An Insight into Indonesia’s Challenges in Implementing Newborn Screening Programs and Their Future Implications
Abstract
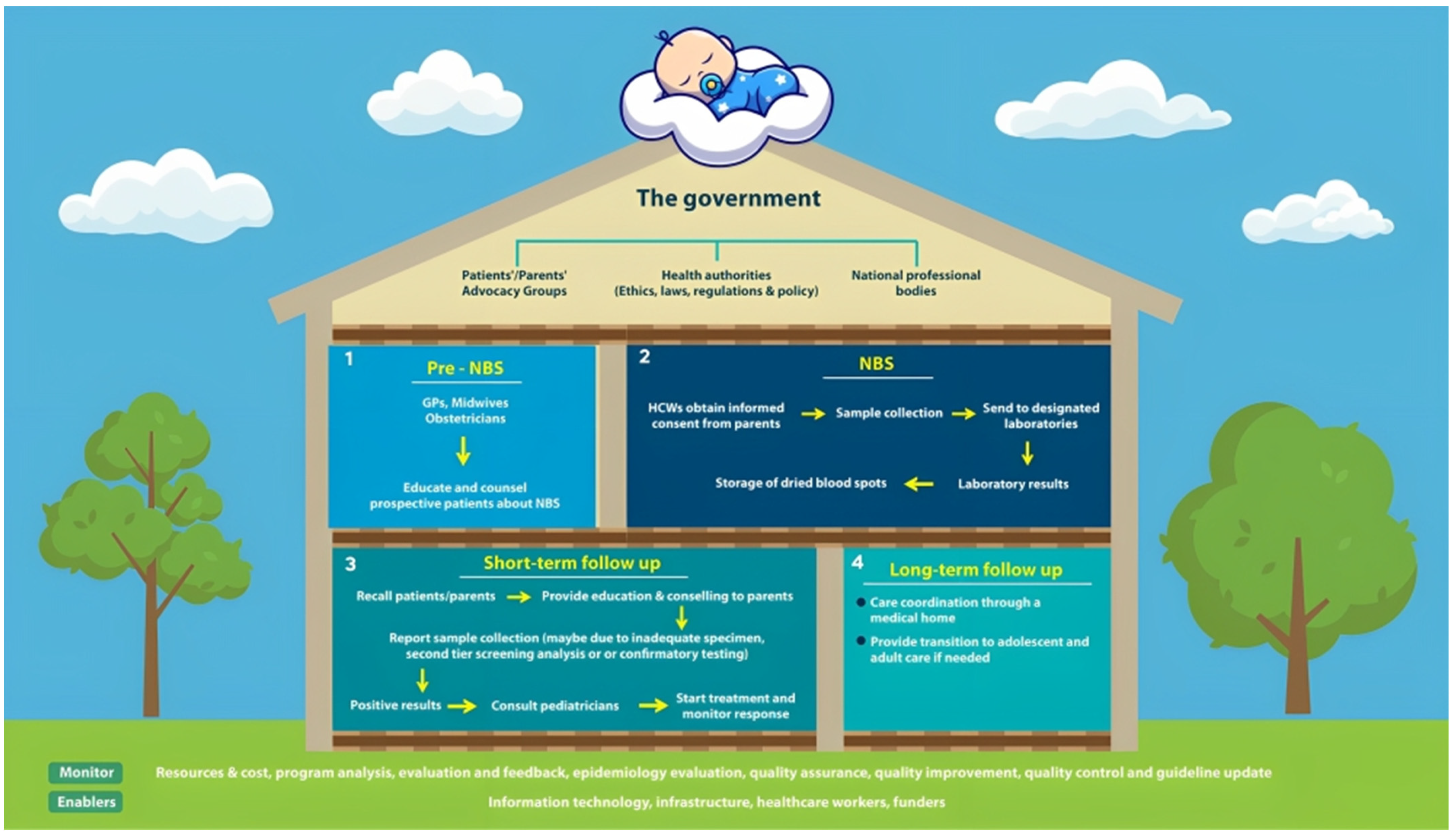
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Brief Description of Healthcare Profiles in Indonesia
3.2. Challenges of Implementing NBS
3.2.1. Lack of Prevalence Data
3.2.2. Economic Issues
3.2.3. Ethical Issues
3.2.4. Lack of Infrastructure
3.2.5. Logistical Issues
3.2.6. Government Support
3.2.7. Lack of Healthcare Workers, Specialization, and Training
3.2.8. Lack of Commitments
3.2.9. Patient Issues
3.3. Future Implications
3.3.1. Support from the Government and Professional Advocates
3.3.2. Single-Payer System
3.3.3. Upgrading the Information Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Octavius, G.S.; Antonio, F. Antecedents of Intention to Adopt Mobile Health (mHealth) Application and Its Impact on Intention to Recommend: An Evidence from Indonesian Customers. Int. J. Telemed. Appl. 2021, 2021, 6698627. [Google Scholar] [CrossRef] [PubMed]
- Badan Pusat Statistik. Available online: https://www.bps.go.id/ (accessed on 1 June 2023).
- Triyana, M. The effects of Indonesia’s ‘Midwife in the Village’ programme 10 years post-launch. Popul. Stud. 2016, 70, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, K.; Joshy, G.; Browne, J.; Bartini, I.; Hull, T.H.; Lokuge, K. Outcomes by birth setting and caregiver for low risk women in Indonesia: A systematic literature review. Reprod. Health 2019, 16, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efendi, F.; Ni’mah, A.R.; Hadisuyatmana, S.; Kuswanto, H.; Lindayani, L.; Berliana, S.M. Determinants of Facility-Based Childbirth in Indonesia. Sci. World J. 2019, 2019, 9694602. [Google Scholar] [CrossRef] [Green Version]
- Pulungan, A.B.; Soesanti, F.; Utari, A.; Pritayati, N.; Julia, M.; Annisa, D.; Bikin, I.W. Preliminary Study of Newborn Screening for Congenital Hypothyroidsm and Con-genital Adrenal Hyperplasia in Indonesia. Ejournal Kedokt. Indones. 2020, 8, 98–103. [Google Scholar] [CrossRef]
- Rochmah, N.; Faizi, M.; Dewanti, C.; Suryawan, C.D.A.A. Pediatric Quality of Life in Congenital Hypothyroidism: An Indonesian Study. Int. J. Thyroid. 2020, 13, 150–154. [Google Scholar] [CrossRef]
- Deng, K.; He, C.; Zhu, J.; Liang, J.; Li, X.; Xie, X.; Yu, P.; Li, N.; Li, Q.; Wang, Y. Incidence of congenital hypothyroidism in China: Data from the national newborn screening program, 2013–2015. J. Pediatr. Endocrinol. Metab. 2018, 31, 601–608. [Google Scholar] [CrossRef]
- Rustama, D.; Fadil, M.R.; Harahap, E.R.; Primadi, A. Newborn screening in Indonesia. Southeast Asian J. Trop. Med. Public Health 2003, 34, 76–79. [Google Scholar]
- Therrell, B.L.; Padilla, C.D. Newborn screening in the developing countries. Curr. Opin. Pediatr. 2018, 30, 734–739. [Google Scholar] [CrossRef]
- Stoddard, J.J. State-to-State Variations in Newborn Screening Policies. Arch. Pediatr. Adolesc. Med. 1997, 151, 561–564. [Google Scholar] [CrossRef]
- Ciorba, A.; Hatzopoulos, S.; Busi, M.; Guerrini, P.; Petruccelli, J.; Martini, A. The universal newborn hearing screening program at the University Hospital of Ferrara: Focus on costs and software solutions. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 807–816. [Google Scholar] [CrossRef] [PubMed]
- White, P.C. Neonatal screening for congenital adrenal hyperplasia. Nat. Rev. Endocrinol. 2009, 5, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Speiser, P.W. Improving neonatal screening for congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2004, 89, 3685–3686. [Google Scholar] [CrossRef] [Green Version]
- Güran, T.; Tezel, B.; Gürbüz, F.; Eklioğlu, B.S.; Hatipoğlu, N.; Kara, C.; Şimşek, E.; Çizmecioğlu, F.M.; Ozon, A.; Baş, F.; et al. Neonatal Screening for Congenital Adrenal Hyperplasia in Turkey: A Pilot Study with 38,935 Infants. J. Clin. Res. Pediatr. Endocrinol. 2019, 11, 13–23. [Google Scholar] [CrossRef]
- Tobe, R.G.; Martin, G.R.; Li, F.; Moriichi, A.; Wu, B.; Mori, R. Cost-effectiveness analysis of neonatal screening of critical congenital heart defects in China. Medicine 2017, 96, e8683. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.E.; Downs, S.M. Comprehensive Cost-Utility Analysis of Newborn Screening Strategies. Pediatrics 2006, 117, S287–S295. [Google Scholar] [CrossRef] [Green Version]
- Schoen, E.J.; Baker, J.C.; Colby, C.J.; To, T.T. Cost-Benefit Analysis of Universal Tandem Mass Spectrometry for Newborn Screening. Pediatrics 2002, 110, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Farrell, P.M.; Langfelder-Schwind, E.; Farrell, M.H. Challenging the dogma of the healthy heterozygote: Implications for newborn screening policies and practices. Mol. Genet. Metab. 2021, 134, 8–19. [Google Scholar] [CrossRef]
- Kementerian Kesehatan Republik Indonesia. Peraturan Menteri Kesehatan Republik Indonesia Nomor 78 Tahun 2014 Tentang Skrining Hipotiroid Kongenital. Available online: https://persi.or.id/wp-content/uploads/2020/11/pmk782014.pdf (accessed on 1 June 2023).
- Anggraini, A.; Suryawati, C.; Fatmasari, E.Y. Evaluasi Pelaksanaan Program Skrining Hipotiroid Kongenital Oleh Puskesmas Karangrejo Kota Metro, Lampung. J. Kesehat. Masy. 2019, 2019, 1–10. [Google Scholar]
- ICMR Task Force on Inherited Metabolic Disorders. Newborn Screening for Congenital Hypothyroidism and Congenital Adrenal Hyperplasia. Indian J. Pediatr. 2018, 85, 935–940. [Google Scholar] [CrossRef]
- Farrell, M.H.; Sims, A.M.; Kirschner, A.L.P.; Farrell, P.M.; Tarini, B.A. Vulnerable Child Syndrome and Newborn Screening Carrier Results for Cystic Fibrosis or Sickle Cell. J. Pediatr. 2020, 224, 44–50.e1. [Google Scholar] [CrossRef] [PubMed]
- Hom, L.A.; Silber, T.J.; Ennis-Durstine, K.; Hilliard, M.A.; Martin, G.R. Legal and Ethical Considerations in Allowing Parental Exemptions From Newborn Critical Congenital Heart Disease (CCHD) Screening. Am. J. Bioeth. 2016, 16, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Trafimow, D. Parents Do Not Always Have to Get Their Way: Why Critical Congenital Heart Disease Screening for Newborns Should Be Mandatory. Am. J. Bioeth. 2016, 16, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Beal, J.; Lewis, J.; Kenner, C. Should Informed Consent be Required for Routine Newborn Screening and for the Storage of Blood Samples? MCN Am. J. Matern. Nurs. 2014, 39, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Tchervenkov, C.I.; Jacobs, J.P.; Bernier, P.-L.; Stellin, G.; Kurosawa, H.; Mavroudis, C.; Jonas, R.A.; Cicek, S.M.; Al-Halees, Z.; Elliott, M.J.; et al. The improvement of care for paediatric and congenital cardiac disease across the World: A challenge for the World Society for Pediatric and Congenital Heart Surgery. Cardiol. Young 2008, 18, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Zheleva, B.; Nair, S.M.; Dobrzycka, A.; Saarinen, A. Considerations for Newborn Screening for Critical Congenital Heart Disease in Low- and Middle-Income Countries. Int. J. Neonatal Screen. 2020, 6, 49. [Google Scholar] [CrossRef]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal Screen. 2021, 7, 15. [Google Scholar] [CrossRef]
- Neumann, K.; Euler, H.A.; Chadha, S.; White, K.R. A Survey on the Global Status of Newborn and Infant Hearing Screening. J. Early Hear. Detect. Interv. 2020, 5, 63–84. [Google Scholar]
- Kementerian Kesehatan Republik Indonesia. Dukung Peningkatan Layanan Jantung Anak di Indonesia, IDAI-PERKI Tanda-tangani MoU. Available online: https://www.kemkes.go.id/article/view/23020600002/dukung-peningkatan-layanan-jantung-anak-di-indonesia-idai-perki-tandatangani-mou.html (accessed on 1 June 2023).
- Rahajoe, A.U. Management of patients with congenitally malformed hearts in Indonesia. Cardiol. Young 2007, 17, 584–588. [Google Scholar] [CrossRef]
- Garniasih, D.; Susanah, S.; Sribudiani, Y.; Hilmanto, D. The incidence and mortality of childhood acute lymphoblastic leukemia in Indonesia: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0269706. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Wulandari, R.D. The Evaluation of Congenital Hypothyroidism Screening Program in Indonesia: A Liter-ature Review. J. Aisyah J. Ilmu Kesehat. 2022, 7, 495–502. [Google Scholar]
- Hiola, F.A.A.; Hilamuhu, F.; Katili, D.N.O. Faktor-Faktor yang Mempengaruhi Cakupan Pelaksanaan Skrining Hipotiroid Kongenital di Rsu Prof. Dr. H. Aloe Saboe Kota Gorontalo. Media Publ. Promosi Kesehat. Indones. 2022, 5, 435–440. [Google Scholar] [CrossRef]
- Kayton, A. Newborn Screening: A Literature Review. Neonatal Netw. 2007, 26, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.; Adams, J. Current status of newborn screening worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikonja, J.; Groselj, U.; Scarpa, M.; la Marca, G.; Cheillan, D.; Kölker, S.; Zetterström, R.H.; Kožich, V.; Le Cam, Y.; Gumus, G.; et al. Towards Achieving Equity and Innovation in Newborn Screening across Europe. Int. J. Neonatal Screen. 2022, 8, 31. [Google Scholar] [CrossRef]
- Sudhanshu, S.; Riaz, I.; Sharma, R.; Desai, M.P.; Parikh, R.; Bhatia, V. Newborn Screening Guidelines for Congenital Hypothyroidism in India: Recommendations of the Indian Society for Pediatric and Adolescent Endocrinology (ISPAE)—Part II: Imaging, Treatment and Follow-up. Indian J. Pediatr. 2018, 85, 448–453. [Google Scholar] [CrossRef]
- Anggraini, R.; Patria, S.Y.; Julia, M. Ketepatan Waktu Pelayanan Skrining Hipotiroidism Kongenital di Yogyakarta. Sari Pediatri 2017, 18, 436. [Google Scholar] [CrossRef] [Green Version]
- Mwansisya, T.; Mbekenga, C.; Isangula, K.; Mwasha, L.; Mbelwa, S.; Lyimo, M.; Kisaka, L.; Mathias, V.; Pallangyo, E.; Edwards, G.; et al. The impact of training on self-reported performance in reproductive, maternal, and newborn health service delivery among healthcare workers in Tanzania: A baseline- and endline-survey. Reprod. Health 2022, 19, 143. [Google Scholar] [CrossRef]
- Hu, X.-J.; Ma, X.-J.; Zhao, Q.-M.; Yan, W.-L.; Ge, X.-L.; Jia, B.; Liu, F.; Wu, L.; Ye, M.; Liang, X.-C.; et al. Pulse Oximetry and Auscultation for Congenital Heart Disease Detection. Pediatrics 2017, 140, e20171154. [Google Scholar] [CrossRef] [Green Version]
- Kemper, A.R.; Uren, R.L.; Moseley, K.L.; Clark, S.J.; Iii, A.E.D.; Sharek, P.J.; Mickas, N.A.; Coker, K.L.; Duncan, J.; McLendon, D.; et al. Primary Care Physicians’ Attitudes Regarding Follow-up Care for Children With Positive Newborn Screening Results. Pediatrics 2006, 118, 1836–1841. [Google Scholar] [CrossRef]
- Murni, I.K.; Musa, N.L. The Need for Specialized Pediatric Cardiac Critical Care Training Program in Limited Resource Settings. Front. Pediatr. 2018, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Laurino, M.Y.; Leppig, K.A.; Abad, P.J.; Cham, B.; Chu, Y.W.Y.; Kejriwal, S.; Lee, J.M.H.; Sternen, D.L.; Thompson, J.K.; Burgess, M.J.; et al. A Report on Ten Asia Pacific Countries on Current Status and Future Directions of the Genetic Counseling Profession: The Establishment of the Professional Society of Genetic Counselors in Asia. J. Genet. Couns. 2017, 27, 21–32. [Google Scholar] [CrossRef]
- Ariani, Y.; Soeharso, P.; Sjarif, D.R. Genetics and genomic medicine in Indonesia. Mol. Genet. Genom. Med. 2017, 5, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.A.; Brueckner, M. The genetics of congenital heart disease… understanding and improving long-term outcomes in congenital heart disease: A review for the general cardiologist and primary care physician. Curr. Opin. Pediatr. 2017, 29, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Lieu, J.E.C.; Kenna, M.; Anne, S.; Davidson, L. Hearing Loss in Children. JAMA 2020, 324, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.H.; Kirschner, A.L.P.; Tluczek, A.; Farrell, P.M. Experience with Parent Follow-Up for Communication Outcomes after Newborn Screening Identifies Carrier Status. J. Pediatr. 2020, 224, 37–43.e2. [Google Scholar] [CrossRef]
- Fazeriandy, A.; Ali, M.; Saing, J.H.; Tobing, T.C.L.; Adriansyah, R. Consanguinity and congenital heart disease in offspring. Paediatr. Indones. 2018, 58, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Wilamarta, K.V.; Yuniadi, Y.; Rachmat, J.; Fakhri, D.; Hakim, T.; Anwar, M. Adult congenital cardiac surgery in Indonesia. Cardiol. Young 2011, 21, 639–645. [Google Scholar] [CrossRef]
- Disorders, I.T.F.O.I.M. The Journey of Newborn Screening: Inception to Conclusion. Indian J. Pediatr. 2018, 85, 933–934. [Google Scholar] [CrossRef]
- Neumann, K.; Mathmann, P.; Chadha, S.; Euler, H.A.; White, K.R. Newborn Hearing Screening Benefits Children, but Global Disparities Persist. J. Clin. Med. 2022, 11, 271. [Google Scholar] [CrossRef]
- White, K.R.; Forsman, I.; Eichwald, J.; Munoz, K. The Evolution of Early Hearing Detection and Intervention Programs in the United States. Semin. Perinatol. 2010, 34, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Franková, V.; Driscoll, R.O.; Jansen, M.E.; Loeber, J.G.; Kožich, V.; Bonham, J.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Regulatory landscape of providing information on newborn screening to parents across Europe. Eur. J. Hum. Genet. 2020, 29, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Fingerhut, R. Newborn Screening for Congenital Hypothyroidism-Clinical Evaluation and Comparison of Two Different Test Kits for the Determination of TSH in Dried Blood Samples on Two Different Platforms. Int. J. Neonatal Screen. 2021, 7, 51. [Google Scholar] [CrossRef]
- Kosmidou, P.; Tzifas, S.; Lygeros, S.; Danielides, G.; Nikolopoulos, T.; Dimitriou, G.; Angelis, S.; Naxakis, S. Newborn Hearing Screening: Analysing the Effectiveness of Early Detection of Neonatal Hearing Loss in a Hospital in Greece. Cureus 2021, 13, e19807. [Google Scholar] [CrossRef]
- Lee, K.-S.; Perlman, M. The impact of early obstetric discharge on newborn health care. Curr. Opin. Pediatr. 1996, 8, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; Drum, M.A.; Kessel, W. Early Discharge: Risks, Benefits, and Who Decides. Clin. Perinatol. 1998, 25, 539–553. [Google Scholar] [CrossRef]
- Shapira, S.K.; Hinton, C.F.; Held, P.K.; Jones, E.; Hannon, W.H.; Ojodu, J. Single newborn screen or routine second screening for primary congenital hypothyroidism. Mol. Genet. Metab. 2015, 116, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Zung, A.; Palmon, R.B.; Golan, A.; Troitzky, M.; Eventov-Friedman, S.; Marom, R.; Keidar, R.; Kats, N.; Almashanu, S.; Flidel-Rimon, O. Risk Factors for the Development of Delayed TSH Elevation in Neonatal Intensive Care Unit Newborns. J. Clin. Endocrinol. Metab. 2017, 102, 3050–3055. [Google Scholar] [CrossRef]
- Kaluarachchi, D.C.; Allen, D.B.; Eickhoff, J.C.; Dawe, S.J.; Baker, M.W. Increased Congenital Hypothyroidism Detection in Preterm Infants with Serial Newborn Screening. J. Pediatr. 2019, 207, 220–225. [Google Scholar] [CrossRef]
- Loeber, J.G.; Burgard, P.; Cornel, M.C.; Rigter, T.; Weinreich, S.S.; Rupp, K.; Hoffmann, G.F.; Vittozzi, L. Newborn screening programmes in Europe; arguments and efforts regarding harmonization. Part 1—From blood spot to screening result. J. Inherit. Metab. Dis. 2012, 35, 603–611. [Google Scholar] [CrossRef]
- Olney, R.S.; Ailes, E.C.; Sontag, M.K. Detection of critical congenital heart defects: Review of contributions from prenatal and newborn screening. Semin. Perinatol. 2015, 39, 230–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mincarone, P.; Leo, C.G.; Sabina, S.; Costantini, D.; Cozzolino, F.; Wong, J.B.; Latini, G. Evaluating reporting and process quality of publications on UNHS: A systematic review of programmes. BMC Pediatr. 2015, 15, 86. [Google Scholar] [CrossRef] [Green Version]
- Dinarti, L.K.; Anggrahini, D.W.; Lilyasari, O.; Siswanto, B.B.; Hartopo, A.B. Pulmonary Arterial Hypertension in Indonesia: Current Status and Local Application of International Guidelines. Glob. Hear. 2021, 16, 23. [Google Scholar] [CrossRef]
- Agustina, R.; Dartanto, T.; Sitompul, R.; Susiloretni, K.A.; Suparmi; Achadi, E.L.; Taher, A.; Wirawan, F.; Sungkar, S.; Sudarmono, P.; et al. Universal health coverage in Indonesia: Concept, progress, and challenges. Lancet 2018, 393, 75–102. [Google Scholar] [CrossRef]
- Pluscauskas, M.; Henderson, M.; Milburn, J.; Chakraborty, P. Building a Newborn Screening Information Management System from Theory to Practice. Int. J. Neonatal Screen. 2019, 5, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammersen, J.; Bettendorf, M.; Bonfig, W.; Schönau, E.; Warncke, K.; Eckert, A.J.; Fricke-Otto, S.; Palm, K.; Holl, R.W.; Woelfle, J. Twenty years of newborn screening for congenital adrenal hyperplasia and congenital primary hypothyroidism—Experiences from the DGKED/AQUAPE study group for quality improvement in Germany. Med. Genet. 2022, 34, 29–40. [Google Scholar] [CrossRef]
- Lloyd-Puryear, M.A.; Tonniges, T.; van Dyck, P.C.; Mann, M.Y.; Brin, A.; Johnson, K.; McPherson, M. American Academy of Pediatrics Newborn Screening Task Force Recommendations: How Far Have We Come? Pediatrics 2006, 117, S194–S211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla, C.D.; Therrell, B.L. Newborn screening in the Asia Pacific region. J. Inherit. Metab. Dis. 2007, 30, 490–506. [Google Scholar] [CrossRef]
- Howson, C.; Cedergren, B.; Giugliani, R.; Huhtinen, P.; Padilla, C.; Palubiak, C.; Santos; Schwartz, I.; Therrell, B.; Umemoto, A.; et al. Universal newborn screening: A roadmap for action. Mol. Genet. Metab. 2018, 124, 177–183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Octavius, G.S.; Daleni, V.A.; Sagala, Y.D.S. An Insight into Indonesia’s Challenges in Implementing Newborn Screening Programs and Their Future Implications. Children 2023, 10, 1216. https://doi.org/10.3390/children10071216
Octavius GS, Daleni VA, Sagala YDS. An Insight into Indonesia’s Challenges in Implementing Newborn Screening Programs and Their Future Implications. Children. 2023; 10(7):1216. https://doi.org/10.3390/children10071216
Chicago/Turabian StyleOctavius, Gilbert Sterling, Vamela Adman Daleni, and Yulita Delfia Sari Sagala. 2023. "An Insight into Indonesia’s Challenges in Implementing Newborn Screening Programs and Their Future Implications" Children 10, no. 7: 1216. https://doi.org/10.3390/children10071216
APA StyleOctavius, G. S., Daleni, V. A., & Sagala, Y. D. S. (2023). An Insight into Indonesia’s Challenges in Implementing Newborn Screening Programs and Their Future Implications. Children, 10(7), 1216. https://doi.org/10.3390/children10071216





