balloon dilation for pediatric subglottic stenosis. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Jensen, E.A.; White, A.M.; Wang, Y.; Biko, D.M.; Nilan, K.; Fraga, M.V.; Mercer-Rosa, L.; Zhang, H.; Kirpalani, H. Characterization of Disease Phenotype in Very Preterm Infants with Severe Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2020, 201, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Hysinger, E.; Friedman, N.; Jensen, E.; Zhang, H.; Piccione, J. Bronchoscopy in neonates with severe bronchopulmonary dysplasia in the NICU. J. Perinatol. 2019, 39, 263–268. [Google Scholar] [CrossRef]
- Su, Y.-T.; Chiu, C.-C.; Lai, S.-H.; Hsia, S.-H.; Lin, J.-J.; Chan, O.-W.; Chiu, C.-Y.; Tseng, P.-L.; Lee, E.-P. Risk Factors for Tracheobronchomalacia in Preterm Infants With Bronchopulmonary Dysplasia. Front. Pediatr. 2021, 9, 697470. [Google Scholar] [CrossRef]
- Wallis, C.; Alexopoulou, E.; Anton-Pacheco, J.L.; Bhatt, J.M.; Bush, A.; Chang, A.B.; Charatsi, A.-M.; Coleman, C.; Depiazzi, J.; Douros, K.; et al. ERS statement on tracheomalacia and bronchomalacia in children. Eur. Respir. J. 2019, 54, 1900382. [Google Scholar] [CrossRef]
- Hysinger, E.B.; Panitch, H.B. Paediatric Tracheomalacia. Paediatr. Respir. Rev. 2016, 17, 9–15. [Google Scholar] [CrossRef]
- Panitch, H.B.; Keklikian, E.N.; Motley, R.A.; Wolfson, M.R.; Schidlow, D.V. Effect of altering smooth muscle tone on maximal expiratory flows in patients with tracheomalacia. Pediatr. Pulmonol. 1990, 9, 170–176. [Google Scholar] [CrossRef]
- Pan, W.; Peng, D.; Luo, J.; Liu, E.; Luo, Z.; Dai, J.; Fu, Z.; Li, Q.; Huang, Y. Clinical features of airway malacia in children: A retrospective analysis of 459 patients. Int. J. Clin. Exp. Med. 2014, 7, 3005–3012. [Google Scholar]
- Aslam, A.; De Luis Cardenas, J.; Morrison, R.J.; Lagisetty, K.H.; Litmanovich, D.; Sella, E.C.; Lee, E.; Agarwal, P. Tracheobronchomalacia and Excessive Dynamic Airway Collapse: Current Concepts and Future Directions. Radiographics 2022, 42, 1012–1027. [Google Scholar] [CrossRef]
- Murgu, S.D.; Colt, H.G. Tracheobronchomalacia and excessive dynamic airway collapse. Respirology 2006, 11, 388–406. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.V.; Elliott, E.A. Wave-speed limitation on expiratory flow-a unifying concept. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1977, 43, 498–515. [Google Scholar] [CrossRef] [PubMed]
- Vicencio, A.G.; Piccione, J. Lights, Camera, Action: Airway Dynamics Takes Center Stage. Chest 2020, 157, 489–490. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; McKay, K.O.; van Asperen, P.P.; Selvadurai, H.; Fitzgerald, D.A. Normal development of the lung and premature birth. Paediatr. Respir. Rev. 2010, 11, 135–142. [Google Scholar] [CrossRef]
- Jobe, A.H.; Bancalari, E. Bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001, 163, 1723–1729. [Google Scholar] [CrossRef]
- Royce, S.G.; Nold, M.F.; Bui, C.; Donovan, C.; Lam, M.; Lamanna, E.; Rudloff, I.; Bourke, J.E.; Nold-Petry, C.A. Airway Remodeling and Hyperreactivity in a Model of Bronchopulmonary Dysplasia and Their Modulation by IL-1 Receptor Antagonist. Am. J. Respir. Cell Mol. Biol. 2016, 55, 858–868. [Google Scholar] [CrossRef]
- Ha, A.W.; Sudhadevi, T.; Ebenezer, D.L.; Fu, P.; Berdyshev, E.V.; Ackerman, S.J.; Natarajan, V.; Harijith, A. Neonatal therapy with PF543, a sphingosine kinase 1 inhibitor, ameliorates hyperoxia-induced airway remodeling in a murine model of bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L497–L512. [Google Scholar] [CrossRef]
- Surate Solaligue, D.E.; Rodriguez-Castillo, J.A.; Ahlbrecht, K.; Morty, R.E. Recent advances in our understanding of the mechanisms of late lung development and bronchopulmonary dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L1101–L1153. [Google Scholar] [CrossRef]
- Martin, C.; Frija, J.; Burgel, P.R. Dysfunctional lung anatomy and small airways degeneration in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2013, 8, 7–13. [Google Scholar]
- McGrath-Morrow, S.A.; Collaco, J.M. Bronchopulmonary dysplasia: What are its links to COPD? Ther. Adv. Respir. Dis. 2019, 13, 1753466619892492. [Google Scholar] [CrossRef]
- Shepherd, E.G.; Clouse, B.J.; Hasenstab, K.A.; Sitaram, S.; Malleske, D.T.; Nelin, L.D.; Jadcherla, S.R. Infant Pulmonary Function Testing and Phenotypes in Severe Bronchopulmonary Dysplasia. Pediatrics 2018, 141, e20173350. [Google Scholar] [CrossRef]
- Nelin, L.D.; Kielt, M.J.; Jebbia, M.; Jadcherla, S.; Shepherd, E.G. Bronchodilator responsiveness and dysanapsis in bronchopulmonary dysplasia. ERJ Open Res. 2022, 8, 682–2021. [Google Scholar] [CrossRef]
- Satrell, E.; Clemm, H.; Roksund, O.D.; Hufthammer, K.O.; Thorsen, E.; Halvorsen, T.; Vollsæter, M. Development of lung diffusion to adulthood following extremely preterm birth. Eur. Respir. J. 2022, 59, 2004103. [Google Scholar] [CrossRef] [PubMed]
- Bardsen, T.; Roksund, O.D.; Eagan, T.M.; Hufthammer, K.O.; Benestad, M.R.; Clemm, H.S.; Halvorsen, T.; Vollsæter, M. Impaired Lung Function in Extremely Preterm-Born Adults in Their Fourth Decade of Life. Am. J. Respir. Crit. Care Med. 2023. [Google Scholar] [CrossRef]
- Bardsen, T.; Roksund, O.D.; Benestad, M.R.; Hufthammer, K.O.; Clemm, H.H.; Mikalsen, I.B.; Øymar, K.; Markestad, T.; Halvorsen, T.; Vollsæter, M. Tracking of lung function from 10 to 35 years after being born extremely preterm or with extremely low birth weight. Thorax 2022, 77, 790–798. [Google Scholar] [CrossRef]
- Doyle, L.W. Respiratory function at age 8–9 years in extremely low birthweight/very preterm children born in Victoria in 1991–1992. Pediatr. Pulmonol. 2006, 41, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Thunqvist, P.; Tufvesson, E.; Bjermer, L.; Winberg, A.; Fellman, V.; Domellof, M.; Melén, E.; Norman, M.; Hallberg, J. Lung function after extremely preterm birth-A population-based cohort study (EXPRESS). Pediatr. Pulmonol. 2018, 53, 64–72. [Google Scholar] [CrossRef]
- Simpson, S.J.; Turkovic, L.; Wilson, A.C.; Verheggen, M.; Logie, K.M.; Pillow, J.J.; Hall, G.L. Lung function trajectories throughout childhood in survivors of very preterm birth: A longitudinal cohort study. Lancet Child Adolesc. Health 2018, 2, 350–359. [Google Scholar] [CrossRef]
- Doyle, L.W.; Andersson, S.; Bush, A.; Cheong, J.L.Y.; Clemm, H.; Evensen, K.A.I.; Gough, A.; Halvorsen, T.; Hovi, P.; Kajantie, E.; et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with controls born at term or with normal birthweight: A meta-analysis of individual participant data. Lancet Respir. Med. 2019, 7, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Urs, R.; Kotecha, S.; Hall, G.L.; Simpson, S.J. Persistent and progressive long-term lung disease in survivors of preterm birth. Paediatr. Respir. Rev. 2018, 28, 87–94. [Google Scholar] [CrossRef]
- Ioan, I.; Gemble, A.; Hamon, I.; Schweitzer, C.; Metche, S.; Bonabel, C.; Nguyen-thi, P.L.; Hascoet, J.-M.; Demoulin-Alexikova, S.; Marchal, F.; et al. Expiratory Flow—Vital Capacity: Airway—Lung Dysanapsis in 7 Year Olds Born Very Preterm? Front. Physiol. 2018, 9, 650. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.S.; Perret, J.L.; Walters, E.H.; Lodge, C.J.; Bowatte, G.; Hamilton, G.S.; Thompson, B.R.; Frith, P.; Erbas, B.; Thomas, P.S.; et al. Association between very to moderate preterm births, lung function deficits, and COPD at age 53 years: Analysis of a prospective cohort study. Lancet Respir. Med. 2022, 10, 478–484. [Google Scholar] [CrossRef]
- Broström, E.B.; Thunqvist, P.; Adenfelt, G.; Borling, E.; Katz-Salamon, M. Obstructive lung disease in children with mild to severe BPD. Respir. Med. 2010, 104, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Spielberg, D.R.; Walkup, L.L.; Stein, J.M.; Crotty, E.J.; Rattan, M.S.; Hossain, M.M.; Brody, A.S.; Woods, J.C. Quantitative CT scans of lung parenchymal pathology in premature infants ages 0–6 years. Pediatr. Pulmonol. 2018, 53, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Logie, K.M.; O’Dea, C.A.; Banton, G.L.; Murray, C.; Wilson, A.C.; Pillow, J.J.; Hall, G.L. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax 2017, 72, 702–711. [Google Scholar] [CrossRef]
- Ronkainen, E.; Perhomaa, M.; Mattila, L.; Hallman, M.; Dunder, T. Structural Pulmonary Abnormalities Still Evident in Schoolchildren with New Bronchopulmonary Dysplasia. Neonatology 2018, 113, 122–130. [Google Scholar] [CrossRef]

| Airway Region | Diagnosis | Etiology | Obstruction Type | Signs/Symptoms | Diagnostic Studies |
|---|---|---|---|---|---|
| Subglottis | Subglottic stenosis * | Acquired/Congenital | Fixed | Biphasic stridor/wheezing | FB, RB |
| Subglottic hemangioma | Congenital | Fixed | Biphasic stridor/wheezing | FL, FB, RB | |
| Trachea | Tracheoesophageal fistula | Congenital | Dynamic | Coughing with feeding, recurrent pneumonia | CXR, FB, or RB with methylene blue instillation |
| Tracheomalacia * | Acquired | Dynamic | Expiratory wheezing | FB, MRI | |
| Tracheomegaly | Acquired | Dynamic | Wheezing | FB, RB, CT | |
| Tracheal compression (extrinsic) * | Acquired/Congenital | Dynamic or Fixed | Expiratory wheezing/Biphasic wheezing | FB, RB, CT | |
| Tracheal web | Congenital | Fixed | Biphasic wheezing | FB, RB | |
| Tracheal ring/stenosis | Acquired/Congenital | Fixed | Biphasic wheezing | RB, RB, CT | |
| Vascular ring, Pulmonary artery sling | Congenital | Fixed | Biphasic wheezing | FB, RB, CTA | |
| Tracheal bronchus | Congenital | Dynamic (regional) | Coughing, Retained secretions, Recurrent pneumonia | FB, RB, CT | |
| Bronchi | Bronchomalacia * | Acquired | Dynamic | Expiratory wheezing | FB |
| Bronchial stenosis | Acquired/Congenital | Fixed | Wheezing, air trapping | FB | |
| Bronchial compression (extrinsic) * | Acquired/Congenital | Dynamic or Fixed | Wheezing, air trapping | FB | |
| Airway granuloma | Acquired | Fixed | Wheezing, air trapping | FB, RB | |
| Bronchioles | Obstructive airways disease * | Acquired | Fixed/Reversible ^ | Wheezing, air trapping | PFT |
| Atelectasis * | Acquired | Reversible | Reduced air entry, crackles | CXR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, D.; Juliano, C.; Bowler, S.; Tiozzo, C. Development and Disorders of the Airway in Bronchopulmonary Dysplasia. Children 2023, 10, 1127. https://doi.org/10.3390/children10071127
Bush D, Juliano C, Bowler S, Tiozzo C. Development and Disorders of the Airway in Bronchopulmonary Dysplasia. Children. 2023; 10(7):1127. https://doi.org/10.3390/children10071127
Chicago/Turabian StyleBush, Douglas, Courtney Juliano, Selina Bowler, and Caterina Tiozzo. 2023. "Development and Disorders of the Airway in Bronchopulmonary Dysplasia" Children 10, no. 7: 1127. https://doi.org/10.3390/children10071127
APA StyleBush, D., Juliano, C., Bowler, S., & Tiozzo, C. (2023). Development and Disorders of the Airway in Bronchopulmonary Dysplasia. Children, 10(7), 1127. https://doi.org/10.3390/children10071127







