Intensive Care Clinicians’ Perspectives on Ethical Challenges Raised by Rapid Genomic Testing in Critically Ill Infants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment
2.3. Data Collection and Analysis
3. Results
3.1. Demographics
3.2. Obtaining Parental Consent in the Context of Rapid Genomic Testing—Responses to Scenario A
“The decision may also be influenced by how rapidly he [Alex] needed life sustaining surgery and how soon a geneticist could get there.”—P22, neonatal intensivist, Canada, 0–4 years of experience.
“Healthcare professionals taking consent for genomic testing should have had training from genetic colleagues and only take consent if confident to do so, otherwise consent taking should be supported by a genetics health professional.”—P40, neonatal intensivist, United Kingdom, 15–19 years of experience.
“Having a child admitted to a NICU infers consent for ‘usual treatment’. Discovery of an underlying genetic cause for a condition is ‘usual treatment’. It is only that the technology being used is different.”—P35, neonatal intensivist, Australia, 20+ years of experience.
“Clear disagreement may result in further harms to this family.”—P3, neonatal intensivist, Armenia, 5–9 years of experience.
“It is common for families in our care to be overwhelmed and distressed. We must trust that they are making the best decision they can at the time.”—P8, neonatal intensivist, Australia, 10–14 years of experience.
“If consent is being sought–testing cannot go ahead without being able to verify that parents understand.”—P2, neonatologist, United Kingdom, 15–19 years of experience.
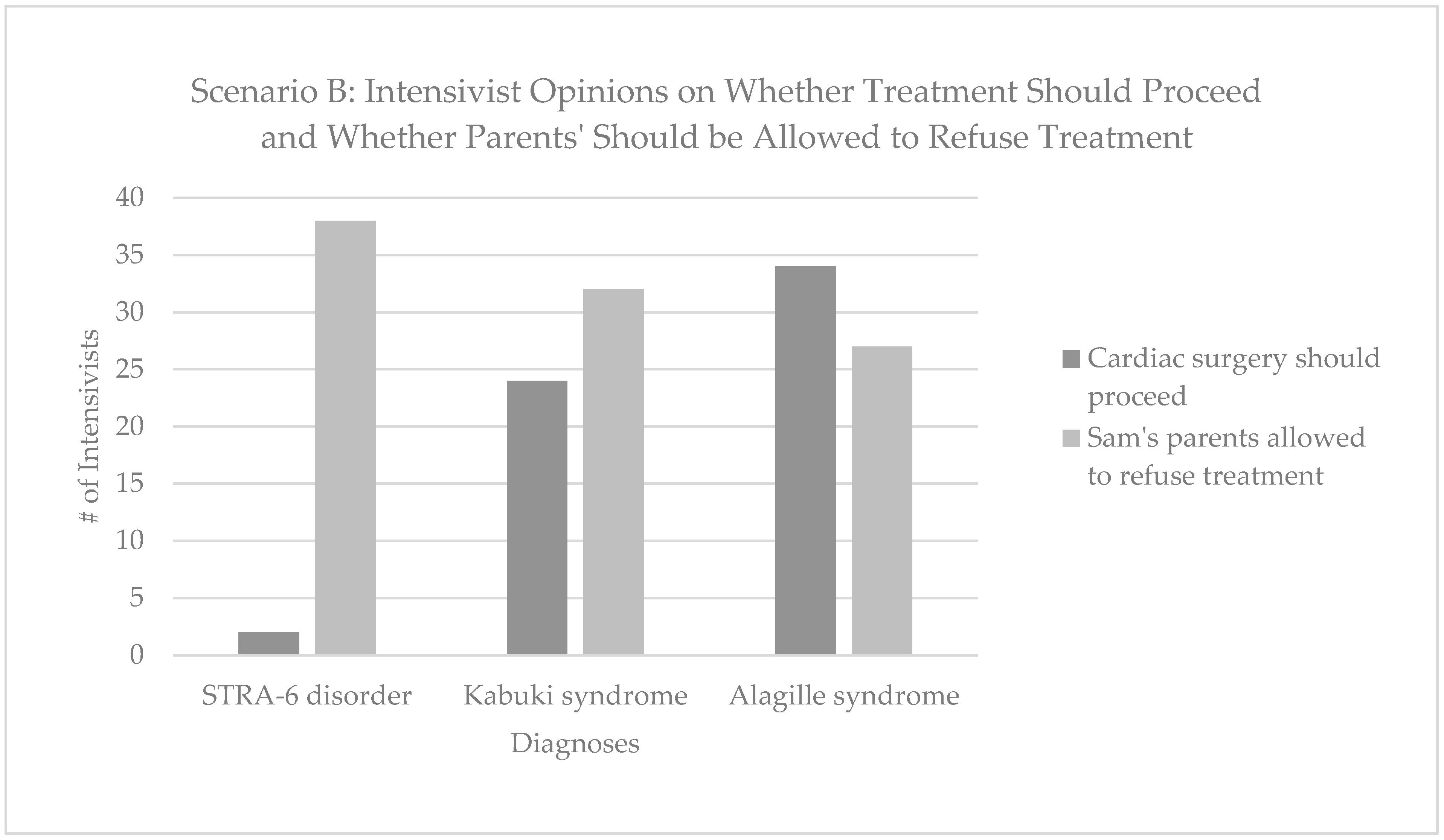
3.3. Withdrawal of Life-Sustaining Treatment in the Context of Rapid Genomic Testing—Responses to Scenario B
“It may be appropriate for a relatively simple procedure to be done but the focus should be on this child’s quality of life. Care should be directed towards making his short life as happy and distress free as possible.”–P39, neonatologist, United Kingdom, 20+ years of experience.
“These are 2 serious conditions—it depends on exactly what the complex heart disease is and the morbidity associated with that. I think it’s a finely balanced decision whether to operate or not—and his parents should be involved in that decision.”—P39, neonatologist, United Kingdom, 20+ years of experience.
“Will definitely need help from geneticist and ethical committee.”—P14, neonatologist, Belgium, 5–9 years of experience.
4. Discussion
4.1. Intensivists Are Divided on Whether Rapid Genomic Testing Requires Specific Consent
4.2. Impact of Genetic Diagnosis on Life-Sustaining Care
4.3. Impact of Demographic Differences on Parental Discretion
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Survey
- have any concerns or complaints about the project
- are worried about your rights as a research participant
- would like to speak to someone independent of the project.
- Neonatal intensivist or trainee
- Paediatric Intensivist or trainee
- General paediatrician or trainee
- Paediatric anaesthetist or trainee
- Other role not listed above (please specify)
Alex was born prematurely at 36 weeks’ gestation with multiple dysmorphic features and complex congenital anomalies, which will require surgery. The intensive care team decide rapid genomic testing is the test most likely to identify a diagnosis and avoid any unnecessary invasive procedures.
- (a)
- consent from the parents should be obtained by a genetic health professional (genetic counsellor or clinical geneticist), even if this means waiting an additional day for someone to be available
- (b)
- consent from the parents should be obtained by whichever non-genetic health professional (ICU doctor, nurse) is available the soonest
- (a)
- the treating clinician should be able to order rapid genomic testing without parental consent
- (b)
- the team should wait for one of the parents to be available and obtain their consent, even though the delay may mean the infant’s condition deteriorates
- (c)
- the team should wait until both parents are available and obtain both their consent, even though the delay may mean the infant’s condition deteriorates
- (a)
- rapid genomic testing should proceed, even when one parent declines
- (b)
- rapid genomic testing should not proceed unless both parents consent
- (a)
- rapid genomic testing should proceed because the parents have given consent to it
- (b)
- rapid genomic testing should be delayed until the parents are able to give informed consent
- (a)
- rapid genomic testing should proceed
- (b)
- rapid genomic testing should not proceed until an interpreter can speak with the family
Sam was born at 32 weeks with multiple dysmorphic features, and a complex heart condition for which surgery is indicated. Surgery has a 50% chance of successfully treating the heart problem, though the overall prognosis is unclear. Rapid Genomic Testing is ordered with the hope of learning more about the prognosis. The test identifies two mutations in the STRA6 gene, which cause a recessive syndromic disorder associated with alveolar capillary dysplasia, diaphragmatic eventration, microphthalmia and profound intellectual disability. This means that even if infant B survives the cardiac surgery, there is a 95% chance they will die in the first year of life, likely secondary to pulmonary issues. In your view, should cardiac surgery proceed?
- (a)
- Yes
- (b)
- No
- (a)
- Yes
- (b)
- No
- (a)
- Yes
- (b)
- No
- (a)
- Yes
- (b)
- No
- (a)
- Yes
- (b)
- No
- (a)
- Yes
- (b)
- No
References
- Elliott, A.M. Genetic Counseling and Genome Sequencing in Pediatric Rare Disease. Cold Spring Harb. Perspect. Med. 2020, 10, a036632. [Google Scholar] [CrossRef] [PubMed]
- Farnaes, L.; Hildreath, A.; Sweeney, M.; Clark, M.; Chowdhury, S.; Nahas, S.; Cakici, J.; Benson, W.; Kaplan, R.; Kronick, R.; et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom. Med. 2018, 3, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestek-Boukhibar, L.; Clement, E.; Jones, W.; Drury, S.; Ocaka, L.; Gagunashvili, A.; Le Quesne Stabej, P.; Bacchelli, C.; Jani, N.; Rahman, S.; et al. Rapid Paediatric Sequencing (RaPS): Comprehensive real-life workflow for rapid diagnosis of critically ill children. J. Med. Genet. 2018, 55, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, M.J.; Wright, M.S.; Batalov, S.; Kwon, Y.; Ding, Y.; Chau, K.K.; Chowdhury, S.; Sweeney, N.M.; Kiernan, E.; Richarson, A.; et al. Reclassification of the Etiology of Infant Mortality With Whole-Genome Sequencing. JAMA Netw. Open 2023, 6, e2254069. [Google Scholar] [CrossRef]
- Kingsmore, S.F.; Petrikin, J.; Willig, L.K.; Guest, E. Emergency medical genomes: A breakthrough application of precision medicine. Genome Med. 2015, 7, 82. [Google Scholar] [CrossRef] [Green Version]
- Jezkova, J.; Shaw, S.; Taverner, N.; Williams, H. Rapid genome sequencing for pediatrics. Hum. Mutat. 2022, 43, 1507–1518. [Google Scholar] [CrossRef]
- Owen, M.J.; Nieme, A.; Dimmock, D.; Speziale, M.; Nespeca, M.; Chau, K.; Van Der Kraan, L.; Wright, M.; Hansen, C.; Veeraraghavan, N.; et al. Rapid Sequencing-Based Diagnosis of Thiamine Metabolism Dysfunction Syndrome. N. Engl. J. Med. 2021, 384, 2159–2161. [Google Scholar] [CrossRef]
- Buchan, J.G.; White, S.; Joshi, R.; Ashley, E. Rapid Genome Sequencing in the Critically Ill. Clin. Chem. 2019, 65, 723–726. [Google Scholar] [CrossRef]
- Clark, M.M.; Stark, Z.; Farnaes, L.; Tan, T.; White, S.; Dimmock, D.; Kingsmore, S. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. NPJ Genom. Med. 2018, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- van Diemen, C.C.; Kerstjens-Frederikse, W.S.; Bergman, K.A.; de Koning, T.J.; Sikkema-Raddatz, B.; van der Velde, J.K.; Abbott, K.M.; Herket, J.C.; Lönner, K.; Rump, P.; et al. Rapid Targeted Genomics in Critically Ill Newborns. Pediatrics 2017, 140, e20162854. [Google Scholar] [CrossRef] [Green Version]
- Best, M.C.; Butow, P.; Jacobs, C.; Savard, J.; Biesecker, B.; Ballinger, M.L.; Bartley, N.; Davies, G.; Napier, C.E.; Smit, A.; et al. Who should access germline genome sequencing? A mixed methods study of patient views. Clin. Genet. 2020, 97, 329–337. [Google Scholar] [CrossRef]
- Goranitis, I.; Wu, Y.; Lunke, S.; White, S.M.; Tan, T.; Yeung, A.; Hunter, M.; Martyn, M.; Gaff, C.; Stark, Z.; et al. Is faster better? An economic evaluation of rapid and ultra-rapid genomic testing in critically ill infants and children. Genet. Med. 2022, 24, 1037–1044. [Google Scholar] [CrossRef]
- Beaman, M.; Fisher, K.; McDonald, M.; Tan, Q.; Jackson, D.; Cocanougher, B.; Landstrom, A.; Hobbs, C.; Cotton, M.; Cohen, J.; et al. Rapid Whole Genome Sequencing in Critically Ill Neonates Enables Precision Medicine Pipeline. J. Pers. Med. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Berrios, C.; Koertje, C.; Noel-MacDonnell, J.; Soden, S.; Lantos, J. Parents of newborns in the NICU enrolled in genome sequencing research: Hopeful, but not naïve. Genet. Med. 2020, 22, 416–422. [Google Scholar] [CrossRef]
- Brett, G.R.; Martyn, M.; Lynch, F.; de Silva, M.G.; Ayres, S.; Gallacher, L.; Boggs, K.; Baxendale, A.; Schenscher, S.; King-Smith, S.; et al. Parental experiences of ultrarapid genomic testing for their critically unwell infants and children. Genet. Med. 2020, 22, 1976–1985. [Google Scholar] [CrossRef]
- Cakici, J.A.; Dimmock, D.; Caylor, S.; Gaughran, M.; Clarke, C.; Triplett, C.; Clark, M.; Kingsmore Bloss, C. A Prospective Study of Parental Perceptions of Rapid Whole-Genome and -Exome Sequencing among Seriously Ill Infants. Am. J. Hum. Genet. 2020, 107, 953–962. [Google Scholar] [CrossRef]
- Char, D.S.; Lee, S.; Magnus, D.; Cho, M. Anticipating uncertainty and irrevocable decisions: Provider perspectives on implementing whole-genome sequencing in critically ill children with heart disease. Genet. Med. 2018, 20, 1455–1461. [Google Scholar] [CrossRef]
- Lynch, F.; Nisselle, A.; Stark, Z.; Gaff, C.; McClaren, B. Parents’ experiences of decision making for rapid genomic sequencing in intensive care. Eur. J. Hum. Genet. 2021, 29, 1804–1810. [Google Scholar] [CrossRef]
- Bowman-Smart, H.; Vears, D.; Brett, G.; Martyn, M.; Stark, Z.; Gyngell, C. ‘Diagnostic shock’: The impact of results from ultrarapid genomic sequencing of critically unwell children on aspects of family functioning. Eur. J. Hum. Genet. 2022, 30, 1036–1043. [Google Scholar] [CrossRef]
- Hill, M.; Hammond, J.; Lewis, C.; Mellis, R.; Clement, E.; Chitty, L. Delivering genome sequencing for rapid genetic diagnosis in critically ill children: Parent and professional views, experiences and challenges. Eur. J. Hum. Genet. 2020, 28, 1529–1540. [Google Scholar] [CrossRef]
- Stark, Z.; Ellard, S. Rapid genomic testing for critically ill children: Time to become standard of care? Eur. J. Hum. Genet. 2022, 30, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, N.M.; Nahas, S.A.; Chowdhury, S.; Campo, M.D.; Jones, M.C.; Dimmock, D.P.; Kingsmore, S.F. The case for early use of rapid whole-genome sequencing in management of critically ill infants: Late diagnosis of Coffin-Siris syndrome in an infant with left congenital diaphragmatic hernia, congenital heart disease, and recurrent infections. Mol. Case Stud. 2018, 4, a002469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayres, S.; Gallacher, L.; Stark, Z.; Brett, G. Genetic counseling in pediatric acute care: Reflections on ultra-rapid genomic diagnoses in neonates. J. Genet. Couns. 2019, 28, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Knapp, B.; Decker, C.; Lantos, J.D. Neonatologists’ Attitudes About Diagnostic Whole-Genome Sequencing in the NICU. Pediatrics 2019, 143 (Suppl. S1), S54–S57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arkell, K.; Gyngell, C.; Stark, Z.; Vears, D. Rapid Genomic Testing in Intensive Care: Health Professionals’ Perspectives on Ethical Challenges. Children 2023, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- ACMG American College of Medical Genetics and Genomics: Policy Statement. 2023. Available online: https://www.acmg.net/ACMG/Advocacy/Policy-Statements/ACMG/Advocacy/Policy-Statements.aspx?hkey=31d4ab23-4888-412f-953e-b5a2be3af63d (accessed on 26 May 2023).
- CCMG Our Mission. 2023. Available online: https://www.ccmg-ccgm.org/about-ccmg/ (accessed on 26 May 2023).
- ESHG. ESHG Policy Statements. 2023. Available online: https://www.eshg.org/public-and-professional-policy/policy-statements (accessed on 26 May 2023).
- HGSA. HGSA Policies and Position Statements. 2023. Available online: https://hgsa.imiscloud.com/Web/Consumer-resources/Policies-Position-Statements.aspx (accessed on 26 May 2023).
- Borghesi, A.; Mencarelli, M.; Memo, L.; Ferrero, G.; Bartulli, A.; Genuardi, M.; Stronati, M.; Villani, A.; Renieri, A.; Corsello, G. Intersociety policy statement on the use of whole-exome sequencing in the critically ill newborn infant. Italian J. Pediatr. 2017, 43, 100. [Google Scholar] [CrossRef] [Green Version]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- R Team. RStudio:Integrated Development for R; RStudio, PBC: Boston, MA, USA, 2020. [Google Scholar]
- Vears, D.F.; Gillam, L. Inductive content analysis: A guide for beginning qualitative researchers. Focus Health Prof. Educ. Multi-Prof. J. 2022, 23, 111–127. [Google Scholar] [CrossRef]
- Borry, P.; Fryns, J.; Schotsmans, P.; Dierickx, K. Carrier testing in minors: A systematic review of guidelines and position papers. Eur. J. Hum. Genet. 2006, 14, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Lynch, F.; Prentice, T.; Gillam, L.; Stark, Z.; Gyngell, C. Rapid Genome Sequencing: Consent for New Technologies in the Neonatal Intensive Care Context. Pediatrics 2022, 150, e2022058222. [Google Scholar] [CrossRef]
- Gyngell, C.; Lynch, F.; Stark, Z.; Vears, D. Consent for rapid genomic sequencing for critically ill children: Legal and ethical issues. Monash Bioeth. Rev. 2021, 39 (Suppl. S1), 117–129. [Google Scholar] [CrossRef]
- Kmietowicz, Z. Down’s children received “less favourable” hospital treatment. BMJ 2001, 322, 815. [Google Scholar] [CrossRef]
- Champagne, C.R.; Lewis, M.; Gilchrist, D.M. Should we mend their broken hearts? The history of cardiac repairs in children with Down syndrome. Pediatrics 2014, 134, 1048–1050. [Google Scholar] [CrossRef] [Green Version]
- Gillam, L.; Sullivan, J. Ethics at the end of life: Who should make decisions about treatment limitation for young children with life-threatening or life-limiting conditions? J. Paediatr. Child Health 2011, 47, 594–598. [Google Scholar] [CrossRef]
- Nuffield Corporation of Bioethics. Disagreements in the Care of Critically Ill Children; Nuffield Corporation of Bioethics: London, UK, 2019. [Google Scholar]
- Nawaz, F.A.; Deo, N.; Surani, S.; Maynard, W.; Gibbs, M.; Kashyap, R. Critical care practices in the world: Results of the global intensive care unit need assessment survey 2020. World J. Crit. Care Med. 2022, 11, 169–177. [Google Scholar] [CrossRef]
- Stark, Z.; Nisselle, A.; McClaren, B.; Lynch, F.; Best, S.; Long, J.; Martyn, M.; Patel, C.; Schlapbach, L.; Barnett, C.; et al. Attitudes of Australian health professionals towards rapid genomic testing in neonatal and paediatric intensive care. Eur. J. Hum. Genet. 2019, 27, 1493–1501. [Google Scholar] [CrossRef]
- Wahlster, S.; Sharma, M.; Coruh, B.; Town, J.; Lewis, A.; Lobo, S.; Maia, I.; Hartog, C.; Patel, P.; Kross, E.; et al. A Global Survey of the Effect of COVID-19 on Critical Care Training. ATS Sch. 2021, 2, 508–520. [Google Scholar] [CrossRef]
- ACMG. Points to consider for informed consent for genome/exome sequencing. Genet. Med. 2013, 15, 748–749. [Google Scholar] [CrossRef] [Green Version]
| Australasia | Europe | North America | All | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Years’ experience in specialty | ||||
| Trainee | 0 (0) | 3 (18.8) | 1 (11.1) | 4 (10) |
| 0–4 | 3 (20) | 0 (0) | 3 (33.3) | 6 (15) |
| 5–9 | 1 (6.7) | 4 (26) | 1 (11.1) | 6 (15) |
| 10–14 | 4 (26.7) | 2 (12) | 1 (11.1) | 7 (18) |
| 15–20 | 1 (6.7) | 5 (31.3) | 0 (0) | 6 (15) |
| 20+ | 6 (40) | 2 (12) | 3 (33.3) | 11 (27) |
| Number of standard genomic tests previously ordered | ||||
| 0 | 3 (20) | 4 (26) | 0 (0) | 7 (17.5) |
| 1–4 | 2 (13.3) | 2 (12) | 0 (0) | 3 (7.5) |
| 5–9 | 4 (26.7) | 5 (31.3) | 1 (11.1) | 10 (25) |
| 10–14 | 2 (13.3) | 0 (0) | 1 (11.1) | 4 (10) |
| 15–20 | 2 (13.3) | 2 (12) | 3 (33.3) | 7 (17.5) |
| 20+ | 2 (13.3) | 3 (18.8) | 4 (44.4) | 9 (22.5) |
| Number of rapid genomic tests previously ordered | ||||
| 0 | 5 (33.3) | 6 (37.5) | 0 (0) | 11 (27.5) |
| 1–4 | 6 (40) | 7 (43.8) | 2 (22.2) | 15 (37.5) |
| 5–9 | 2 (13.3) | 2 (12) | 2 (22.2) | 6 (15) |
| 10–14 | 1 (6.7) | 0 (0) | 3 (33.3) | 4 (10) |
| 15–20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 20+ | 1 (6.7) | 1 (6.3) | 2 (22.2) | 4 (10) |
| TOTAL | 15 | 16 | 9 | 40 |
| rGT Should/Should Not Proceed under the Following Circumstances: | Agree n (%) | Disagree n (%) |
|---|---|---|
| We should wait an extra day to allow the GHP obtain consent from family | 20 (50) | 20 (50) |
| We should wait for at least one parent’s consent before ordering rGT | 30 (75) | 10 (25) |
| rGT should NOT proceed if one parent has refused to provide consent | 23 (57) | 17 (43) |
| rGT should proceed if parents are overwhelmed but have given (possibly uninformed) consent | 22 (55) | 18 (45) |
| If the parents do not speak English rGT should NOT proceed until an interpreter is available | 29 (72) | 11 (28) |
| Scenario A Questions | Scenario B Questions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 6 | ||
| Continent | Chi-squared | 1.32 | 0.35 | 0.83 | 0.79 | 3.75 | 0.614 | 1.55 | 2.67 | 4.74 | 5.67 | 6.84 |
| DF | 2 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| p-value | 0.518 | 0.987 | 0.66 | 0.675 | 0.153 | 0.736 | 0.461 | 0.263 | 0.093 | 0.059 | 0.033 * | |
| Years Practising | Chi-squared | 2.23 | 7.62 | 2.94 | 5.95 | 6.56 | 4.41 | 8.48 | 7.18 | 14.58 | 3.64 | 6.71 |
| DF | 5 | 10 | 5 | 5 | 5 | 5 | 10 | 5 | 5 | 5 | 5 | |
| p-value | 0.816 | 0.666 | 0.71 | 0.311 | 0.256 | 0.492 | 0.582 | 0.207 | 0.012 * | 0.601 | 0.243 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poogoda, S.; Lynch, F.; Stark, Z.; Wilkinson, D.; Savulescu, J.; Vears, D.; Gyngell, C. Intensive Care Clinicians’ Perspectives on Ethical Challenges Raised by Rapid Genomic Testing in Critically Ill Infants. Children 2023, 10, 970. https://doi.org/10.3390/children10060970
Poogoda S, Lynch F, Stark Z, Wilkinson D, Savulescu J, Vears D, Gyngell C. Intensive Care Clinicians’ Perspectives on Ethical Challenges Raised by Rapid Genomic Testing in Critically Ill Infants. Children. 2023; 10(6):970. https://doi.org/10.3390/children10060970
Chicago/Turabian StylePoogoda, Sachini, Fiona Lynch, Zornitza Stark, Dominic Wilkinson, Julian Savulescu, Danya Vears, and Christopher Gyngell. 2023. "Intensive Care Clinicians’ Perspectives on Ethical Challenges Raised by Rapid Genomic Testing in Critically Ill Infants" Children 10, no. 6: 970. https://doi.org/10.3390/children10060970
APA StylePoogoda, S., Lynch, F., Stark, Z., Wilkinson, D., Savulescu, J., Vears, D., & Gyngell, C. (2023). Intensive Care Clinicians’ Perspectives on Ethical Challenges Raised by Rapid Genomic Testing in Critically Ill Infants. Children, 10(6), 970. https://doi.org/10.3390/children10060970






