Translating Precision Health for Pediatrics: A Scoping Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Search Strategy
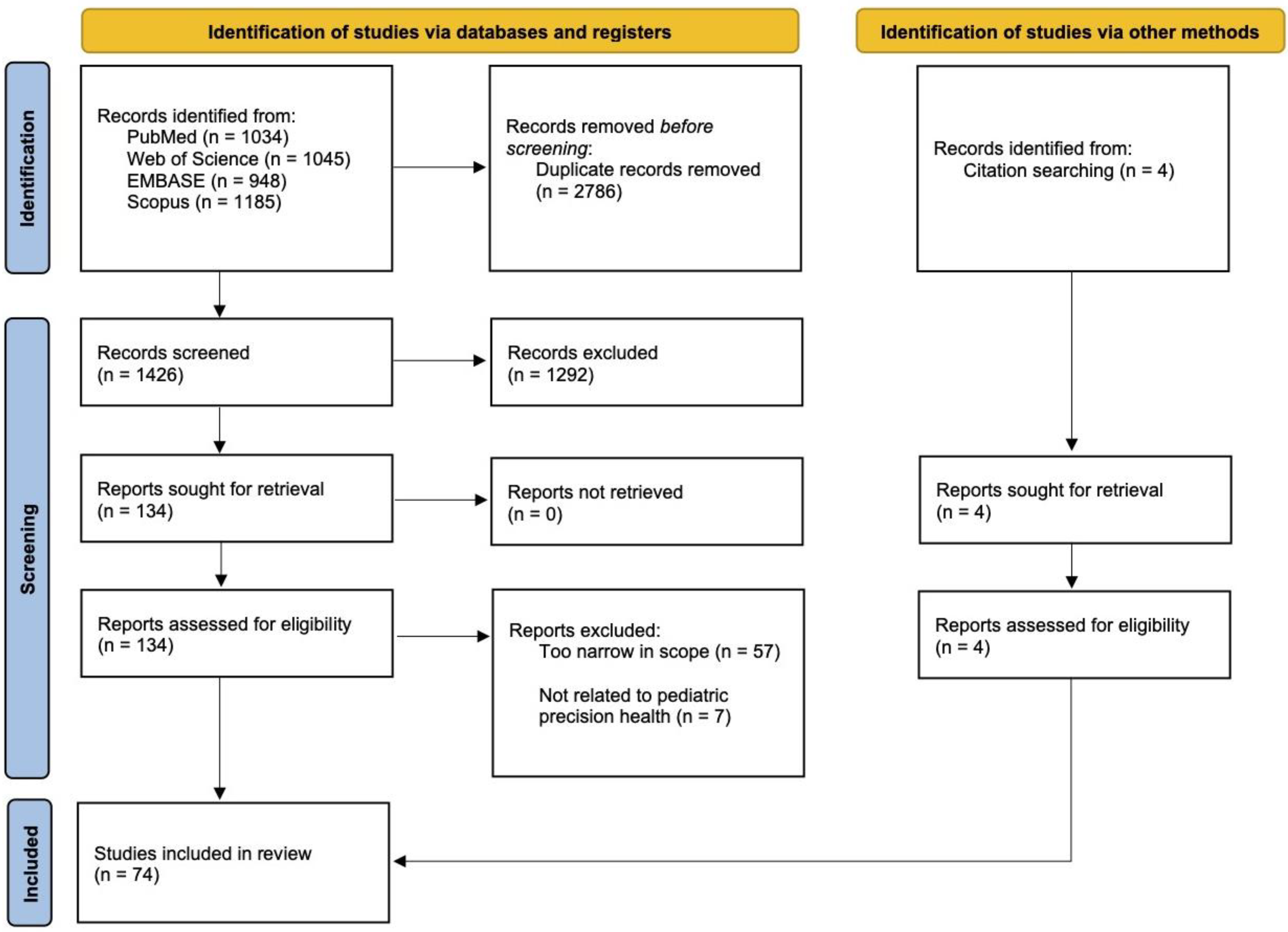
2.3. Study Selection
2.4. Data Extraction and Coding
3. Results
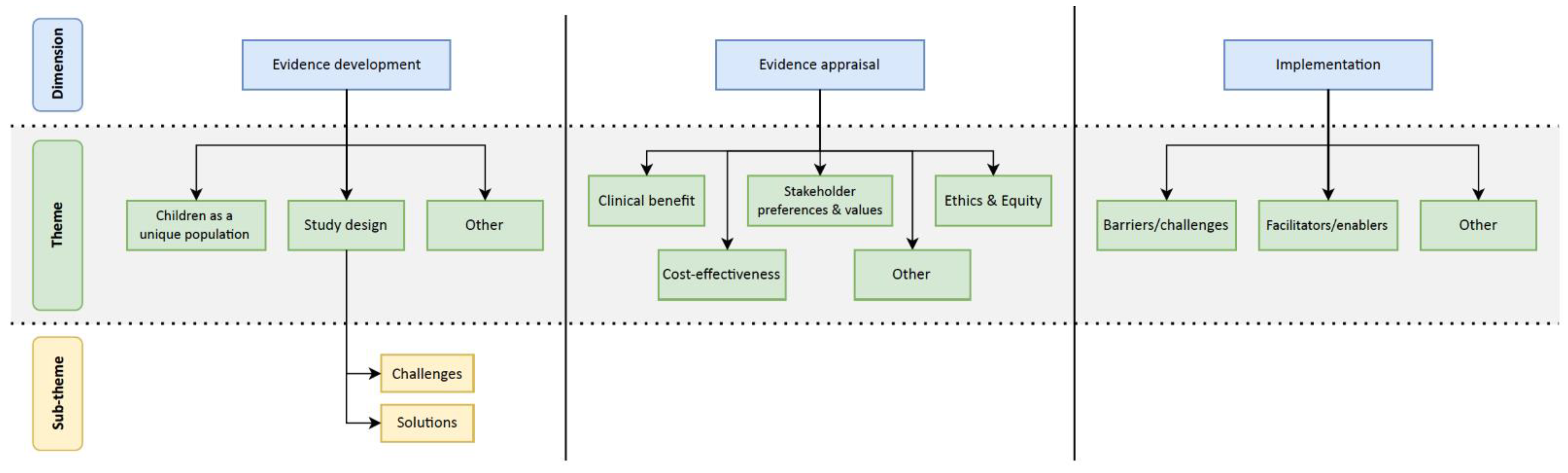
3.1. Thematic Analysis
3.2. Evidence Development
3.2.1. Children Are a Unique Population
3.2.2. Study Design Challenges and Potential Solutions
3.3. Evidence Appraisal
3.3.1. Clinical Benefit
3.3.2. Cost-Effectiveness
3.3.3. Stakeholder Preferences and Values
3.3.4. Ethics and Equity in Value Assessments
3.4. Implementation
Barriers and Enablers to Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Search Strategy
| PubMed Search (5 November 2022): ((pediatric[Title] OR paediatric[Title] OR child*[Title] OR adolescen*[Title] OR youth[Title] OR young[Title] OR teen*[Title] OR juvenile[Title]) AND (Personalized[Title] OR Personalised[Title] OR Precision[Title] OR Stratified[Title] OR Individuali*[Title] OR Tailor*[Title] OR Pharmacogen*[Title] OR “Health Technolog*”[Title])) AND (priori*[Title/Abstract] OR assess*[Title/Abstract] OR evaluat*[Title/Abstract] OR implement*[Title/Abstract] OR framework*[Title/Abstract] OR guideline*[Title/Abstract]) Timespan: 2000–2022 Language: English Article Type: Exclude “Corrected and Republished Article”, “Letter”, “News”, “Newspaper Article”, “Retracted Publication”, “Retraction of Publication” Web of Science Search (5 November 2022): (TI=(pediatric OR paediatric OR child* OR adolescen* OR youth OR young OR teen* OR juvenile) AND TI=(Personalized OR Personalised OR Precision OR Stratified OR Individuali* OR Tailor* OR Pharmacogen* OR ”Health Technolog*”) AND ((TI=(priori* OR assess* OR evaluat* OR implement* OR framework* OR guideline*) OR (AB=(priori* OR assess* OR evaluat* OR implement* OR framework* OR guideline*))))) Timespan: 2000–2022 Document Types: Exclude: Meeting Abstracts, Letter, Correction Language: English Embase Search (5 November 2022):
Scopus Search (5 November 2022): TITLE (pediatric OR paediatric OR child* OR adolescen* OR youth OR young OR teen* OR juvenile) AND TITLE (personalized OR personalised OR precision OR stratified OR individuali* OR tailor* OR pharmacogen* OR “Health Technolog*”) AND TITLE-ABS (priori* OR assess* OR evaluat* OR implement* OR framework* OR guideline*) AND (EXCLUDE (DOCTYPE, “no”) OR EXCLUDE ( DOCTYPE, “le”) OR EXCLUDE (DOCTYPE, “er”) OR EXCLUDE (DOCTYPE, “tb”) OR EXCLUDE (DOCTYPE, “Undefined”)) AND (LIMIT-TO (PUBYEAR, 2022) OR (LIMIT-TO (PUBYEAR, 2021) OR LIMIT-TO (PUBYEAR, 2020) OR LIMIT-TO (PUBYEAR, 2019) OR LIMIT-TO (PUBYEAR, 2018) OR LIMIT-TO (PUBYEAR, 2017) OR LIMIT-TO (PUBYEAR, 2016) OR LIMIT-TO (PUBYEAR, 2015) OR LIMIT-TO (PUBYEAR, 2014) OR LIMIT-TO (PUBYEAR, 2013) OR LIMIT-TO (PUBYEAR, 2012) OR LIMIT-TO (PUBYEAR, 2011) OR LIMIT-TO (PUBYEAR, 2010) OR LIMIT-TO (PUBYEAR, 2009) OR LIMIT-TO (PUBYEAR, 2008) OR LIMIT-TO (PUBYEAR, 2007) OR LIMIT-TO (PUBYEAR, 2006) OR LIMIT-TO (PUBYEAR, 2005) OR LIMIT-TO (PUBYEAR, 2004) OR LIMIT-TO (PUBYEAR, 2003) OR LIMIT-TO (PUBYEAR, 2002) OR LIMIT-TO (PUBYEAR, 2001) OR LIMIT-TO (PUBYEAR, 2000)) AND (LIMIT-TO (LANGUAGE, “English”)) |
References
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Veit, G.; Avramescu, R.G.; Chiang, A.N.; Houck, S.A.; Cai, Z.; Peters, K.W.; Hong, J.S.; Pollard, H.B.; Guggino, W.B.; Balch, W.E.; et al. From CFTR biology toward combinatorial pharmacotherapy: Expanded classification of cystic fibrosis mutations. Mol. Biol. Cell 2016, 27, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Castellani, C.; Massie, J.; Sontag, M.; Southern, K.W. Newborn screening for cystic fibrosis. Lancet Respir. Med. 2016, 4, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Zaher, A.; ElSaygh, J.; Elsori, D.; ElSaygh, H.; Sanni, A. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy. Cureus 2021, 13, e16144. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; de Luca, V.; Kostikova, A.; Laramie, J.; Kennedy, S.; Ferrero, E.; Siegel, R.; Fink, M.; Ahmed, S.; Millholland, J.; et al. Translational precision medicine: An industry perspective. J. Transl. Med. 2021, 19, 245. [Google Scholar] [CrossRef]
- Krahn, M.; Miller, F.; Bayoumi, A.; Brooker, A.S.; Wagner, F.; Winsor, S.; Giacomini, M.; Goeree, R.; Schünemann, H.; van der Velde, G.; et al. Development Of The Ontario Decision Framework: A Values Based Framework For Health Technology Assessment. Int. J. Technol. Assess. Health Care 2018, 34, 290–299. [Google Scholar] [CrossRef]
- Burls, A.; Caron, L.; Cleret de Langavant, G.; Dondorp, W.; Harstall, C.; Pathak-Sen, E.; Hofmann, B. Tackling ethical issues in health technology assessment: A proposed framework. Int. J. Technol. Assess. Health Care 2011, 27, 230–237. [Google Scholar] [CrossRef]
- Lehoux, P.; Daudelin, G.; Demers-Payette, O.; Boivin, A. Fostering deliberations about health innovation: What do we want to know from publics? Soc. Sci. Med. 2009, 68, 2002–2009. [Google Scholar] [CrossRef]
- Oortwijn, W.; Husereau, D.; Abelson, J.; Barasa, E.; Bayani, D.D.; Santos, V.C.; Culyer, A.; Facey, K.; Grainger, D.; Kieslich, K.; et al. Designing and Implementing Deliberative Processes for Health Technology Assessment: A Good Practices Report of a Joint HTAi/ISPOR Task Force. Int. J. Technol. Assess. Health Care 2022, 38, e37. [Google Scholar] [CrossRef]
- Brown, H.L.; Sherburn, I.A.; Gaff, C.; Taylor, N.; Best, S. Structured approaches to implementation of clinical genomics: A scoping review. Genet. Med. 2022, 24, 1415–1424. [Google Scholar] [CrossRef]
- Faulkner, E.; Holtorf, A.P.; Walton, S.; Liu, C.Y.; Lin, H.; Biltaj, E.; Brixner, D.; Barr, C.; Oberg, J.; Shandhu, G.; et al. Being Precise About Precision Medicine: What Should Value Frameworks Incorporate to Address Precision Medicine? A Report of the Personalized Precision Medicine Special Interest Group. Value Health 2020, 23, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Elzagallaai, A.; Barker, C.; Lewis, T.; Cohn, R.; Rieder, M. Advancing Precision Medicine in Paediatrics: Past, present and future. Camb. Prism. Precis. Med. 2023, 1, e11. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ontario, H.Q. Health Technology Assessments: Methods and Process Guide, Version 2.0; King’s Printer for Ontario: Toronto, ON, Canada, 2018; p. 66. [Google Scholar]
- Thorne, S.E. Applied Interpretive Approaches. In The Oxford Handbook of Qualitative Research; Oxford University Press: New York, NY, USA, 2014; pp. 99–115. [Google Scholar] [CrossRef]
- Freund, C.L.; Clayton, E.W. Pharmacogenomics and children: Meeting the ethical challenges. Am. J. Pharm. 2003, 3, 399–404. [Google Scholar] [CrossRef]
- Maagdenberg, H.; Vijverberg, S.J.; Bierings, M.B.; Carleton, B.C.; Arets, H.G.; de Boer, A.; Maitland-van der Zee, A.H. Pharmacogenomics in Pediatric Patients: Towards Personalized Medicine. Paediatr. Drugs 2016, 18, 251–260. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Wagner, J.; Shakhnovich, V.; Carleton, B.; Leeder, J.S. Considerations for Implementing Precision Therapeutics for Children. Clin. Transl. Sci. 2019, 12, 140–150. [Google Scholar] [CrossRef]
- Costa, V.; Ungar, W.J. Health technology assessment in child health. In Economic Evaluation in Child Health; Ungar, W.J., Ed.; Oxford University Press: New York, NY, USA, 2009; pp. 289–302. [Google Scholar] [CrossRef]
- Piana, C.; Surh, L.; Furst-Recktenwald, S.; Iolascon, A.; Jacqz-Aigrain, E.M.; Jonker, I.; Russo, R.; van Schaik, R.H.; Wessels, J.; Della Pasqua, O.E. Integration of pharmacogenetics and pharmacogenomics in drug development: Implications for regulatory and medical decision making in pediatric diseases. J. Clin. Pharmacol. 2012, 52, 704–716. [Google Scholar] [CrossRef]
- Lowry, J.A.; Leeder, J.S. Application of Pharmacogenetics and Pharmacogenomics in Pediatrics: What Makes Children Different? In Principles of Pharmacogenetics and Pharmacogenomics; Flockhart, D., Goldstein, D.B., Altman, R.B., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 249–262. [Google Scholar]
- Leeder, J.S.; Lantos, J.; Spielberg, S.P. Conference scene: Pediatric pharmacogenomics and personalized medicine. Pharmacogenomics 2010, 11, 1691–1702. [Google Scholar] [CrossRef]
- Sing, C.W.; Cheung, C.L.; Wong, I.C. Pharmacogenomics--how close/far are we to practising individualized medicine for children? Br. J. Clin. Pharmacol. 2015, 79, 419–428. [Google Scholar] [CrossRef]
- Hawcutt, D.B.; Cooney, L.; Oni, L.; Pirmohamed, M. Precision Dosing in Children. Expert. Rev. Precis. Med. Drug. Dev. 2016, 1, 69–78. [Google Scholar] [CrossRef]
- Avard, D.; Silverstein, T.; Sillon, G.; Joly, Y. Researchers’ Perceptions of the Ethical Implications of Pharmacogenomics Research with Children. Public Health Genom. 2009, 12, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Cotta, M.; Raman, S.; Graham, N.; Schlapbach, L.; Roberts, J.A. Individualized precision dosing approaches to optimize antimicrobial therapy in pediatric populations. Expert. Rev. Clin. Pharmacol. 2021, 14, 1383–1399. [Google Scholar] [CrossRef]
- Fernandez-Luque, L.; Al Herbish, A.; Al Shammari, R.; Argente, J.; Bin-Abbas, B.; Deeb, A.; Dixon, D.; Zary, N.; Koledova, E.; Savage, M.O. Digital Health for Supporting Precision Medicine in Pediatric Endocrine Disorders: Opportunities for Improved Patient Care. Front. Pediatr. 2021, 9, 715705. [Google Scholar] [CrossRef] [PubMed]
- Gregornik, D.; Salyakina, D.; Brown, M.; Roiko, S.; Ramos, K. Pediatric pharmacogenomics: Challenges and opportunities: On behalf of the Sanford Children’s Genomic Medicine Consortium. Pharm. J. 2021, 21, 8–19. [Google Scholar] [CrossRef]
- Hoshitsuki, K.; Fernandez, C.A.; Yang, J.J. Pharmacogenomics for Drug Dosing in Children: Current Use, Knowledge, and Gaps. J. Clin. Pharmacol. 2021, 61 (Suppl. S1), S188–S192. [Google Scholar] [CrossRef]
- Liko, I.; Lee, Y.M.; Stutzman, D.L.; Blackmer, A.B.; Deininger, K.M.; Reynolds, A.M.; Aquilante, C.L. Providers’ perspectives on the clinical utility of pharmacogenomic testing in pediatric patients. Pharmacogenomics 2021, 22, 263–274. [Google Scholar] [CrossRef]
- Ramsey, L.B.; Namerow, L.B.; Bishop, J.R.; Hicks, J.K.; Bousman, C.; Croarkin, P.E.; Mathews, C.A.; Van Driest, S.L.; Strawn, J.R. Thoughtful Clinical Use of Pharmacogenetics in Child and Adolescent Psychopharmacology. J. Am. Acad. Child. Adolesc. Psychiatry 2021, 60, 660–664. [Google Scholar] [CrossRef]
- Issa, A.M.; Aboushawareb, S.A.; Eisenstat, D.D.; Guilcher, G.M.; Liu, G.; Rassekh, S.R.; Strahlendorf, C.; Tallen, G.; Tanoshima, R.; Carleton, B. Deliberations about clinical pharmacogenetic testing in pediatric oncology. Per. Med. 2021, 18, 399–405. [Google Scholar] [CrossRef]
- Moretti, F.; Ruiz, F.; Bonifazi, F.; Pizzo, E.; Kindblom, J.M. Health technology assessment of paediatric medicines: European landscape, challenges and opportunities inside the conect4children project. Br. J. Clin. Pharmacol. 2022, 88, 5052–5059. [Google Scholar] [CrossRef] [PubMed]
- Blake, M.J.; Gaedigk, A.; Pearce, R.E.; Bomgaars, L.R.; Christensen, M.L.; Stowe, C.; James, L.P.; Wilson, J.T.; Kearns, G.L.; Leeder, J.S. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin. Pharmacol. Ther. 2007, 81, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Koukouritaki, S.B.; Manro, J.R.; Marsh, S.A.; Stevens, J.C.; Rettie, A.E.; McCarver, D.G.; Hines, R.N. Developmental expression of human hepatic CYP2C9 and CYP2C19. J. Pharmacol. Exp. Ther. 2004, 308, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Cacioppo, C.N.; Chandler, A.E.; Towne, M.C.; Beggs, A.H.; Holm, I.A. Expectation versus Reality: The Impact of Utility on Emotional Outcomes after Returning Individualized Genetic Research Results in Pediatric Rare Disease Research, a Qualitative Interview Study. PLoS ONE 2016, 11, e0153597. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.H.; DuBois, S.G.; Glade Bender, J.L.; Kim, A.; Crompton, B.D.; Parker, E.; Dumont, I.P.; Hong, A.L.; Guo, D.; Church, A.; et al. Multicenter Feasibility Study of Tumor Molecular Profiling to Inform Therapeutic Decisions in Advanced Pediatric Solid Tumors: The Individualized Cancer Therapy (iCat) Study. JAMA Oncol. 2016, 2, 608–615. [Google Scholar] [CrossRef]
- Ungar, W.J. Technology Assessment at SickKids (TASK): A Health Technology Assessment Research Unit Devoted to Child Health in Canada. In Hospital-Based Health Technology Assessment: The Next Frontier for Health Technology Assessment; Sampietro-Colom, L., Martin, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 153–165. [Google Scholar]
- Nersting, J.; Borst, L.; Schmiegelow, K. Challenges in implementing individualized medicine illustrated by antimetabolite therapy of childhood acute lymphoblastic leukemia. Clin. Proteom. 2011, 8, 8. [Google Scholar] [CrossRef]
- Bourgeois, F.T.; Avillach, P.; Kong, S.W.; Heinz, M.M.; Tran, T.A.; Chakrabarty, R.; Bickel, J.; Sliz, P.; Borglund, E.M.; Kornetsky, S.; et al. Development of the Precision Link Biobank at Boston Children’s Hospital: Challenges and Opportunities. J. Pers. Med. 2017, 7, 21. [Google Scholar] [CrossRef]
- Worst, B.C.; van Tilburg, C.M.; Balasubramanian, G.P.; Fiesel, P.; Witt, R.; Freitag, A.; Boudalil, M.; Previti, C.; Wolf, S.; Schmidt, S.; et al. Next-generation personalised medicine for high-risk paediatric cancer patients—The INFORM pilot study. Eur. J. Cancer 2016, 65, 91–101. [Google Scholar] [CrossRef]
- Seibel, N.L.; Janeway, K.; Allen, C.E.; Chi, S.N.; Cho, Y.J.; Glade Bender, J.L.; Kim, A.; Laetsch, T.W.; Irwin, M.S.; Takebe, N.; et al. Pediatric oncology enters an era of precision medicine. Curr. Probl. Cancer 2017, 41, 194–200. [Google Scholar] [CrossRef]
- Tang Girdwood, S.C.; Rossow, K.M.; Van Driest, S.L.; Ramsey, L.B. Perspectives from the Society for Pediatric Research: Pharmacogenetics for pediatricians. Pediatr. Res. 2022, 91, 529–538. [Google Scholar] [CrossRef]
- Ramos, K.N.; Gregornik, D.; Ramos, K.S. Pharmacogenomics insights into precision pediatric oncology. Curr. Opin. Pediatr. 2021, 33, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.; Thummel, K. Precision medicine and health disparities: The case of pediatric acute lymphoblastic leukemia. Nurs. Outlook 2019, 67, 331–336. [Google Scholar] [CrossRef]
- Forrest, S.J.; Geoerger, B.; Janeway, K.A. Precision medicine in pediatric oncology. Curr. Opin. Pediatr. 2018, 30, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.J.; Prensner, J.R.; Everett, J.; Parsons, D.W.; Chinnaiyan, A.M. Precision medicine in pediatric oncology: Lessons learned and next steps. Pediatr. Blood Cancer 2017, 64, e26288. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, S.J.H.; Brinkman, P.; Rutjes, N.W.P.; Maitland-van der Zee, A.H. Precision medicine in severe pediatric asthma: Opportunities and challenges. Curr. Opin. Pulm. Med. 2020, 26, 77–83. [Google Scholar] [CrossRef]
- Denburg, A.E.; Giacomini, M.; Ungar, W.J.; Abelson, J. ‘The problem is small enough, the problem is big enough’: A qualitative study of health technology assessment and public policy on drug funding decisions for children. Int. J. Equity Health 2020, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.E.; Pui, C.H.; Yang, J.J. The Promise and the Reality of Genomics to Guide Precision Medicine in Pediatric Oncology: The Decade Ahead. Clin. Pharmacol. Ther. 2020, 107, 176–180. [Google Scholar] [CrossRef]
- Subbiah, V. Prospects and pitfalls of personalizing therapies for sarcomas: From children, adolescents, and young adults to the elderly. Curr. Oncol. Rep. 2014, 16, 401. [Google Scholar] [CrossRef]
- Renfro, L.A.; Ji, L.; Piao, J.; Onar-Thomas, A.; Kairalla, J.A.; Alonzo, T.A. Trial Design Challenges and Approaches for Precision Oncology in Rare Tumors: Experiences of the Children’s Oncology Group. JCO Precis. Oncol. 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Cahaney, C.; Dhir, A.; Ghosh, T. Role of Precision Medicine in Pediatric Oncology. Pediatr. Ann. 2022, 51, e8–e14. [Google Scholar] [CrossRef]
- Maamari, D.; El-Khoury, H.; Saifi, O.; Muwakkit, S.A.; Zgheib, N.K. Implementation of Pharmacogenetics to Individualize Treatment Regimens for Children with Acute Lymphoblastic Leukemia. Pharm. Pers. Med. 2020, 13, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, S.J.; Pijnenburg, M.W.; Hövels, A.M.; Koppelman, G.H.; Maitland-van der Zee, A.H. The need for precision medicine clinical trials in childhood asthma: Rationale and design of the PUFFIN trial. Pharmacogenomics 2017, 18, 393–401. [Google Scholar] [CrossRef]
- Vanakker, O.M.; De Paepe, A. Pharmacogenomics in children: Advantages and challenges of next generation sequencing applications. Int. J. Pediatr. 2013, 2013, 136524. [Google Scholar] [CrossRef]
- Claudio-Campos, K.; Padrón, A.; Jerkins, G.; Nainaparampil, J.; Nelson, R.; Martin, A.; Wiisanen, K.; Smith, D.M.; Strekalova, Y.; Marsiske, M.; et al. Acceptability, Feasibility, and Utility of Integrating Pharmacogenetic Testing into a Child Psychiatry Clinic. Clin. Transl. Sci. 2021, 14, 589–598. [Google Scholar] [CrossRef]
- Ramsey, L.B.; Prows, C.A.; Zhang, K.; Saldaña, S.N.; Sorter, M.T.; Pestian, J.P.; Wenstrup, R.J.; Vinks, A.A.; Glauser, T.A. Implementation of Pharmacogenetics at Cincinnati Children’s Hospital Medical Center: Lessons Learned Over 14 Years of Personalizing Medicine. Clin. Pharmacol. Ther. 2019, 105, 49–52. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.C.; De Abreu Lourenco, R.; McMillan, L.J.; Meshcheriakova, E.; Cao, A.; Gillam, L. Finding Out What Matters in Decision-Making Related to Genomics and Personalized Medicine in Pediatric Oncology: Developing Attributes to Include in a Discrete Choice Experiment. Patient 2020, 13, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Hansford, J.R. Personalised medicine in paediatric oncology: Ethical practice outside the clinical trial framework? J. Paediatr. Child Health 2019, 55, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, N.A.; Melham, K.; Kaye, J.; van Delden, J.J.; Bredenoord, A.L. Personalized assent for pediatric biobanks. BMC Med. Ethics 2016, 17, 59. [Google Scholar] [CrossRef]
- Saldaña, S.N.; Hooper, D.K.; Froehlich, T.E.; Campbell, K.M.; Prows, C.A.; Sadhasivam, S.; Nick, T.G.; Seid, M.; Vinks, A.A.; Glauser, T.A. Characteristics of successful recruitment in prospective pediatric pharmacogenetic studies. Clin. Ther. 2011, 33, 2072–2081. [Google Scholar] [CrossRef]
- Bowdin, S.C.; Hayeems, R.Z.; Monfared, N.; Cohn, R.D.; Meyn, M.S. The SickKids Genome Clinic: Developing and evaluating a pediatric model for individualized genomic medicine. Clin. Genet. 2016, 89, 10–19. [Google Scholar] [CrossRef]
- Jessel, C.D.; Al Maruf, A.; Oomen, A.; Arnold, P.D.; Bousman, C.A. Pharmacogenetic Testing Knowledge and Attitudes among Pediatric Psychiatrists and Pediatricians in Alberta, Canada. J. Can. Acad. Child. Adolesc. Psychiatry 2022, 31, 18–27. [Google Scholar]
- Roberts, T.A.; Wagner, J.A.; Sandritter, T.; Black, B.T.; Gaedigk, A.; Stancil, S.L. Retrospective Review of Pharmacogenetic Testing at an Academic Children’s Hospital. Clin. Transl. Sci. 2021, 14, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, D.; Caylor, S.; Waldman, B.; Benson, W.; Ashburner, C.; Carmichael, J.L.; Carroll, J.; Cham, E.; Chowdhury, S.; Cleary, J.; et al. Project Baby Bear: Rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am. J. Hum. Genet. 2021, 108, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Dagar, A.; Cherlopalle, S.; Ahuja, V.; Senko, L.; Butler, R.S.; Austerman, J.; Anand, A.; Falcone, T. Real-world experience of using combinatorial pharmacogenomic test in children and adolescents with depression and anxiety. J. Psychiatr. Res. 2022, 146, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.E.; Chowdhury, S.; Pashayan, N.; Hallowell, N.; Pharoah, P.; Burton, H. What ethical and legal principles should guide the genotyping of children as part of a personalised screening programme for common cancer? J. Med. Ethics 2014, 40, 163–167. [Google Scholar] [CrossRef]
- Cohn, I.; Manshaei, R.; Liston, E.; Okello, J.B.A.; Khan, R.; Curtis, M.R.; Krupski, A.J.; Jobling, R.K.; Kalbfleisch, K.; Paton, T.A.; et al. Assessment of the Implementation of Pharmacogenomic Testing in a Pediatric Tertiary Care Setting. JAMA Netw. Open. 2021, 4, e2110446. [Google Scholar] [CrossRef]
- Gargallo, P.; Bautista, F.; Juan-Ribelles, A.; Izquierdo, E.; Soriano, A.; de Rojas, T.; Escudero, A.; Lavarino, C.; Solano, P.; Hladun, R.; et al. Current status of precision medicine in pediatric oncology in Spain: A consensus report by the Spanish Society of Paediatric Haematology and Oncology (SEHOP). Clin. Transl. Oncol. 2022, 24, 809–815. [Google Scholar] [CrossRef]
- Lee, J.; Gillam, L.; Visvanathan, K.; Hansford, J.R.; McCarthy, M.C. Clinical Utility of Precision Medicine in Pediatric Oncology: A Systematic Review. JCO Precis. Oncol. 2021, 5, 1088–1102. [Google Scholar] [CrossRef]
- Sisk, B.A.; Antes, A.L.; Burrous, S.; DuBois, J.M. Parental Attitudes toward Artificial Intelligence-Driven Precision Medicine Technologies in Pediatric Healthcare. Children 2020, 7, 145. [Google Scholar] [CrossRef]
- Longo, C.; Rahimzadeh, V.; Bartlett, G. Communication of Pharmacogenomic test results and treatment plans in pediatric oncology: Deliberative stakeholder consultations with parents. BMC Palliat. Care 2021, 20, 15. [Google Scholar] [CrossRef]
- Waldman, L.; Hancock, K.; Gallinger, B.; Johnstone, B.; Brunga, L.; Malkin, D.; Barrera, M.; Villani, A. Perspectives and Experiences of Parents and Adolescents Who Participate in a Pediatric Precision Oncology Program: “When You Feel Helpless, This Kind of Thing Is Very Helpful”. JCO Precis. Oncol. 2022, 6, e2100444. [Google Scholar] [CrossRef] [PubMed]
- McGill, B.C.; Wakefield, C.E.; Hetherington, K.; Munro, L.J.; Warby, M.; Lau, L.; Tyrrell, V.; Ziegler, D.S.; O’Brien, T.A.; Marshall, G.M.; et al. “Balancing Expectations with Actual Realities”: Conversations with Clinicians and Scientists in the First Year of a High-Risk Childhood Cancer Precision Medicine Trial. J. Pers. Med. 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Denburg, A.E.; Giacomini, M.; Ungar, W.; Abelson, J. Ethical and Social Values for Paediatric Health Technology Assessment and Drug Policy. Int. J. Health Policy Manag. 2022, 11, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Bégo-Le Bagousse, G.; Jia, X.; Wolowacz, S.; Eckert, L.; Tavi, J.; Hudson, R. Health utility estimation in children and adolescents: A review of health technology assessments. Curr. Med. Res. Opin. 2020, 36, 1209–1224. [Google Scholar] [CrossRef]
- Ungar, W.J.; Prosser, L.A.; Burnett, H.F. Values and evidence colliding: Health technology assessment in child health. Expert. Rev. Pharm. Outcomes Res. 2013, 13, 417–419. [Google Scholar] [CrossRef]
- Brown, J.T.; Ramsey, L.B.; Van Driest, S.L.; Aka, I.; Colace, S.I. Characterizing Pharmacogenetic Testing Among Children’s Hospitals. Clin. Transl. Sci. 2021, 14, 692–701. [Google Scholar] [CrossRef]
- Sobkowiak-Sobierajska, A.; Lindemans, C.; Sykora, T.; Wachowiak, J.; Dalle, J.H.; Bonig, H.; Gennery, A.; Lawitschka, A. Management of Chronic Graft-vs.-Host Disease in Children and Adolescents With ALL: Present Status and Model for a Personalised Management Plan. Front. Pediatr. 2022, 10, 808103. [Google Scholar] [CrossRef]
- Gill, P.S.; Yu, F.B.; Porter-Gill, P.A.; Boyanton, B.L.; Allen, J.C.; Farrar, J.E.; Veerapandiyan, A.; Prodhan, P.; Bielamowicz, K.J.; Sellars, E.; et al. Implementing Pharmacogenomics Testing: Single Center Experience at Arkansas Children’s Hospital. J. Pers. Med. 2021, 11, 394. [Google Scholar] [CrossRef]
- Tucker, E.R.; George, S.; Angelini, P.; Bruna, A.; Chesler, L. The Promise of Patient-Derived Preclinical Models to Accelerate the Implementation of Personalised Medicine for Children with Neuroblastoma. J. Pers. Med. 2021, 11, 248. [Google Scholar] [CrossRef]
- Manzi, S.F.; Fusaro, V.A.; Chadwick, L.; Brownstein, C.; Clinton, C.; Mandl, K.D.; Wolf, W.A.; Hawkins, J.B. Creating a scalable clinical pharmacogenomics service with automated interpretation and medical record result integration—Experience from a pediatric tertiary care facility. J. Am. Med. Inform. Assoc. 2017, 24, 74–80. [Google Scholar] [CrossRef]
- Mordechai, O.; Weyl-Ben-Arush, M. Precision Medicine in Relapsed and Refractory Childhood Cancers: Single-center Experience, Literature Review, and Meta-analysis. Rambam Maimonides Med. J. 2018, 9, e0019. [Google Scholar] [CrossRef] [PubMed]
- Mack, S.C.; Northcott, P.A. Genomic Analysis of Childhood Brain Tumors: Methods for Genome-Wide Discovery and Precision Medicine Become Mainstream. J. Clin. Oncol. 2017, 35, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Jose, R.; Rooney, R.; Nagisetty, N.; Davis, R.; Hains, D. Biorepository and integrative genomics initiative: Designing and implementing a preliminary platform for predictive, preventive and personalized medicine at a pediatric hospital in a historically disadvantaged community in the USA. Epma. J. 2018, 9, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Cathaoir, K.Ó. The invisible child of personalized medicine. J. Law. Biosci. 2021, 8, lsab029. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Boucher, M.; Noorani, H. Priority setting for health technology assessment at CADTH. Int. J. Technol. Assess. Health Care 2010, 26, 341–347. [Google Scholar] [CrossRef]
- Noorani, H.Z.; Husereau, D.R.; Boudreau, R.; Skidmore, B. Priority setting for health technology assessments: A systematic review of current practical approaches. Int. J. Technol. Assess. Health Care 2007, 23, 310–315. [Google Scholar] [CrossRef]
- Advice to the Profession: Consent to Treatment. Available online: https://www.cpso.on.ca/en/Physicians/Policies-Guidance/Policies/Consent-to-Treatment/Advice-to-the-Profession-Consent-to-Treatment (accessed on 1 February 2023).
- Informed Consent: Draft Guidance for IRBs, Clinical Investigators, and Sponsors. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/informed-consent (accessed on 1 February 2023).
- Research Ethics Board: Consent Process. Available online: https://www.canada.ca/en/health-canada/services/science-research/science-advice-decision-making/research-ethics-board/consent-process.html (accessed on 1 February 2023).
- Canada, D.O.J. The Voice of the Child in Family Law: Exploring Strategies, Challenges, and Best Practices for Canada; Department of Justice Canada: Ottawa, ON, Canada, 2019; CanLIIDocs 4522. [Google Scholar]
- Gauvreau, C.L.; Wight, L.; Subasri, M.; Palmer, A.; Hayeems, R.; Croker, A.; Abelson, J.; Fraser, B.; Bombard, Y.; Moore Hepburn, C.; et al. Access to novel drugs and therapeutics for children and youth: Eliciting citizens’ values to inform public funding decisions. Health Expect. 2023, 26, 715–727. [Google Scholar] [CrossRef]
- Fung, A.; Yue, X.; Wigle, P.R.; Guo, J.J. Off-label medication use in rare pediatric diseases in the United States. Intractable Rare Dis. Res. 2021, 10, 238–245. [Google Scholar] [CrossRef]
- Precision Medicine. Available online: https://www.fda.gov/medical-devices/in-vitro-diagnostics/precision-medicine (accessed on 1 February 2023).
- NICE Health Technology Evaluations: The Manual. Available online: https://www.nice.org.uk/process/pmg36/chapter/introduction-to-health-technology-evaluation (accessed on 1 February 2023).
- Health Technology Assessment and the Pharmaceutical Benefits Advisory Committee. Available online: https://www.aph.gov.au/Parliamentary_Business/Committees/House/Health_Aged_Care_and_Sport/Newdrugs/Report/section?id=committees%2freportrep%2f024755%2f77593 (accessed on 1 February 2023).
- Improving Paediatric Medications: A Prescription for Canadian Children and Youth. Available online: https://cps.ca/en/documents/position/improving-paediatric-medications (accessed on 1 February 2023).
- Silent Genomes: Reducing Health-Care Disparities and Improving Diagnostic Success for Indigenous Children with Genetic Disease. Available online: https://genomecanada.ca/project/silent-genomes-reducing-health-care-disparities-and-improving-diagnostic-success-indigenous-children/ (accessed on 1 February 2023).
- Genome UK: The Future of Healthcare. Available online: https://www.gov.uk/government/publications/genome-uk-the-future-of-healthcare/genome-uk-the-future-of-healthcare (accessed on 1 February 2023).
- S.3239—RACE for Children Act. Available online: https://www.congress.gov/bill/114th-congress/senate-bill/3239 (accessed on 1 February 2023).
- The European Paediatric Initiative: History of the Paediatric Regulation. Available online: https://www.ema.europa.eu/en/documents/other/european-paediatric-initiative-history-paediatric-regulation_en.pdf (accessed on 1 February 2023).
- Zettler, M.E. The RACE for Children Act at One Year: Progress in Pediatric Development of Molecularly Targeted Oncology Drugs. Expert. Rev. Anticancer. Ther. 2022, 22, 317–321. [Google Scholar] [CrossRef]
- Pochopień, M.; Paterak, E.; Clay, E.; Janik, J.; Aballea, S.; Biernikiewicz, M.; Toumi, M. An overview of health technology assessments of gene therapies with the focus on cost-effectiveness models. J. Mark. Access. Health Policy 2021, 9, 2002006. [Google Scholar] [CrossRef]
- Rawson, N.S.B. Health technology assessment and price negotiation alignment for rare disorder drugs in Canada: Who benefits? Orphanet. J. Rare Dis. 2022, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Kirwin, E.; Round, J.; Bond, K.; McCabe, C. A Conceptual Framework for Life-Cycle Health Technology Assessment. Value Health 2022, 25, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Regier, D.A.; Pollard, S.; McPhail, M.; Bubela, T.; Hanna, T.P.; Ho, C.; Lim, H.J.; Chan, K.; Peacock, S.J.; Weymann, D. A perspective on life-cycle health technology assessment and real-world evidence for precision oncology in Canada. NPJ Precis. Oncol. 2022, 6, 76. [Google Scholar] [CrossRef]
- Wadmann, S. Disease classification: A framework for analysis of contemporary developments in precision medicine. SSM Qual. Res. Health 2023, 3, 100217. [Google Scholar] [CrossRef]
- Lange, C.; Aarnoutse, R.; Chesov, D.; van Crevel, R.; Gillespie, S.H.; Grobbel, H.P.; Kalsdorf, B.; Kontsevaya, I.; van Laarhoven, A.; Nishiguchi, T.; et al. Perspective for Precision Medicine for Tuberculosis. Front. Immunol. 2020, 8, 566608. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Williams, L.M.; Steiner, J.; Leboyer, M.; Carvalho, A.F.; Berk, M. The new field of ‘precision psychiatry’. BMC Med. 2017, 15, 80. [Google Scholar] [CrossRef] [PubMed]
| Number | Percentage | |
|---|---|---|
| Year of publication | ||
| Before 2010 | 2 | 2.7 |
| 2010–2015 | 10 | 13.5 |
| 2015–2020 | 24 | 32.4 |
| 2020–2022 | 38 | 51.4 |
| Type of article | ||
| Review article | 37 | 50 |
| Primary literature | 28 | 37.8 |
| Book chapter | 3 | 4.1 |
| Opinion piece | 2 | 2.7 |
| Editorial | 1 | 1.35 |
| Conference proceedings | 1 | 1.35 |
| Best practice guidelines | 1 | 1.35 |
| Special Report | 1 | 1.35 |
| Jurisdiction | ||
| United States of America | 33 | 44.6 |
| Multinational | 10 | 13.5 |
| Canada | 12 | 16.2 |
| Australia | 4 | 5.4 |
| The Netherlands | 4 | 5.4 |
| United Kingdom | 3 | 4.1 |
| Denmark | 2 | 2.7 |
| Israel | 1 | 1.35 |
| Lebanon | 1 | 1.35 |
| Germany | 1 | 1.35 |
| China | 1 | 1.35 |
| Belgium | 1 | 1.35 |
| Spain | 1 | 1.35 |
| Medical discipline (if applicable) | ||
| Oncology | 29 | 76.3 |
| Psychiatry | 4 | 10.5 |
| Pulmonology | 2 | 5.3 |
| Rare Diseases | 1 | 2.6 |
| Infectious Disease | 1 | 2.6 |
| Multidisciplinary | 1 | 2.6 |
| Dimension | Theme | Description |
|---|---|---|
| Evidence Development | Children are a unique population | Children have a unique physiology that is distinct from adults and that changes with age and development. Developmental progression can have large impacts on gene expression and function, ultimately impacting the efficacy of targeted interventions. |
| Study design challenges and potential solutions | Small sample sizes, diversity of study protocols, and safety are all barriers to conducting effective clinical trials in the pediatric population. Employing novel trial designs (e.g., umbrella, basket, or n-of-1 trials) and institutional collaboration are potential avenues to circumvent major study design challenges. | |
| Evidence Appraisal | Clinical benefit | As in adult populations, disease domains with sufficient evidence have clear indications for precision health interventions in children. Unique to pediatrics are the potentially greater life course implications and impact on family members through a greater prevalence of germline mutations in cancer predisposition genes. |
| Cost-effectiveness | Similar to value assessments for adult interventions, cost-effectiveness was deemed important. Specifically, preemptive pharmacogenomic testing was highlighted as a strategy for health system savings. | |
| Stakeholder preferences and values | HTA typically incorporates the values and preferences of various stakeholders, including patients, health care providers, and disease domain experts. Parents and providers were generally accepting of precision health interventions. Parents viewed the intervention as an opportunity to improve care but also felt little choice as their children often had advanced stages of disease. Key decision-makers were critical of existing tools and frameworks to evaluate precision health for children. | |
| Ethics and equity | Substantive and procedural ethical challenges were identified, which included metrics for measuring value for children and the process of incorporating child voices into value assessments, respectively. Equity concerns related to the cost of precision health interventions were raised, as were concerns related to precision health literacy. | |
| Implementation | Implementation barriers and enablers | Challenges with the implementation of precision health interventions relate to (1) providers’ confidence to prescribe and use these interventions, and (2) ensuring patients can make informed decisions regarding the use of these interventions in their care. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subasri, M.; Cressman, C.; Arje, D.; Schreyer, L.; Cooper, E.; Patel, K.; Ungar, W.J.; Barwick, M.; Denburg, A.; Hayeems, R.Z. Translating Precision Health for Pediatrics: A Scoping Review. Children 2023, 10, 897. https://doi.org/10.3390/children10050897
Subasri M, Cressman C, Arje D, Schreyer L, Cooper E, Patel K, Ungar WJ, Barwick M, Denburg A, Hayeems RZ. Translating Precision Health for Pediatrics: A Scoping Review. Children. 2023; 10(5):897. https://doi.org/10.3390/children10050897
Chicago/Turabian StyleSubasri, Mathushan, Celine Cressman, Danielle Arje, Leighton Schreyer, Erin Cooper, Komal Patel, Wendy J. Ungar, Melanie Barwick, Avram Denburg, and Robin Z. Hayeems. 2023. "Translating Precision Health for Pediatrics: A Scoping Review" Children 10, no. 5: 897. https://doi.org/10.3390/children10050897
APA StyleSubasri, M., Cressman, C., Arje, D., Schreyer, L., Cooper, E., Patel, K., Ungar, W. J., Barwick, M., Denburg, A., & Hayeems, R. Z. (2023). Translating Precision Health for Pediatrics: A Scoping Review. Children, 10(5), 897. https://doi.org/10.3390/children10050897






