Sono-Elastography: An Ultrasound Quantitative Non-Invasive Measurement to Guide Bacterial Pneumonia Diagnosis in Children
Abstract
:1. Introduction
2. Materials and Methods
3. Lung Ultrasound: Where Do We Start
- The presence of A-lines.
- The presence of B-lines, their characteristics (short or long, spared or confluent) and their location (peri-lesional, monolateral/bilateral).
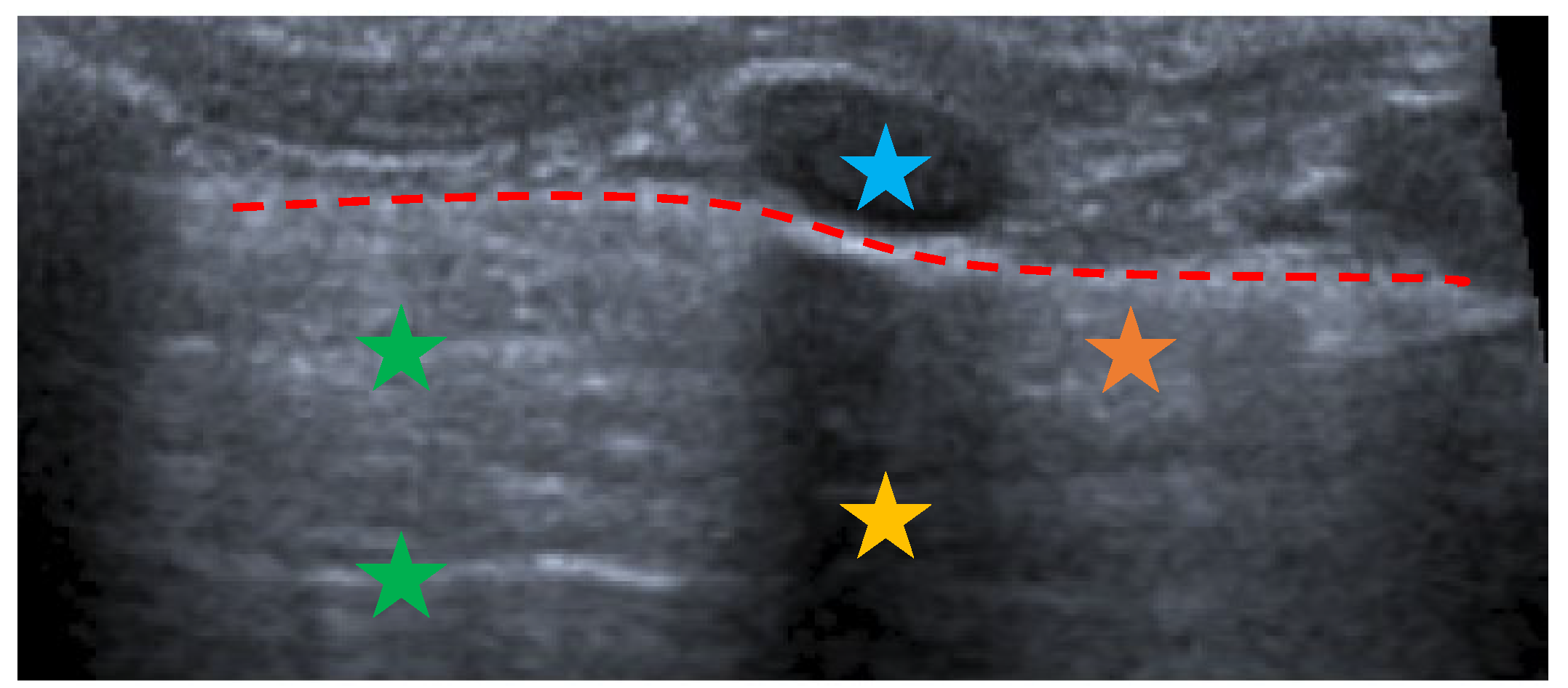
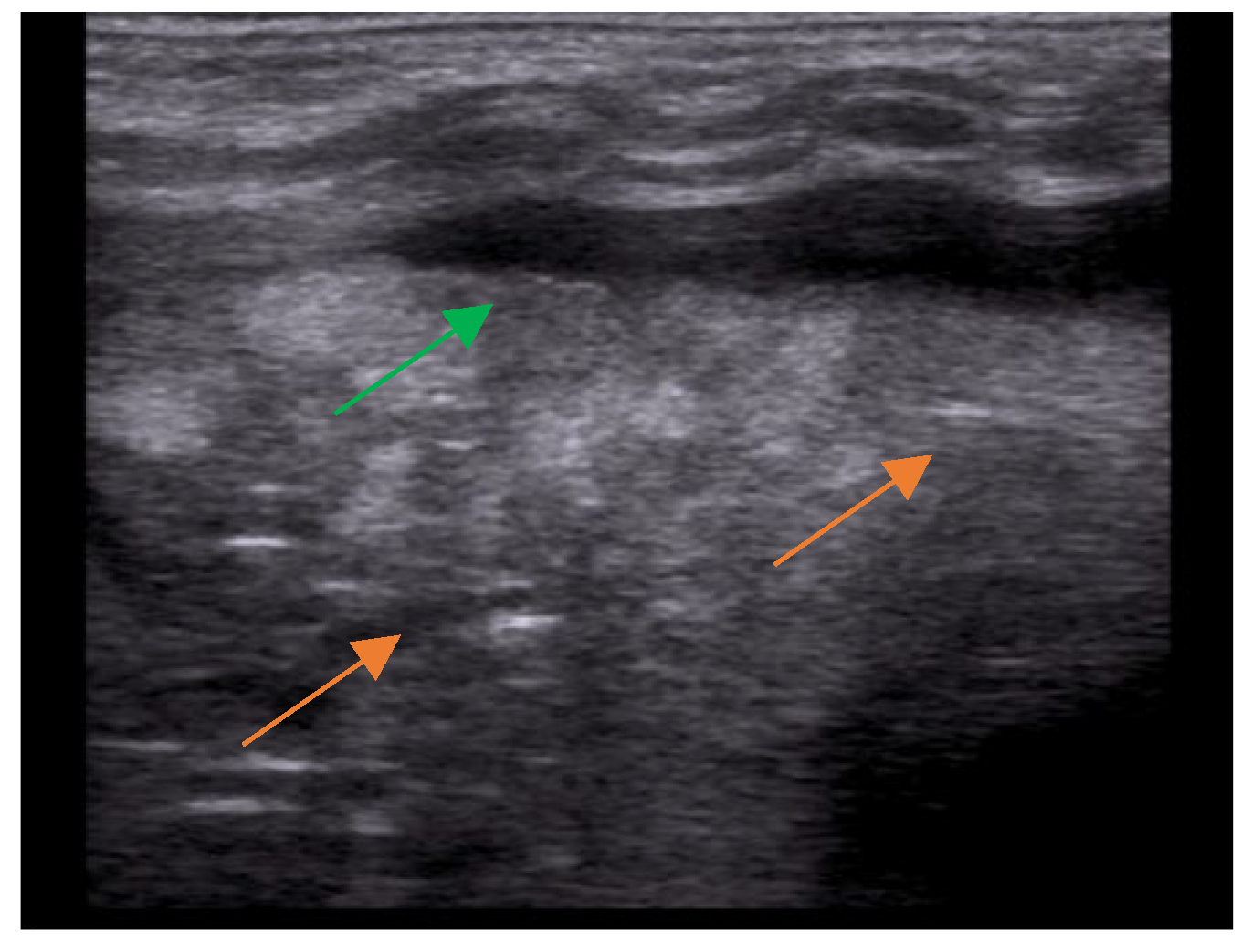
- The main lesion (consolidation): size, whether it is single or multiple, location (monolateral or bilateral). The presence of small subpleural consolidations (<1 cm).
- The presence of a bronchogram and its characteristics (air or fluid), morphology (branched or dot-like), dynamics during breathing (poorly or clearly dynamic); vascular pattern, presence of lung point and pulmonary pulse.
- The presence of lung sliding (M-mode).
- The presence and type of pleural effusion.
4. Ultrasound Elastography or Sono-Elastography
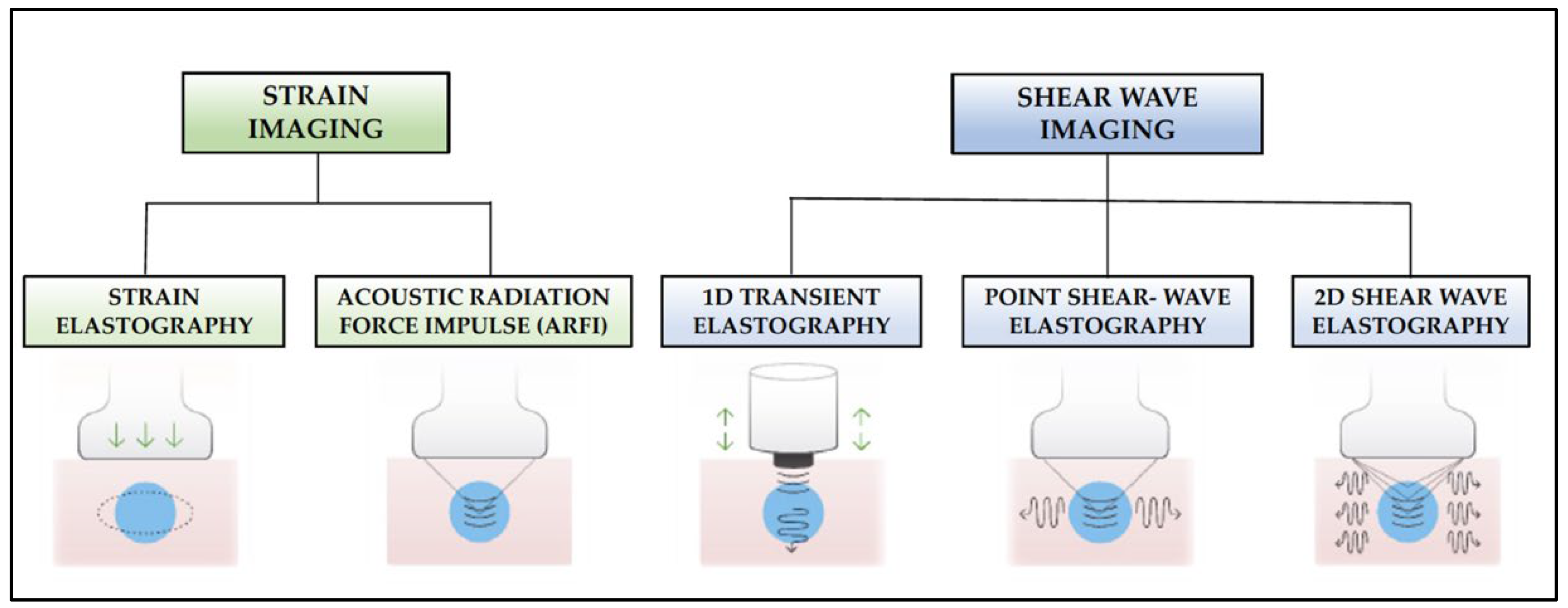
4.1. Fundamentals and Modalities of Sono-Elastography
- Strain elastography: Depending on the type of stimulation, there are different strain elastography modalities. There are modes that require gentle compression with the transducer by the examiner, and other modes where pressure is generated by physiological movements of the patient, such as breathing. Whatever the mode of stimulation, there is a displacement of the tissue in the same direction as the pulse. This displacement (strain measurement) is recorded by the device as an indirect measure of tissue elasticity and plotted on a color map called an elastogram.
- Acoustic Radiation Force Impulse (ARFI): Focused acoustic radiation pulses of short duration (0.1–0.5 ms) achieve tissue displacement in the same direction as the impulse, i.e., perpendicular to the skin surface. As a result, the generated waves are captured and displayed on a greyscale elasticity map, where the brightest areas correspond to those with the highest elasticity (soft tissues) and the darkest areas to those with the lowest elasticity (hard tissues). Thus, it does not require manual external compression and it provides a one-dimensional measure of tissue elasticity on a measurement area that can be positioned in a B-mode image plane.
- 1D-Transient elastography (FibroscanTM): This is based on the generation of an external vibration (50 Hz) that is transmitted from the body surface to the target tissue, where compression is produced. The speed of transmission of the resulting shear waves, which is proportional to the stiffness of the tissue, is then recorded (expressed in kPa). It is used mostly for the assessment of liver fibrosis in chronic liver disease (assessment of a tissue volume of 1 cm wide × 4 cm long). The advantages of this method is that it is fast and that it can be repeated throughout the patient’s follow-up. The main disadvantage is that, unlike other SE modalities, the measurement is not accompanied by a B-mode ultrasound image.
- Point Shear Wave Elastography (pSWE): A pulse of acoustic radiation causes tissue displacement, in the normal direction and at a particular tissue location. However, the tissue displacement itself is not measured in this case. Instead, a portion of the longitudinal waves generated by the ARFI are converted into shear waves by the absorption of acoustic energy within the tissue. The shear wave velocity perpendicular to excitation plane is measured and used as a quantitative estimation of tissue elasticity. The higher the stiffness of the tissue, the higher the velocity of the resulting shear waves. In this modality, only a quantitative result is provided as no elasticity map is generated.
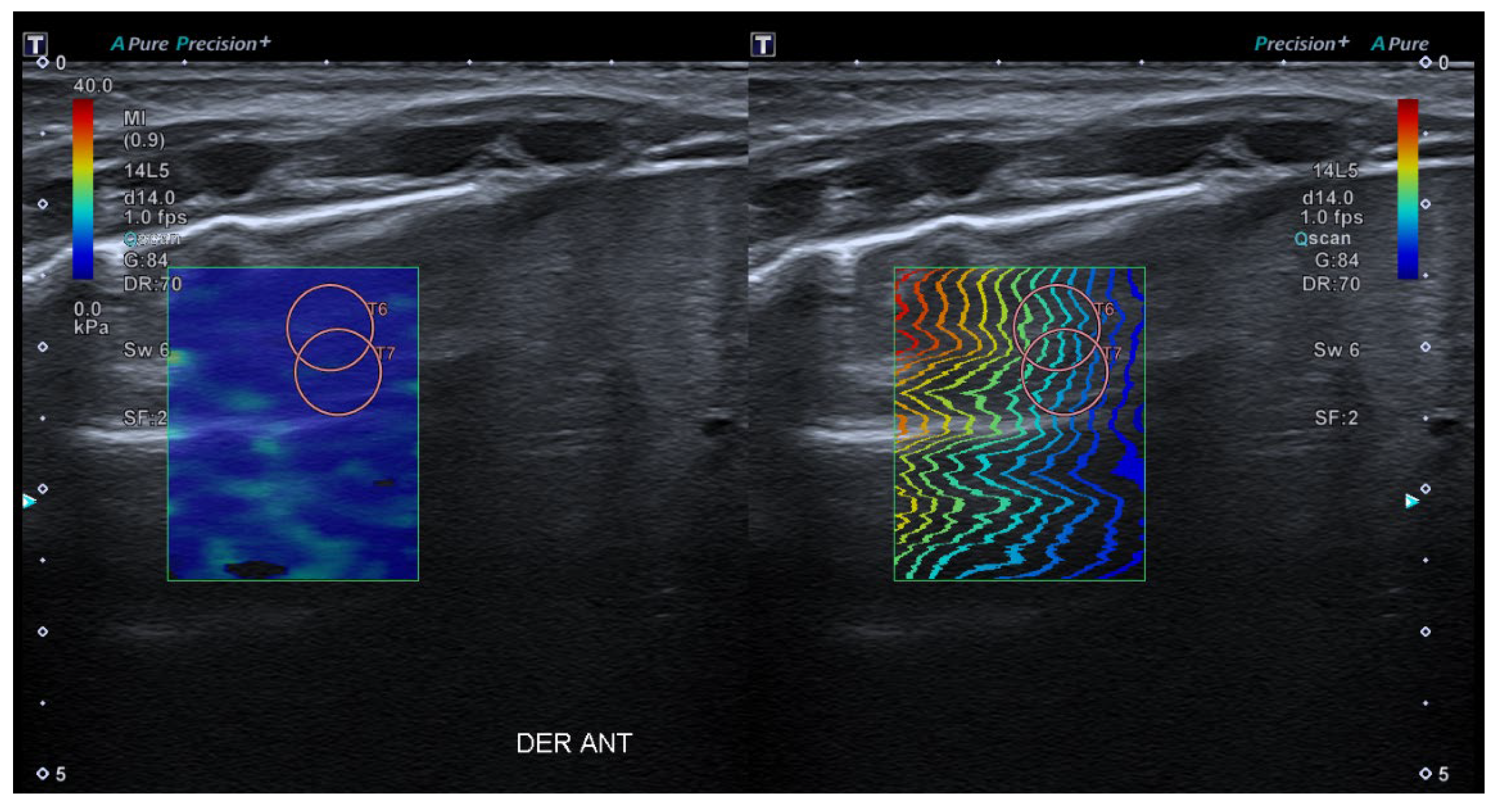
- 2D-Shear Wave Elastography (SWE): This is the latest and newest shear wave imaging technique. Like ARFI or pSWE, it uses acoustic radiation stimulation but, in this case, it rapidly scans multiple focal areas. This creates a virtual cylindrical shear wave cone that allows for the real-time monitoring of shear waves in 2D to measure their velocity, which is displayed on a quantitative colour map superimposed on a B-mode image (Figure 5). SWE has been extensively applied to characterize liver fibrosis [30,31], breast masses [32,33], prostate cancer lesions [34], thyroid nodules [35] and cervical lymph nodes [36]. In these contexts, SWE displayed low variability with respect to SE [37].
4.2. What Role Does SE Play in the Study of Pulmonary Conditions?
5. Lung SE Image Acquisition Protocol Proposal
6. Are There Other Elastography-Based Research Opportunities for Diagnosing Lung Processes?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committing to Child Survival: A Promise Renewed; Progress Report 2015; UNICEF: New York, NY, USA, 2015; ISBN 978-92-806-4815-7.
- Demi, L.; Egan, T.; Muller, M. Lung Ultrasound Imaging, a Technical Review. Appl. Sci. 2020, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.W.J.C.; Wong, J.J.-M.; Sultana, R.; Wong, P.P.C.; Mok, Y.H.; Lee, J.H. Risk Factors for Mortality in Children with Pneumonia Admitted to the Pediatric Intensive Care Unit: Risk Factors for Mortality in Pediatric Pneumonia. Pediatr. Pulmonol. 2017, 52, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H.; Moore, M.R.; et al. The Management of Community-Acquired Pneumonia in Infants and Children Older than 3 Months of Age: Clinical Practice Guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, e25–e76. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.N.; Bachur, R.G.; Simel, D.L.; Neuman, M.I. Does This Child Have Pneumonia? The Rational Clinical Examination Systematic Review. JAMA 2017, 318, 462. [Google Scholar] [CrossRef]
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; McKean, M.; Thomson, A.; On behalf of the British Thoracic Society Standards of Care Committee. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Children: Update 2011. Thorax 2011, 66, ii1–ii23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marangu, D.; Zar, H.J. Childhood Pneumonia in Low-and-Middle-Income Countries: An Update. Paediatr. Respir. Rev. 2019, 32, 3–9. [Google Scholar] [CrossRef]
- Berce, V.; Tomazin, M.; Gorenjak, M.; Berce, T.; Lovrenčič, B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci. Rep. 2019, 9, 17957. [Google Scholar] [CrossRef] [Green Version]
- Huijskens, E.G.W.; Koopmans, M.; Palmen, F.M.H.; Van Erkel, A.J.M.; Mulder, P.G.H.; Rossen, J.W.A. The Value of Signs and Symptoms in Differentiating between Bacterial, Viral and Mixed Aetiology in Patients with Community-Acquired Pneumonia. J. Med. Microbiol. 2014, 63, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Isaacs, D. Problems in Determining the Etiology of Community-Acquired Childhood Pneumonia. Pediatr. Infect. Dis. J. 1989, 8, 143–148. [Google Scholar]
- Alejandre, C.; Balaguer, M.; Guitart, C.; Torrús, I.; Felipe, A.; Launes, C.; Cambra, F.J.; Jordan, I. Procalcitonin-guided Protocol Decreased the Antibiotic Use in Paediatric Patients with Severe Bronchiolitis. Acta Paediatr. 2020, 109, 1190–1195. [Google Scholar] [CrossRef]
- Rodríguez-Fanjul, J.; Guitart, C.; Bobillo-Perez, S.; Balaguer, M.; Jordan, I. Procalcitonin and Lung Ultrasound Algorithm to Diagnose Severe Pneumonia in Critical Paediatric Patients (PROLUSP Study). A Randomised Clinical Trial. Respir. Res. 2020, 21, 255. [Google Scholar] [CrossRef]
- Jones, B.P.; Tay, E.T.; Elikashvili, I.; Sanders, J.E.; Paul, A.Z.; Nelson, B.P.; Spina, L.A.; Tsung, J.W. Feasibility and Safety of Substituting Lung Ultrasonography for Chest Radiography When Diagnosing Pneumonia in Children. Chest 2016, 150, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Conlon, T.W.; Nishisaki, A.; Singh, Y.; Bhombal, S.; De Luca, D.; Kessler, D.O.; Su, E.R.; Chen, A.E.; Fraga, M.V. Moving beyond the Stethoscope: Diagnostic Point-of-Care Ultrasound in Pediatric Practice. Pediatrics 2019, 144, e20191402. [Google Scholar] [CrossRef]
- Guzmán Aroca, F.; Abellán Rivera, D.; Reus Pintado, M. La elastografía: Una nueva aplicación de la ecografía. ¿Cuál es su utilidad clínica? Radiología 2014, 56, 290–294. [Google Scholar] [CrossRef]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; Chiaretti, A.; Biasucci, D.G.; Spanu, T.; et al. Role of lung ultrasound for the etiological diagnosis of community-acquired pneumonia in children: A prospective study. medRXiv 2020, 25, 185–197. [Google Scholar] [CrossRef]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; De Sanctis, R.; Chiaretti, A.; Biasucci, D.G.; et al. Role of Lung Ultrasound for the Etiological Diagnosis of Acute Lower Respiratory Tract Infection (ALRTI) in Children: A Prospective Study. J. Ultrasound 2022, 25, 185–197. [Google Scholar] [CrossRef]
- Guitart, C.; Esteban, E.; Becerra, J.; Rodríguez-Fanjul, J.; Cambra, F.J.; Balaguer, M.; Jordan, I. A Training Plan to Implement Lung Ultrasound for Diagnosing Pneumonia in Children. Pediatr. Res. 2022, 92, 1115–1121. [Google Scholar] [CrossRef]
- Shah, V.P.; Tunik, M.G.; Tsung, J.W. Prospective Evaluation of Point-of-Care Ultrasonography for the Diagnosis of Pneumonia in Children and Young Adults. JAMA Pediatr. 2013, 167, 119. [Google Scholar] [CrossRef] [Green Version]
- Copetti, R.; Cattarossi, L. Ultrasound Diagnosis of Pneumonia in Children. Radiol. Med. 2008, 113, 190–198. [Google Scholar] [CrossRef]
- Gullett, J.; Donnelly, J.P.; Sinert, R.; Hosek, B.; Fuller, D.; Hill, H.; Feldman, I.; Galetto, G.; Auster, M.; Hoffmann, B. Interobserver Agreement in the Evaluation of B-Lines Using Bedside Ultrasound. J. Crit. Care 2015, 30, 1395–1399. [Google Scholar] [CrossRef]
- Musolino, A.M.; Tomà, P.; Supino, M.C.; Scialanga, B.; Mesturino, A.; Scateni, S.; Battaglia, M.; Pirozzi, N.; Bock, C.; Buonsenso, D. Lung Ultrasound Features of Children with Complicated and Noncomplicated Community Acquired Pneumonia: A Prospective Study. Pediatr. Pulmonol. 2019, 54, 1479–1486. [Google Scholar] [CrossRef]
- Varshney, T.; Mok, E.; Shapiro, A.J.; Li, P.; Dubrovsky, A.S. Point-of-Care Lung Ultrasound in Young Children with Respiratory Tract Infections and Wheeze. Emerg. Med. J. 2016, 33, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Franquet, T. Imaging of Pulmonary Viral Pneumonia. Radiology 2011, 260, 18–39. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.L.; Nightingale, K.R. What Challenges Must Be Overcome before Ultrasound Elasticity Imaging Is Ready for the Clinic? Imaging Med. 2011, 3, 433–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Lim, C.-K.; Chung, C.-L.; Lin, Y.-T.; Chang, C.-H.; Lai, Y.-C.; Wang, H.-C.; Yu, C.-J. Transthoracic Ultrasound Elastography in Pulmonary Lesions and Diseases. Ultrasound Med. Biol. 2017, 43, 145–152. [Google Scholar] [CrossRef]
- Dietrich, C.; Barr, R.; Farrokh, A.; Dighe, M.; Hocke, M.; Jenssen, C.; Dong, Y.; Saftoiu, A.; Havre, R. Strain Elastography—How to Do It? Ultrasound Int. Open 2017, 3, E137–E149. [Google Scholar] [CrossRef] [Green Version]
- Frulio, N.; Trillaud, H. Ultrasound Elastography in Liver. Diagn. Interv. Imaging 2013, 94, 515–534. [Google Scholar] [CrossRef] [Green Version]
- Quarato, C.M.I.; Venuti, M.; Dimitri, L.; Lacedonia, D.; Simeone, A.; Mirijello, A.; De Cosmo, S.; Maiello, E.; Taurchini, M.; Scioscia, G.; et al. Transthoracic Ultrasound Shear Wave Elastography for the Study of Subpleural Lung Lesions. Ultrasonography 2022, 41, 93–105. [Google Scholar] [CrossRef]
- Trout, A.T.; Anupindi, S.A.; Gee, M.S.; Khanna, G.; Xanthakos, S.A.; Serai, S.D.; Baikpour, M.; Calle-Toro, J.S.; Ozturk, A.; Zhang, B.; et al. Normal Liver Stiffness Measured with MR Elastography in Children. Radiology 2020, 297, 663–669. [Google Scholar] [CrossRef]
- Barr, J.; Fraser, G.L.; Puntillo, K.; Ely, E.W.; Gélinas, C.; Dasta, J.F.; Davidson, J.E.; Devlin, J.W.; Kress, J.P.; Joffe, A.M.; et al. Clinical Practice Guidelines for the Management of Pain, Agitation, and Delirium in Adult Patients in the Intensive Care Unit: Crit. Care Med. 2013, 41, 263–306. [Google Scholar] [CrossRef]
- Ricci, P.; Maggini, E.; Mancuso, E.; Lodise, P.; Cantisani, V.; Catalano, C. Clinical Application of Breast Elastography: State of the Art. Eur. J. Radiol. 2014, 83, 429–437. [Google Scholar] [CrossRef]
- Correas, J.-M.; Tissier, A.-M.; Khairoune, A.; Vassiliu, V.; Méjean, A.; Hélénon, O.; Memo, R.; Barr, R.G. Prostate Cancer: Diagnostic Performance of Real-Time Shear-Wave Elastography. Radiology 2015, 275, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Asteria, C.; Giovanardi, A.; Pizzocaro, A.; Cozzaglio, L.; Morabito, A.; Somalvico, F.; Zoppo, A. US-Elastography in the Differential Diagnosis of Benign and Malignant Thyroid Nodules. Thyroid 2008, 18, 523–531. [Google Scholar] [CrossRef]
- Dudea, S.M. Differentiating Benign from Malignant Superficial Lymph Nodes with Sonoelastography. Med. Ultrason. 2013, 15, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Pareda, M.A.; Chavez, M.A.; Hooper-Miele, C.C.; Gilman, R.H.; Steinhoff, M.C.; Ellington, L.E.; Gross, M.; Price, C.; Tielsch, J.M.; Checkley, W. Lung Ultrasound for the Diagnosis of Pneumonia in Children: A Meta-Analysis. Pediatrics 2015, 135, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Clay, R.; Bartholmai, B.J.; Zhou, B.; Karwoski, R.; Peikert, T.; Osborn, T.; Rajagopalan, S.; Kalra, S.; Zhang, X. Assessment of Interstitial Lung Disease Using Lung Ultrasound Surface Wave Elastography: A Novel Technique With Clinicoradiologic Correlates. J. Thorac. Imaging 2019, 34, 313–319. [Google Scholar] [CrossRef]
- Zhou, B.; Bartholmai, B.J.; Kalra, S.; Osborn, T.G.; Zhang, X. Lung US Surface Wave Elastography in Interstitial Lung Disease Staging. Radiology 2019, 291, 479–484. [Google Scholar] [CrossRef]
- Kuo, Y.-W.; Chen, Y.-L.; Wu, H.-D.; Chien, Y.-C.; Huang, C.-K.; Wang, H.-C. Application of Transthoracic Shear-Wave Ultrasound Elastography in Lung Lesions. Eur. Respir. J. 2021, 57, 2002347. [Google Scholar] [CrossRef]
- Ozgokce, M.; Yavuz, A.; Akbudak, I.; Durmaz, F.; Uney, I.; Aydin, Y.; Yildiz, H.; Batur, A.; Arslan, H.; Dundar, I. Usability of Transthoracic Shear Wave Elastography in Differentiation of Subpleural Solid Masses. Ultrasound Q. 2018, 34, 233–237. [Google Scholar] [CrossRef]
- Wei, H.; Lu, Y.; Ji, Q.; Zhou, H.; Zhou, X. The Application of Conventional Us and Transthoracic Ultrasound Elastography in Evaluating Peripheral Pulmonary Lesions. Exp. Ther. Med. 2018, 16, 1203–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koda, R.; Taniguchi, H.; Konno, K.; Yoshiki, Y. B-Line Elastography Measurement of Lung Parenchymal Elasticity. Ultrason. Imaging 2023, 45, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Wan, Y.-D.; Sun, T.-W.; Kan, Q.-C.; Wang, L.-X. Association between Vitamin D Deficiency and Mortality in Critically Ill Adult Patients: A Meta-Analysis of Cohort Studies. Crit. Care 2014, 18, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Osborn, T.; Zhou, B.; Meixner, D.; Kinnick, R.R.; Bartholmai, B.; Greenleaf, J.F.; Kalra, S. Lung Ultrasound Surface Wave Elastography: A Pilot Clinical Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1298–1304. [Google Scholar] [CrossRef]
- Fakhouri, F.; Matthew, J.; Ballinger, M.; Shukla, V.; Weimar, D.; Novak, C.; Ghadali, S.; Kolipaka, A. Magnetic Resonance Elastography (MRE) of Bleomycin-Induced Pulmonary Fibrosis in an Animal Model. Investig. Radiol. 2023, 58, 299–306. [Google Scholar] [CrossRef]



| Chest X-ray | Lung Ultrasound | ||
|---|---|---|---|
| Irradiation | Yes | No | |
| Bedside | No | Yes | |
| Reproducibility | Not operator-dependent | Operator-dependent | |
| Exploration areas | Central and peripheral, with possibility of visualising the perihilar region | Peripheral, without possibility to visualise perihilar region | |
| Characteristic imaging findings |
| Bacterial pneumonia | Viral pneumonia |
|
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta-Calpe, S.; Salas, B.; Inarejos Clemente, E.J.; Guitart, C.; Balaguer, M.; Jordan, I. Sono-Elastography: An Ultrasound Quantitative Non-Invasive Measurement to Guide Bacterial Pneumonia Diagnosis in Children. Children 2023, 10, 1335. https://doi.org/10.3390/children10081335
Huerta-Calpe S, Salas B, Inarejos Clemente EJ, Guitart C, Balaguer M, Jordan I. Sono-Elastography: An Ultrasound Quantitative Non-Invasive Measurement to Guide Bacterial Pneumonia Diagnosis in Children. Children. 2023; 10(8):1335. https://doi.org/10.3390/children10081335
Chicago/Turabian StyleHuerta-Calpe, Sergi, Bárbara Salas, Emilio J. Inarejos Clemente, Carmina Guitart, Mònica Balaguer, and Iolanda Jordan. 2023. "Sono-Elastography: An Ultrasound Quantitative Non-Invasive Measurement to Guide Bacterial Pneumonia Diagnosis in Children" Children 10, no. 8: 1335. https://doi.org/10.3390/children10081335






