An Unusual Case of Neonatal Hypotonia and Femur Fracture: Neuromuscular Variant of Glycogen Storage Disease Type IV
Abstract
1. Introduction
2. Case
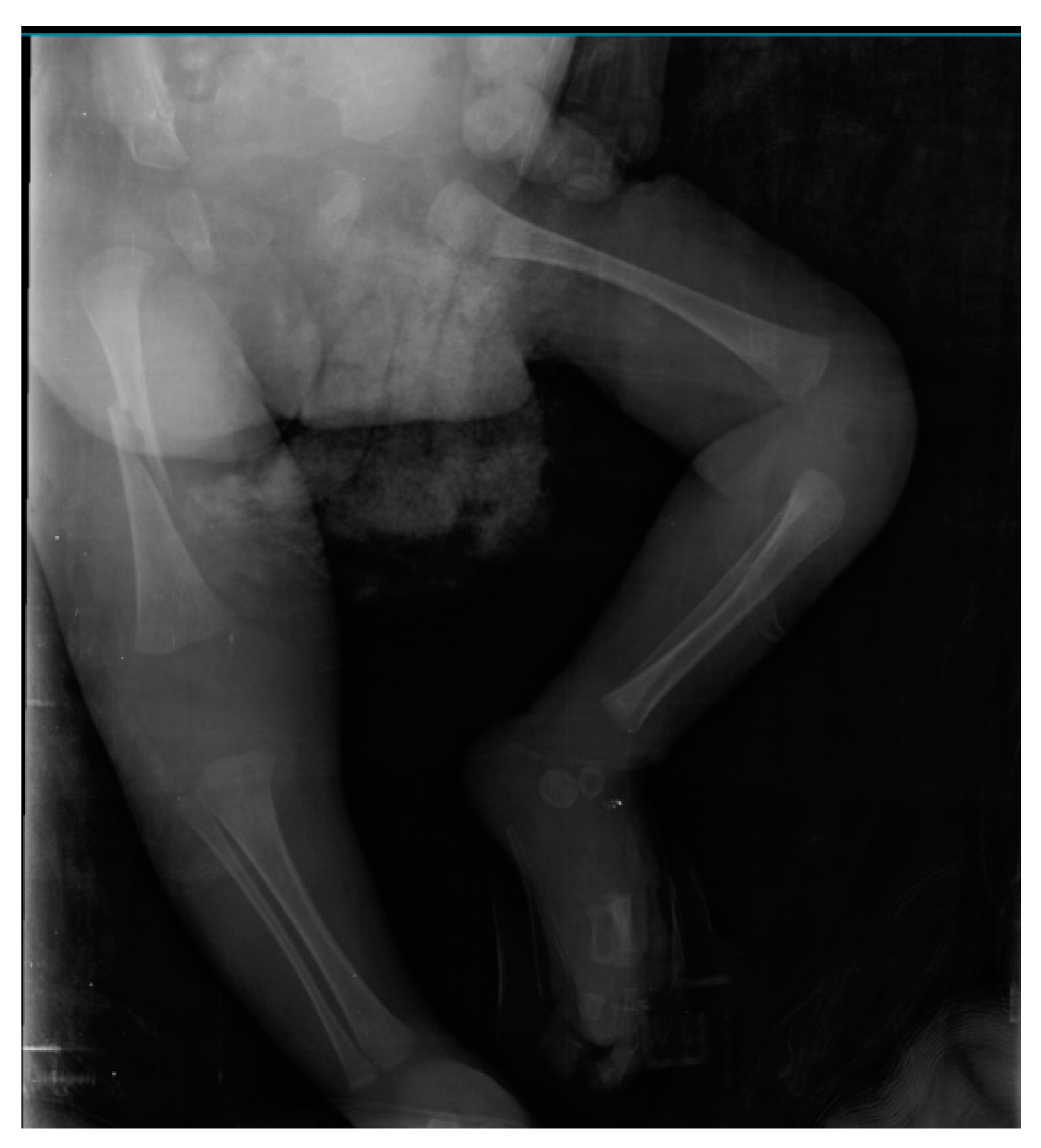
2.1. Initial Presentation
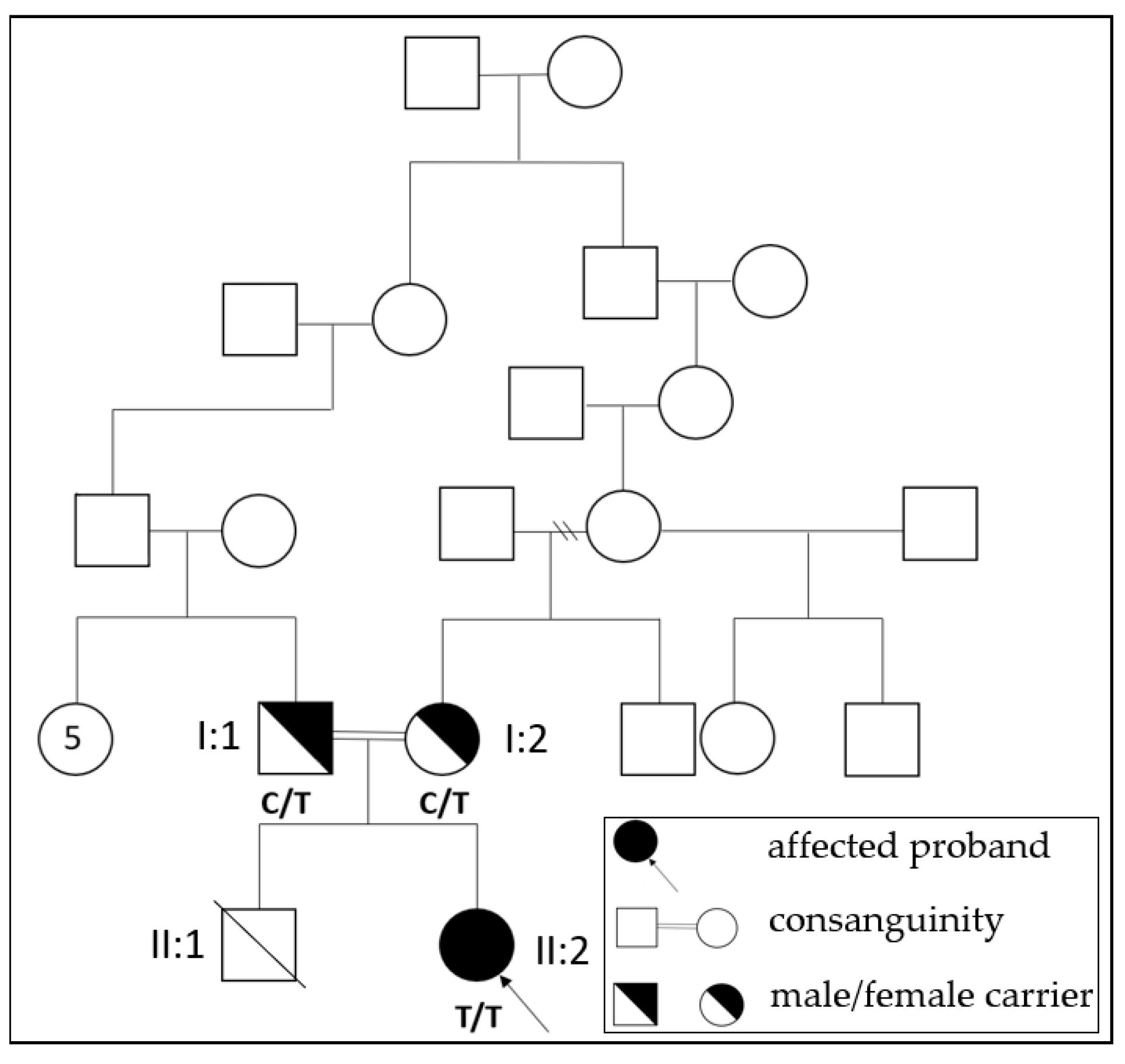
2.2. Family History
2.3. Investigation
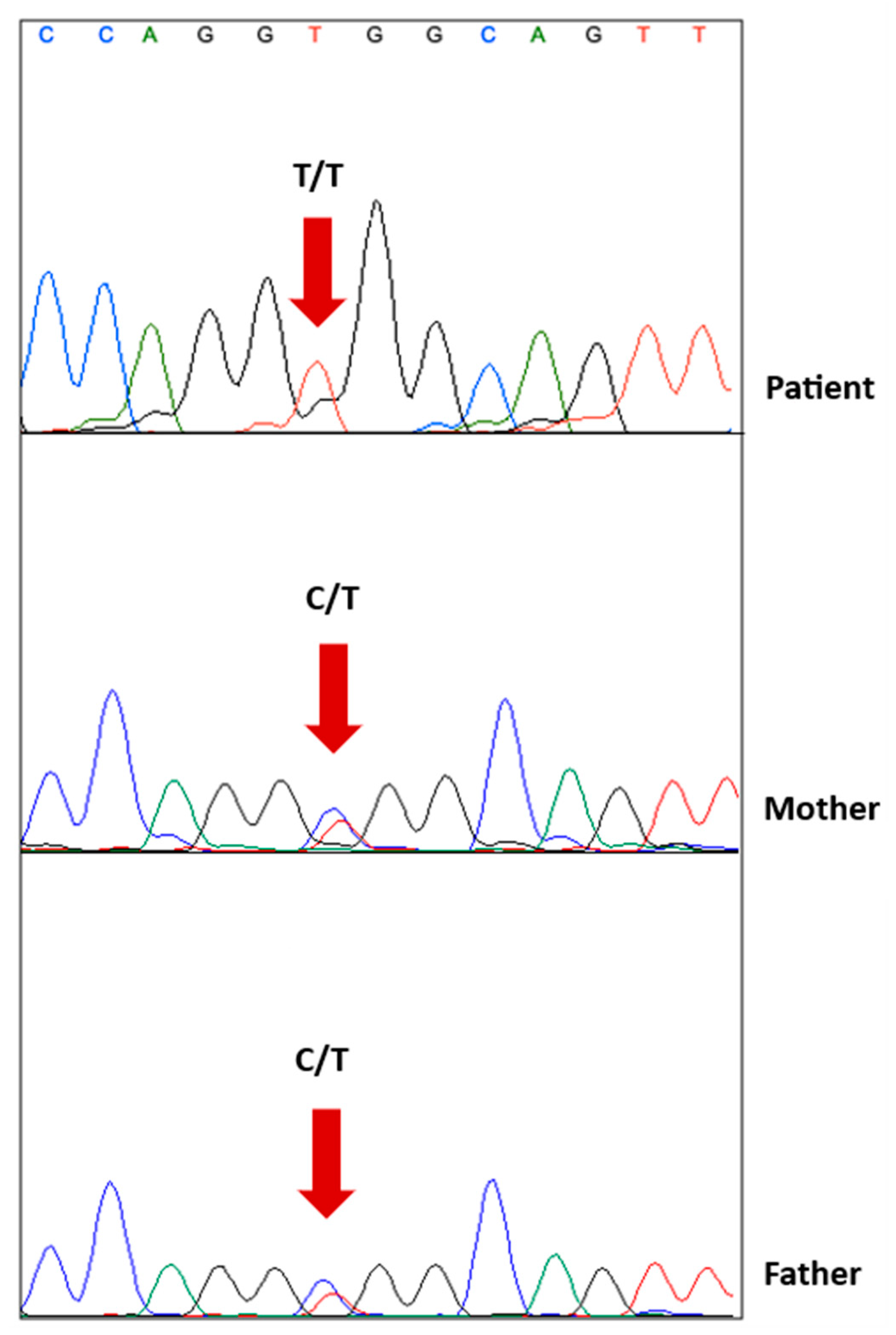
2.4. Genetic Testing
2.5. Genetic Analysis and Functional Interpretation
2.6. Follow-Up and Outcome
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ozen, H. Glycogen storage diseases: New perspectives. World J. Gastroenterol. 2007, 13, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Segura, A.D.; Chen, Y.T.; Ricci, L.M.; Franciosi, R.A.; Splaingard, M.L.; Lubinsky, M.S. Neonatal hyopotonia and cardiomyopathy secondary to type IV glycogenosis. Acta Neuropathol. 1994, 87, 531–536. [Google Scholar] [CrossRef]
- Moses, S.W.; Parvari, S.W.M.A.R. The Variable Presentations of Glycogen Storage Disease Type IV: A Review of Clinical, Enzymatic and Molecular Studies. Curr. Mol. Med. 2002, 2, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Kiely, B.T.; Koch, R.L.; Flores, L.; Burner, D.; Kaplan, S.; Kishnani, P.S. A novel approach to characterize phenotypic variation in GSD IV: Reconceptualizing the clinical continuum. Front. Genet. 2022, 13, 992406. [Google Scholar] [CrossRef] [PubMed]
- Escobar, L.F.; Wagner, S.; Tucker, M.; Wareham, J. Neonatal presentation of lethal neuromuscular glycogen storage disease type IV. J. Perinatol. 2012, 32, 810–813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taratuto, A.; Akman, H.; Saccoliti, M.; Riudavets, M.; Arakaki, N.; Mesa, L.; Sevlever, G.; Goebel, H.; DiMauro, S. Branching enzyme deficiency/glycogenosis storage disease type IV presenting as a severe congenital hypotonia: Muscle biopsy and autopsy findings, biochemical and molecular genetic studies. Neuromuscul. Disord. 2010, 20, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, G.; Thompson, E.M.; Todd, E.J.; Yau, K.S.; Kresoje, N.; Sivadorai, P.; Friend, K.; Riley, K.; Manton, N.D.; Blumbergs, P.; et al. Whole exome sequencing in foetal akinesia expands the genotype-phenotype spectrum of GBE1 glycogen storage disease mutations. Neuromuscul. Disord. 2013, 23, 165–169. [Google Scholar] [CrossRef]
- Pergande, M.; Motameny, S.; Özdemir, Ö.; Kreutzer, M.; Wang, H.; Msc, H.-S.D.; Becker, K.; Karakaya, M.; Ehrhardt, H.; Elcioglu, N.; et al. The genomic and clinical landscape of fetal akinesia. Genet. Med. 2020, 22, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Sergi, C. Biochemistry, Amino Acid Synthesis and Degradation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK559250/ (accessed on 11 January 2023).
- Venselaar, H.; Beek, T.A.T.; Kuipers, R.K.; Hekkelman, M.L.; Vriend, G. Protein structure analysis of mutations causing inheritable diseases. An e-Science approach with life scientist friendly interfaces. BMC Bioinform. 2010, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Froese, D.S.; Michaeli, A.; McCorvie, T.J.; Krojer, T.; Sasi, M.; Melaev, E.; Goldblum, A.; Zatsepin, M.; Lossos, A.; Álvarez, R.; et al. Structural basis of glycogen branching enzyme deficiency and pharmacologic rescue by rational peptide design. Hum. Mol. Genet. 2015, 24, 5667–5676. [Google Scholar] [CrossRef] [PubMed]
- Magoulas, P.L.; El-Hattab, A.W.; Roy, A.; Bali, D.S.; Finegold, M.J.; Craigen, W.J. Diffuse reticuloendothelial system involvement in type IV glycogen storage disease with a novel GBE1 mutation: A case report and review. Hum. Pathol. 2012, 43, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Iwano, R.; Tanaka, Y.; Muroya, K.; Fukuda, T.; Sugie, H.; Kurosawa, K.; Adachi, M. Analysis of GBE1 mutations via protein expression studies in glycogen storage disease type IV: A report on a non-progressive form with a literature review. Mol. Genet. Metab. Rep. 2018, 17, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, P.; Moirangthem, A.; Nayak, S.S.; Shukla, A.; Mathew, M.; Girisha, K.M. Novel pathogenic variants in GBE1 causing fetal akinesia deformation sequence and severe neuromuscular form of glycogen storage disease type IV. Clin. Dysmorphol. 2019, 28, 17–21. [Google Scholar] [CrossRef]
- Li, S.-C.; Chen, C.-M.; Goldstein, J.L.; Wu, J.-Y.; Lemyre, E.; Burrow, T.A.; Kang, P.B.; Chen, Y.-T.; Bali, D.S. Glycogen storage disease type IV: Novel mutations and molecular characterization of a heterogeneous disorder. J. Inherit. Metab. Dis. 2010, 33 (Suppl. 3), S83–S90. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Cassandrini, D.; Assereto, S.; Akman, H.O.; Minetti, C.; Di Mauro, S. Neuromuscular forms of glycogen branching enzyme deficiency. Acta Myol. 2007, 26, 75–78. [Google Scholar]
- Bruno, C.; van Diggelen, O.P.; Cassandrini, D.; Gimpelev, M.; Giuffrè, B.; Donati, M.A.; Introvini, P.; Alegria, A.; Assereto, S.; Morandi, L.; et al. Clinical and genetic heterogeneity of branching enzyme deficiency (glycogenosis type IV). Neurology 2004, 63, 1053–1058. [Google Scholar] [CrossRef]
- Koch, R.L.; Soler-Alfonso, C.; Kiely, B.T.; Asai, A.; Smith, A.L.; Bali, D.S.; Kang, P.B.; Landstrom, A.P.; Akman, H.O.; Burrow, T.A.; et al. Diagnosis and management of glycogen storage disease type IV, including adult polyglucosan body disease: A clinical practice resource. Mol. Genet. Metab. 2023, 138, 107525. [Google Scholar] [CrossRef] [PubMed]
- Van Toorn, R.; Davies, J.; Wilmshurst, J.M. Spinal muscular atrophy with congenital fractures: Postmortem analysis. J. Child. Neurol. 2002, 17, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Lacson, A.G.; Donaldson, G.; Barness, E.G.; Ranells, J.D.; Pomerance, H.H. Infant with high arched palate, bell-shaped chest, joint contractures, and intrauterine fractures. Pediatr. Pathol. Mol. Med. 2002, 21, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Abbott, M.; Jain, M.; Pferdehirt, R.; Chen, Y.; Tran, A.; Duz, M.B.; Seven, M.; Gibbs, R.A.; Muzny, D.; Lee, B.; et al. Neonatal fractures as a presenting feature of LMOD3-associated congenital myopathy. Am. J. Med. Genet. A 2017, 173, 2789–2794. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bezirganoglu, H.; Adanur Saglam, K. An Unusual Case of Neonatal Hypotonia and Femur Fracture: Neuromuscular Variant of Glycogen Storage Disease Type IV. Children 2023, 10, 1375. https://doi.org/10.3390/children10081375
Bezirganoglu H, Adanur Saglam K. An Unusual Case of Neonatal Hypotonia and Femur Fracture: Neuromuscular Variant of Glycogen Storage Disease Type IV. Children. 2023; 10(8):1375. https://doi.org/10.3390/children10081375
Chicago/Turabian StyleBezirganoglu, Handan, and Kubra Adanur Saglam. 2023. "An Unusual Case of Neonatal Hypotonia and Femur Fracture: Neuromuscular Variant of Glycogen Storage Disease Type IV" Children 10, no. 8: 1375. https://doi.org/10.3390/children10081375
APA StyleBezirganoglu, H., & Adanur Saglam, K. (2023). An Unusual Case of Neonatal Hypotonia and Femur Fracture: Neuromuscular Variant of Glycogen Storage Disease Type IV. Children, 10(8), 1375. https://doi.org/10.3390/children10081375




