Does Increased Femoral Anteversion Can Cause Hip Abductor Muscle Weakness?
Abstract
:1. Introduction
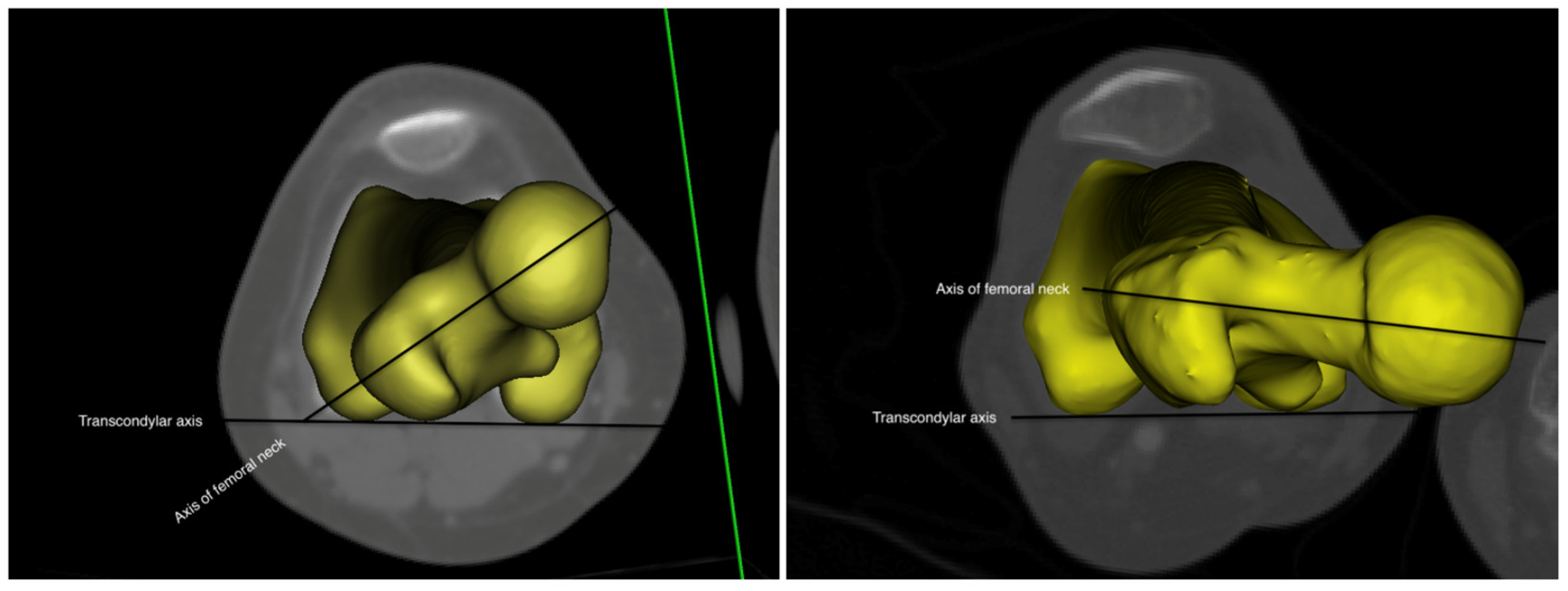
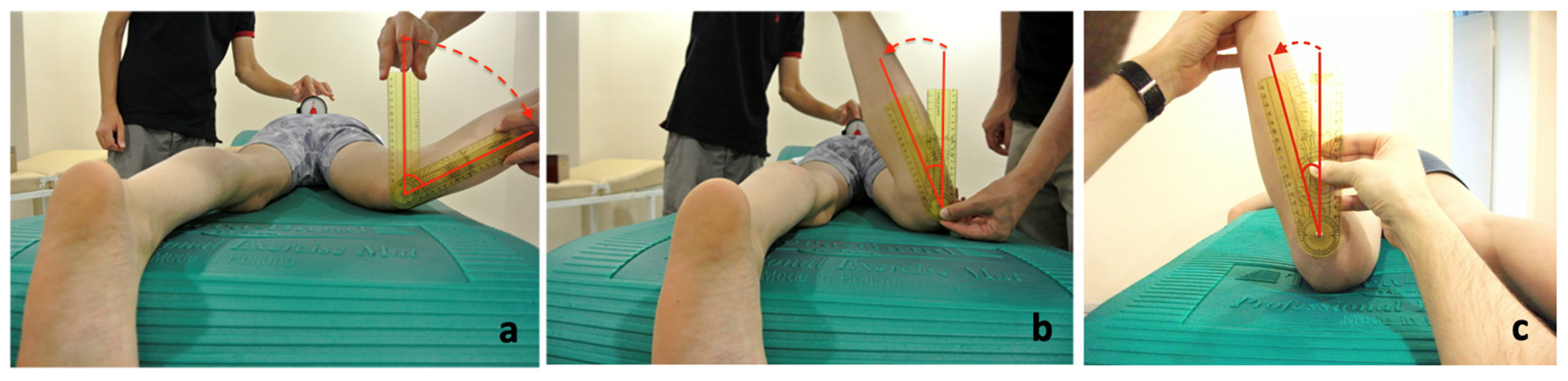
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mooney, J.F. Lower Extremity Rotational and Angular Issues in Children. Pediatr. Clin. N. Am. 2014, 61, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, G.D. Anteversion of the femur. Postgrad. Med. 1976, 60, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Leblebici, G.; Akalan, E.; Apti, A.; Kuchimov, S.; Kurt, A.; Onerge, K.; Temelli, Y.; Miller, F. Increased femoral anteversion-related biomechanical abnormalities: Lower extremity function, falling frequencies, and fatigue. Gait Posture 2019, 70, 336–340. [Google Scholar] [CrossRef]
- Alexander, N.; Studer, K.; Lengnick, H.; Payne, E.; Klima, H.; Wegener, R. The impact of increased femoral antetorsion on gait deviations in healthy adolescents. J. Biomech. 2019, 86, 167–174. [Google Scholar] [CrossRef]
- Byrnes, S.K.; Kunic, D.; Rethwilm, R.; Böhm, H.; Horstmann, T.; Dussa, C.U. Compensatory mechanisms in children with idiopathic lower extremity internal rotational malalignment during walking and running. Gait Posture 2020, 79, 46–52. [Google Scholar] [CrossRef]
- Apti, A.; Akalan, E.; Leblebici, G.; Kuchimov, S.; Kurt, A.; Kilicoglu, O.; Temelli, Y.; Miller, F. Frontal kinematic deviations between hyper and hypomobile children with increased femoral anteversion. Gait Posture 2021, 90, 9–10. [Google Scholar] [CrossRef]
- Powers, C.M. The Influence of Altered Lower-Extremity Kinematics on Patellofemoral Joint Dysfunction: A Theoretical Perspective. J. Orthop. Sports Phys. Ther. 2003, 33, 639–646. [Google Scholar] [CrossRef]
- Bedi, A.; Dolan, M.; Leunig, M.; Kelly, B.T. Static and Dynamic Mechanical Causes of Hip Pain. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 235–251. [Google Scholar] [CrossRef]
- Milani, C.J.; Moley, P.J. Advanced Concepts in Hip Morphology, Associated Pathologies, and Specific Rehabilitation for Athletic Hip Injuries. Curr. Sports Med. Rep. 2018, 17, 199–207. [Google Scholar] [CrossRef]
- Arnold, A.S.; Komallu, A.V.; Delp, S.L. Internal rotation gait: A compensatory mechanism to restore abduction capacity decreased by bone deformity? Dev. Med. Child Neurol. 1997, 39, 40–44. [Google Scholar] [CrossRef]
- Nyland, J.; Kuzemchek, S.; Parks, M.; Caborn, D. Femoral anteversion influences vastus medialis and gluteus medius EMG amplitude: Composite hip abductor EMG amplitude ratios during isometric combined hip abduction-external rotation. J. Electromyogr. Kinesiol. 2004, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Marostica, A.D.; Pizzolatti, A.L.A.; Adam, G.P.; Codonho, D.; Canella, R.P.; Ganev, G.G. Is Femoral Version Associated with Changes in Hip Muscle Strength in Females with Symptomatic Femoroacetabular Impingement? Rev. Bras. Ortop. Sao Paulo 2019, 54, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M. The Influence of Abnormal Hip Mechanics on Knee Injury: A Biomechanical Perspective. J. Orthop. Sports Phys. Ther. 2010, 40, 42–51. [Google Scholar] [CrossRef]
- Selistre, L.F.A.; Gonçalves, G.H.; Nakagawa, T.H.; Petrella, M.; Jones, R.K.; Mattiello, S.M. The role of hip abductor strength on the frontal plane of gait in subjects with medial knee osteoarthritis. Physiother. Res. Int. 2019, 24, e1779. [Google Scholar] [CrossRef]
- Allison, K.; Wrigley, T.V.; Vicenzino, B.; Bennell, K.L.; Grimaldi, A.; Hodges, P.W. Kinematics and kinetics during walking in individuals with gluteal tendinopathy. Clin. Biomech. 2016, 32, 56–63. [Google Scholar] [CrossRef]
- Liu, J.; Lewton, K.L.; Colletti, P.M.; Powers, C.M. Hip Adduction during Running: Influence of Sex, Hip Abductor Strength and Activation, and Pelvis and Femur Morphology. Med. Sci. Sports Exerc. 2021, 53, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Smits-Engelsman, B.; Klerks, M.; Kirby, A. Beighton Score: A Valid Measure for Generalized Hypermobility in Children. J. Pediatr. 2011, 158, 119–123, 123.e1–4. [Google Scholar] [CrossRef]
- Ruwe, P.A.; Gage, J.R.; Ozonoff, M.B.; DeLuca, P.A. Clinical determination of femoral anteversion. A comparison with established techniques. J. Bone Jt. Surg. Am. 1992, 74, 820–830. [Google Scholar] [CrossRef]
- Staheli, L.T.; Corbett, M.; Wyss, C.; King, H. Lower-extremity rotational problems in children. Normal values to guide management. J. Bone Jt. Surg. Am. 1985, 67, 39–47. [Google Scholar] [CrossRef]
- Davids, J.R.; Benfanti, P.; Blackhurst, D.W.; Allen, B.L. Assessment of femoral anteversion in children with cerebral palsy: Accuracy of the trochanteric prominence angle test. J. Pediatr. Orthop. 2002, 22, 173–178. [Google Scholar] [CrossRef]
- Kozic, S.; Gulan, G.; Matovinovic, D.; Nemec, B.; Sestan, B.; Ravlic-Gulan, J. Femoral anteversion related to side differences in hip rotation: Passive rotation in 1, 140 children aged 8–9 years. Acta Orthop. Scand. 1997, 68, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Widler, K.S.; Glatthorn, J.F.; Bizzini, M.; Impellizzeri, F.M.; Munzinger, U.; Leunig, M.; Maffiuletti, N.A. Assessment of Hip Abductor Muscle Strength. A Validity and Reliability Study. J. Bone Jt. Surg. Am. 2009, 91, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.B.; Kendall, K.D.; Patel, C.; Wiley, J.P.; Emery, C.; Ferber, R. Experimentally Reduced Hip-Abductor Muscle Strength and Frontal-Plane Biomechanics During Walking. J. Athl. Train. 2015, 50, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.B.; Õunpuu, S.; Tyburski, D.; Gage, J.R. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991, 10, 575–587. [Google Scholar] [CrossRef]
- Browner, W.S.; Newman, T.B.; Hulley, S.B. Estimating sample size and power: Applications and examples. Des. Clin. Res. 2007, 3, 66–85. [Google Scholar]
- Norman, G.R.; Sloan, J.A.; Wyrwich, K.W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med. Care 2003, 41, 582–592. [Google Scholar] [CrossRef]
- Passmore, E.; Graham, H.K.; Pandy, M.G.; Sangeux, M. Hip- and patellofemoral-joint loading during gait are increased in children with idiopathic torsional deformities. Gait Posture 2018, 63, 228–235. [Google Scholar] [CrossRef]
- Jindal, P.; Narayan, A.; Ganesan, S.; MacDermid, J.C. Muscle strength differences in healthy young adults with and without generalized joint hypermobility: A cross-sectional study. BMC Sports Sci. Med. Rehabilit. 2016, 8, 12. [Google Scholar] [CrossRef]
- Simmonds, J.V.; Keer, R.J. Hypermobility and the hypermobility syndrome. Man. Ther. 2007, 12, 298–309. [Google Scholar] [CrossRef]
- Scott, D.A.; Bond, E.Q.; Sisto, S.A.; Nadler, S.F. The intra- and interrater reliability of hip muscle strength assessments using a handheld versus a portable dynamometer anchoring station. Arch. Phys. Med. Rehabilit. 2004, 85, 598–603. [Google Scholar] [CrossRef]
- Bazett-Jones, D.M.; Cobb, S.C.; Joshi, M.N.; Cashin, S.E.; Earl, J.E. Normalizing Hip Muscle Strength: Establishing Body-Size-Independent Measurements. Arch. Phys. Med. Rehabilit. 2011, 92, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.; Aaboe, J.; Simonsen, E.B.; Alkjær, T.; Bliddal, H. Experimentally reduced hip abductor function during walking: Implications for knee joint loads. J. Biomech. 2009, 42, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Boyer, E.R.; Novacheck, T.F.; Schwartz, M.H. Changes in hip abductor moment 3 or more years after femoral derotation osteotomy among individuals with cerebral palsy. Dev. Med. Child Neurol. 2017, 59, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Lee, K.M.; Park, M.S.; Lee, S.H.; Choi, I.H.; Cho, T.-J. Validity and Reliability of Measuring Femoral Anteversion and Neck-Shaft Angle in Patients with Cerebral Palsy. J. Bone Jt. Surg. Am. 2010, 92, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Sangeux, M.; Mahy, J.; Graham, H.K. Do physical examination and CT-scan measures of femoral neck anteversion and tibial torsion relate to each other? Gait Posture 2014, 39, 12–16. [Google Scholar] [CrossRef]

| Control n = 22 Mean ± SD | IFA n = 18 Mean ± SD | p-Value | |
|---|---|---|---|
| Age (year) | 10.25 ± 3.98 | 9.5 ± 2.97 | 0.385 |
| BMI (Kg/m2) | 18.10 ± 6.05 | 18.49 ± 3.5 | 0.461 |
| Maximum hip internal rotation (°) | 38.95 ± 13.66 | 68.33 ± 6.34 | 0.001 * |
| Maximum hip external rotation (°) | 31.55 ± 7.52 | 18.5 ± 4.21 | 0.003 * |
| Craig’s test (°) | 21.25 ± 12.25 | 42.83 ± 10.29 | 0.003 * |
| Normalized hip abductor MIVC (Newton/kg) | 3 ± 0.89 | 4 ± 0.57 | 0.14 |
| Control n = 22 | IFA n = 18 | p-Value | |
|---|---|---|---|
| Gait velocity (m/s.) | 1.27 ± 0.11 | 1.13 ± 0.13 | 0.96 |
| Pelvic obliquity ROM@%GC (°) | 6.33 ± 2.55 | 9.67 ± 5.36 | 0.025 * |
| Peak pelvic depression@%ST (°) | −2.8 ± 1.92 | −4.25 ± 3.67 | 0.149 |
| Peak pelvic rotation@%GC (°) | 8.76 ± 4.56 | 14.12 ± 5.77 | 0.022 * |
| Peak pelvic tilt@%GC (°) | 11.44 ± 2.78 | 12.82 ± 5.89 | 0.371 |
| Peak hip adduction@ST (°) | 4.34 ± 4.03 | 6.12 ± 4.97 | 0.088 |
| Peak hip internal rotation@ST (°) | 3.84 ± 5.31 | 12.24 ± 7.32 | 0.003 * |
| Peak hip extension@ST (°) | −13.01 ± 5.42 | −8.71 ± 6.78 | 0.085 |
| Peak foot internal rotation@ST (°) | −9 ± 7.59 | −3.18 ± 7.29 | 0.022 * |
| Hip adductor moment@ST (N*m/kg) | 0.20 ± 0.15 | −0.32 ± 0.33 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apti, A.; Akalan, N.E. Does Increased Femoral Anteversion Can Cause Hip Abductor Muscle Weakness? Children 2023, 10, 782. https://doi.org/10.3390/children10050782
Apti A, Akalan NE. Does Increased Femoral Anteversion Can Cause Hip Abductor Muscle Weakness? Children. 2023; 10(5):782. https://doi.org/10.3390/children10050782
Chicago/Turabian StyleApti, Adnan, and Nazif Ekin Akalan. 2023. "Does Increased Femoral Anteversion Can Cause Hip Abductor Muscle Weakness?" Children 10, no. 5: 782. https://doi.org/10.3390/children10050782
APA StyleApti, A., & Akalan, N. E. (2023). Does Increased Femoral Anteversion Can Cause Hip Abductor Muscle Weakness? Children, 10(5), 782. https://doi.org/10.3390/children10050782







