Enhancing Post-Operative Recovery in Spastic Diplegia through Physical Therapy Rehabilitation following Selective Dorsal Rhizotomy: A Case Report and Thorough Literature Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Analysis of Co-Occurring Keywords
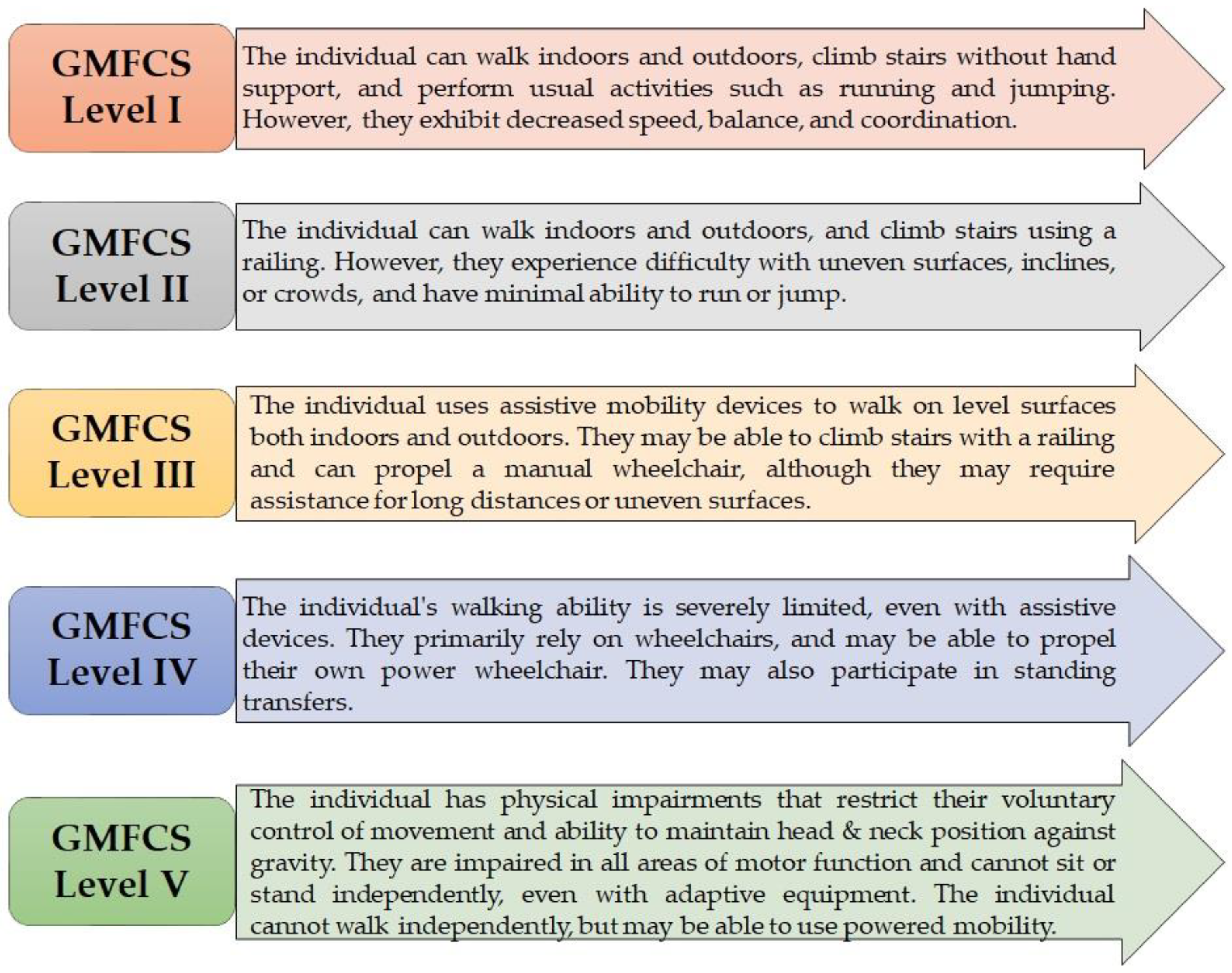
2.4. Case Presentation
3. Framework for Rehabilitation
| Author, Study Design | Post-Operative PT Start Day | Follow-Up Time (Year) | Physical Therapy Intensity and Frequency | Physical Therapy Interventions |
|---|---|---|---|---|
| Graubert et al. [39], prospective randomized trial | - | 1 | The patient underwent 4 weeks of therapy for 10 h per week, followed by 5 months of physiotherapy for 4–5 h per week, and then 6 months of PT for 1–3 h per week. | The patient underwent an intensive physical therapy program, which included the evaluation of gait kinematics and the improvement of ambulation. |
| Buckon et al. [35], RCT study | 4th | 1 | The patient received two PT sessions daily and one OT session daily while in the hospital. After discharge, PT was conducted 3–4 times in a week and OT was conducted 1–2 times per week for a period of six months. PT continued for an additional year, with a frequency of 1–2 times per week. | The treatment plan included the use of assistive devices, occupational therapy, physical therapy, isometric contraction exercises, transfer training, and functional muscle strengthening. |
| Annika Lundkvist Josenby [4] | 1st | 10 | For first 6 months post-surgery, the patients received one-hour sessions of therapy twice a week. Later on, the patients received therapy once a week for 18 months. | Primary goals of therapy were to improve the patient’s posture, enhance their balance control while sitting, standing, transferring, and walking, and also to train them on the use of assistive devices for walking. |
| Sophelia Hoi-shan Chan [38], case series study | 2nd | 1 | At 6 and 12 months post-surgery, the patient underwent four weeks of therapy, consisting of 5 h per day. | The patient received both occupational therapy and physical therapy, with a focus on gait training. |
| Jack R. Engsberg [40] | - | 2 | From the 5th day to the 8th month post-surgery, the patient received therapy four times per week. | The therapy aimed to improve the patient’s gait speed and function, as well as cognitive skills. |
| Petra E. M. van Schie [37] | 1st | 1 | During the first 3 months after surgery, the patient underwent therapy five times a week for 1 h each session. From the 3rd to the 6th month, therapy was conducted four times per week for one hour each session. From the 6th to the 12th month, therapy was conducted three times a week, with each session lasting 30 min. | The therapy program focused on improving the patient’s self-care abilities, gait training with specific emphasis on initial contact and heel-lift, and training in ADLs. |
| Annika Lundkvist Josenby [41], cohort study | 1st | 10 | The patient received one-hour therapy sessions twice a week for 6 months, and then continued with once-a-week sessions thereafter for 18 months. | The therapy program included training the patient on functional ADLs, for example, getting in and out of bed, changing positions, and using assistive devices. |
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Shea, T.M. Diagnosis, Treatment, and Prevention of Cerebral Palsy. Clin. Obstet. Gynecol. 2008, 51, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Radwan, A.; Eltalawy, H.A.; Abdelziem, F.H.; Macaluso, R.; O’Brien, M.K.; Jayaraman, A. Effect of Transcranial Direct Current Stimulation versus Virtual Reality on Gait for Children with Bilateral Spastic Cerebral Palsy: A Randomized Clinical Trial. Children 2023, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Narayan, A.; Muhit, M.; Whitehall, J.; Hossain, I.; Badawi, N.; Khandaker, G.; Jahan, I. Associated Impairments among Children with Cerebral Palsy in Rural Bangladesh—Findings from the Bangladesh Cerebral Palsy Register. J. Clin. Med. 2023, 12, 1597. [Google Scholar] [CrossRef] [PubMed]
- Josenby, A.L.; Wagner, P.; Jarnlo, G.-B.; Westbom, L.; Nordmark, E. Motor Function after Selective Dorsal Rhizotomy: A 10-Year Practice-Based Follow-up Study. Dev. Med. Child Neurol. 2012, 54, 429–435. [Google Scholar] [CrossRef]
- Velnar, T.; Spazzapan, P.; Rodi, Z.; Kos, N.; Bosnjak, R. Selective Dorsal Rhizotomy in Cerebral Palsy Spasticity—A Newly Established Operative Technique in Slovenia: A Case Report and Review of Literature. World J. Clin. Cases 2019, 7, 1133–1141. [Google Scholar] [CrossRef]
- Ismail, A.; Sk Abd Razak, R.; Suddin, L.S.; Mahmud, A.; Kamaralzaman, S.; Yusri, G. The Economic Burden and Determinant Factors of Parents/Caregivers of Children with Cerebral Palsy in Malaysia: A Mixed Methods Study. Int. J. Environ. Res. Public Health 2022, 19, 475. [Google Scholar] [CrossRef]
- Appleton, R.E.; Gupta, R. Cerebral Palsy: Not Always What It Seems. Arch. Dis. Child. 2019, 104, 809–814. [Google Scholar] [CrossRef]
- Hägglund, G.; Wagner, P. Development of Spasticity with Age in a Total Population of Children with Cerebral Palsy. BMC Musculoskelet. Disord. 2008, 9, 150. [Google Scholar] [CrossRef]
- Lindén, O.; Hägglund, G.; Rodby-Bousquet, E.; Wagner, P. The Development of Spasticity with Age in 4162 Children with Cerebral Palsy: A Register-Based Prospective Cohort Study. Acta Orthop. 2019, 90, 286–291. [Google Scholar] [CrossRef]
- de Oliveira Cacho, R.; Cacho, E.W.A.; Loureiro, A.B.; de Medeiros Cirne, G.N.; Pereira, S.A.; de Abreu Freitas, R.P.; Lima, N.M.F.V.; Borges, G. The Spasticity in the Motor and Functional Disability in Adults with Post-Stroke Hemiparetic. Fisioter. Mov. 2017, 30, 745–752. [Google Scholar] [CrossRef]
- Ghotbi, N.; Nakhostin Ansari, N.; Naghdi, S.; Hasson, S. Measurement of Lower-Limb Muscle Spasticity: Intrarater Reliability of Modified Modified Ashworth Scale. J. Rehabil. Res. Dev. 2011, 48, 83–88. [Google Scholar] [CrossRef]
- Nahm, N.J.; Graham, H.K.; Gormley, M.E.J.; Georgiadis, A.G. Management of Hypertonia in Cerebral Palsy. Curr. Opin. Pediatr. 2018, 30, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Givon, U. Management of the Spastic Hip in Cerebral Palsy. Curr. Opin. Pediatr. 2017, 29, 65–69. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, D.; Moussa, A.A.; Ammar, A.; Ingale, H.; Vloeberghs, M. Selective Dorsal Rhizotomy for the Treatment of Severe Spastic Cerebral Palsy: Efficacy and Therapeutic Durability in GMFCS Grade IV and V Children. Acta Neurochir. 2018, 160, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Ahn, J.H.; Rha, D.; Park, E.S. Reliability of the Modified Ashworth and Modified Tardieu Scales with Standardized Movement Speeds in Children with Spastic Cerebral Palsy. Children 2022, 9, 827. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, S.K.; Park, E.S. The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy. Toxins 2019, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Shahid, J.; Kashif, A.; Shahid, M.K. A Comprehensive Review of Physical Therapy Interventions for Stroke Rehabilitation: Impairment-Based Approaches and Functional Goals. Brain Sci. 2023, 13, 717. [Google Scholar] [CrossRef]
- Howard, J.; Soo, B.; Graham, H.K.; Boyd, R.N.; Reid, S.; Lanigan, A.; Wolfe, R.; Reddihough, D.S. Cerebral Palsy in Victoria: Motor Types, Topography and Gross Motor Function. J. Paediatr. Child Health 2005, 41, 479–483. [Google Scholar] [CrossRef]
- Ansari, N.N.; Naghdi, S.; Younesian, P.; Shayeghan, M. Inter- and Intrarater Reliability of the Modified Modified Ashworth Scale in Patients with Knee Extensor Poststroke Spasticity. Physiother. Theory Pract. 2008, 24, 205–213. [Google Scholar] [CrossRef]
- Conner, B.C.; Schwartz, M.H.; Lerner, Z.F. Pilot Evaluation of Changes in Motor Control after Wearable Robotic Resistance Training in Children with Cerebral Palsy. J. Biomech. 2021, 126, 110601. [Google Scholar] [CrossRef]
- Paulson, A.; Vargus-Adams, J. Overview of Four Functional Classification Systems Commonly Used in Cerebral Palsy. Children 2017, 4, 30. [Google Scholar] [CrossRef]
- Nordmark, E.; Hägglund, G.; Lauge-Pedersen, H.; Wagner, P.; Westbom, L. Development of Lower Limb Range of Motion from Early Childhood to Adolescence in Cerebral Palsy: A Population-Based Study. BMC Med. 2009, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Bektaşoğlu, P.K. Neurosurgical Treatment of Cerebral Palsy. In Cerebral Palsy; Bektaşoğlu, P.K., Ed.; IntechOpen: Rijeka, Croatia, 2023; Chapter 4; pp. 1–8. ISBN 978-1-80356-582-8. [Google Scholar]
- Aquilina, K.; Graham, D.; Wimalasundera, N. Selective Dorsal Rhizotomy: An Old Treatment Re-Emerging. Arch. Dis. Child. 2015, 100, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Grunt, S.; Fieggen, A.G.; Vermeulen, R.J.; Becher, J.G.; Langerak, N.G. Selection Criteria for Selective Dorsal Rhizotomy in Children with Spastic Cerebral Palsy: A Systematic Review of the Literature. Dev. Med. Child Neurol. 2014, 56, 302–312. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.E.; Fragala-Pinkham, M.A.; Westcott, S.L.; Martin, K.; Chiarello, L.A.; Valvano, J.; Rose, R.U. Physical Therapy Clinical Management Recommendations for Children with Cerebral Palsy—Spastic Diplegia: Achieving Functional Mobility Outcomes. Pediatr. Phys. Ther. 2006, 18, 49–72. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.E.; Martin, D.K. Qualitative Research and Evidence-Based Physiotherapy Practice. Physiotherapy 2003, 89, 350–358. [Google Scholar] [CrossRef]
- Üstün, T.B.; Chatterji, S.; Bickenbach, J.; Kostanjsek, N.; Schneider, M. The International Classification of Functioning, Disability and Health: A New Tool for Understanding Disability and Health. Disabil. Rehabil. 2003, 25, 565–571. [Google Scholar] [CrossRef]
- Chen, C.C.; Heinemann, A.W.; Bode, R.K.; Granger, C.V.; Mallinson, T. Impact of Pediatric Rehabilitation Services on Children’s Functional Outcomes. Am. J. Occup. Ther. 2004, 58, 44–53. [Google Scholar] [CrossRef]
- Kaminker, M.K.; Chiarello, L.A.; O’Neil, M.E.; Dichter, C.G. Decision Making for Physical Therapy Service Delivery in Schools: A Nationwide Survey of Pediatric Physical Therapists. Phys. Ther. 2004, 84, 919–933. [Google Scholar] [CrossRef]
- Bower, E.; Michell, D.; Burnett, M.; Campbell, M.J.; McLellan, D.L. Randomized Controlled Trial of Physiotherapy in 56 Children with Cerebral Palsy Followed for 18 Months. Dev. Med. Child Neurol. 2001, 43, 4–15. [Google Scholar] [CrossRef]
- Nicolini-Panisson, R.D.; Tedesco, A.P.; Folle, M.R.; Donadio, M.V.F. Rizotomia dorsal seletiva na paralisia cerebral: Critérios de indicação e protocolos de reabilitação fisioterapêutica pós-operatória. Rev. Paul. Pediatr. 2018, 36, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Langerak, N.G.; Lamberts, R.P.; Fieggen, A.G.; Peter, J.C.; Peacock, W.J.; Vaughan, C.L. Functional Status of Patients with Cerebral Palsy According to the International Classification of Functioning, Disability and Health Model: A 20-Year Follow-Up Study after Selective Dorsal Rhizotomy. Arch. Phys. Med. Rehabil. 2009, 90, 994–1003. [Google Scholar] [CrossRef]
- Steinbok, P.; McLeod, K. Comparison of Motor Outcomes after Selective Dorsal Rhizotomy with and without Preoperative Intensified Physiotherapy in Children with Spastic Diplegic Cerebral Palsy. Pediatr. Neurosurg. 2002, 36, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Buckon, C.E.; Thomas, S.S.; Harris, G.E.; Piatt, J.H.; Aiona, M.D.; Sussman, M.D. Objective Measurement of Muscle Strength in Children with Spastic Diplegia after Selective Dorsal Rhizotomy. Arch. Phys. Med. Rehabil. 2002, 83, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Nordmark, E.; Josenby, A.L.; Lagergren, J.; Andersson, G.; Strömblad, L.-G.; Westbom, L. Long-Term Outcomes Five Years after Selective Dorsal Rhizotomy. BMC Pediatr. 2008, 8, 54. [Google Scholar] [CrossRef]
- van Schie, P.E.M.; Vermeulen, R.J.; van Ouwerkerk, W.J.R.; Kwakkel, G.; Becher, J.G. Selective Dorsal Rhizotomy in Cerebral Palsy to Improve Functional Abilities: Evaluation of Criteria for Selection. Child’s Nerv. Syst. 2005, 21, 451–457. [Google Scholar] [CrossRef]
- Chan, S.H.; Yam, K.Y.; Yiu-Lau, B.P.; Poon, C.Y.; Chan, N.N.; Cheung, H.M.; Wu, M.; Chak, W.K. Selective Dorsal Rhizotomy in Hong Kong: Multidimensional Outcome Measures. Pediatr. Neurol. 2008, 39, 22–32. [Google Scholar] [CrossRef]
- Graubert, C.; Song, K.M.; McLaughlin, J.F.; Bjornson, K.F. Changes in Gait at 1 Year Post-Selective Dorsal Rhizotomy: Results of a Prospective Randomized Study. J. Pediatr. Orthop. 2000, 20, 496–500. [Google Scholar] [CrossRef]
- Engsberg, J.R.; Ross, S.A.; Collins, D.R.; Park, T.S. Predicting Functional Change from Preintervention Measures in Selective Dorsal Rhizotomy. J. Neurosurg. Pediatr. 2007, 106, 282–287. [Google Scholar] [CrossRef]
- Josenby, A.L.; Wagner, P.; Jarnlo, G.-B.; Westbom, L.; Nordmark, E. Functional Performance in Self-Care and Mobility after Selective Dorsal Rhizotomy: A 10-Year Practice-Based Follow-Up Study. Dev. Med. Child Neurol. 2015, 57, 286–293. [Google Scholar] [CrossRef]
- Wright, F.V.; Sheil, E.M.H.; Drake, J.M.; Wedge, J.H.; Naumann, S. Evaluation of Selective Dorsal Rhizotomy for the Reduction of Spasticity in Cerebral Palsy: A Randomized Controlled Trial. Dev. Med. Child Neurol. 1998, 40, 239–247. [Google Scholar] [CrossRef]
- Engsberg, J.R.; Ross, S.A.; Wagner, J.M.; Park, T.S. Changes in Hip Spasticity and Strength following Selective Dorsal Rhizotomy and Physical Therapy for Spastic Cerebral Palsy. Dev. Med. Child Neurol. 2002, 44, 220–226. [Google Scholar] [CrossRef]
- Afzal, F.; Gulraiz, Q.; Manzoor, S. Role of Spider Cage in Motor Control in Cerebral Palsy. Int. J. Phys. Med. Rehabil. 2017, 5, 2. [Google Scholar] [CrossRef]
- Engsberg, J.R.; Ross, S.A.; Collins, D.R.; Park, T.S. Effect of Selective Dorsal Rhizotomy in the Treatment of Children with Cerebral Palsy. J. Neurosurg. 2006, 105, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.F.; Ann MacPhail, H.E. Relationships among Measures of Walking Efficiency, Gross Motor Ability, and Isokinetic Strength in Adolescents with Cerebral Palsy. Pediatr. Phys. Ther. 1994, 6, 3–9. [Google Scholar] [CrossRef]
- Mittal, S.; Farmer, J.-P.; Al-Atassi, B.; Gibis, J.; Kennedy, E.; Galli, C.; Courchesnes, G.; Poulin, C.; Cantin, M.-A.; Benaroch, T.E. Long-Term Functional Outcome after Selective Posterior Rhizotomy. J. Neurosurg. 2002, 97, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Farmer, J.-P.; Al-Atassi, B.; Montpetit, K.; Gervais, N.; Poulin, C.; Benaroch, T.E.; Cantin, M.-A. Functional Performance following Selective Posterior Rhizotomy: Long-Term Results Determined Using a Validated Evaluative Measure. J. Neurosurg. 2002, 97, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.-P.; Sabbagh, A.J. Selective Dorsal Rhizotomies in the Treatment of Spasticity Related to Cerebral Palsy. Child’s Nerv. Syst. 2007, 23, 991–1002. [Google Scholar] [CrossRef]
- Geurtsen, G.J.; van Heugten, C.M.; Martina, J.D.; Geurts, A.C.H. Comprehensive Rehabilitation Programmes in the Chronic Phase after Severe Brain Injury: A Systematic Review. J. Rehabil. Med. 2010, 42, 97–110. [Google Scholar] [CrossRef]
- Vostrý, M.; Lanková, B.; Zilcher, L.; Jelinková, J. The Effect of Individual Combination Therapy on Children with Motor Deficits from the Perspective of Comprehensive Rehabilitation. Appl. Sci. 2022, 12, 4270. [Google Scholar] [CrossRef]
- Batool, A.; Kashif, A.; Nawaz, M.H.; Khan, A.A.; Iqbal, N.; Shahid, M.K. Global Overview of SARS-CoV-2 Induced COVID-19 in 2020: Biological Characterization, Epidemiology with Social, Economic and Environmental Implications. RADS J. Biol. Res. Appl. Sci. 2022, 13, 83–122. [Google Scholar] [CrossRef]





| Publisher | No. of Articles | Web of Science Categories | * No. of Articles |
|---|---|---|---|
| Elsevier | 197 | Rehabilitation | 537 |
| Taylor & Francis | 146 | Neurosciences | 217 |
| Lippincott Williams & Wilkins | 86 | Sport Sciences | 200 |
| Springer Nature | 60 | Orthopedics | 188 |
| Frontiers Media SA | 48 | Pediatrics | 155 |
| Wiley | 47 | Surgery | 50 |
| IOS Press | 45 | ||
| Foundation Rehabilitation Information | 43 | ||
| Sage | 41 | ||
| MDPI | 36 | ||
| Edizioni Minerva Medica | 26 | ||
| Oxford Univ Press | 23 | ||
| Others | 193 |
| Physical Limitations | Suggested Stretching Exercises |
|---|---|
| Knee extension | Supine with therapist assisting extension, supine with heels pressing on a block and therapist apply force on knees to extend, fixed leg straps, supine with hip and knee extended, prone with weight hanging on ankle, wedge-board standing, standing in parallel bars |
| Ankle dorsiflexion | Standing, use of TheraBand, heels off step, calf stretch |
| Ankle plantarflexion | Frozen can roll, TheraBand stretch, balance-board-standing exercise |
| Inversion | Locomotor training while practicing loading and extension of lower limb, postural correction with both feet placed in anatomical position, standing balance exercise |
| Eversion | Side stepping, active stretches |
| Muscle | Right | Left |
|---|---|---|
| Quadriceps | - | - |
| Hamstrings | +3 | +3 |
| Adductors | - | - |
| Gastrocnemius | +1 | +1 |
| TA | +1 | +1 |
| Extensor halluces longus | - | - |
| ROMs | Pre-Operative | Post-Operative (2 Weeks) | Post-Operative (8 Months) | |||
|---|---|---|---|---|---|---|
| Right (°) | Left (°) | Right (°) | Left (°) | Right (°) | Left (°) | |
| Knee flexion | 120 | 120 | 120 | 120 | 120 | 120 |
| Knee extension limitation | 90 | 80 | 40 | 40 | 10 | 10 |
| Dorsiflexion | 10 | 10 | 14 | 16 | 20 | 20 |
| Plantarflexion | 40 | 40 | 40 | 40 | 40 | 40 |
| Inversion | 30 | 20 | 20 | 20 | 20 | 20 |
| Eversion | 10 | 10 | 10 | 10 | 16 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, J.; Kashif, A.; Shahid, M.K. Enhancing Post-Operative Recovery in Spastic Diplegia through Physical Therapy Rehabilitation following Selective Dorsal Rhizotomy: A Case Report and Thorough Literature Analysis. Children 2023, 10, 842. https://doi.org/10.3390/children10050842
Shahid J, Kashif A, Shahid MK. Enhancing Post-Operative Recovery in Spastic Diplegia through Physical Therapy Rehabilitation following Selective Dorsal Rhizotomy: A Case Report and Thorough Literature Analysis. Children. 2023; 10(5):842. https://doi.org/10.3390/children10050842
Chicago/Turabian StyleShahid, Jawaria, Ayesha Kashif, and Muhammad Kashif Shahid. 2023. "Enhancing Post-Operative Recovery in Spastic Diplegia through Physical Therapy Rehabilitation following Selective Dorsal Rhizotomy: A Case Report and Thorough Literature Analysis" Children 10, no. 5: 842. https://doi.org/10.3390/children10050842
APA StyleShahid, J., Kashif, A., & Shahid, M. K. (2023). Enhancing Post-Operative Recovery in Spastic Diplegia through Physical Therapy Rehabilitation following Selective Dorsal Rhizotomy: A Case Report and Thorough Literature Analysis. Children, 10(5), 842. https://doi.org/10.3390/children10050842






