Vitamin D Deficiency and Maternal Diseases as Risk Factors for the Development of Macrosomia in Newborns
Abstract
1. Introduction
2. Materials and Methods of Research
2.1. Characteristics of the Object of Study
2.2. Ethical Approval Details
2.3. Laboratory Tests
Determination of the Level of Vitamin D in Cord Blood
2.4. Statistical Analysis
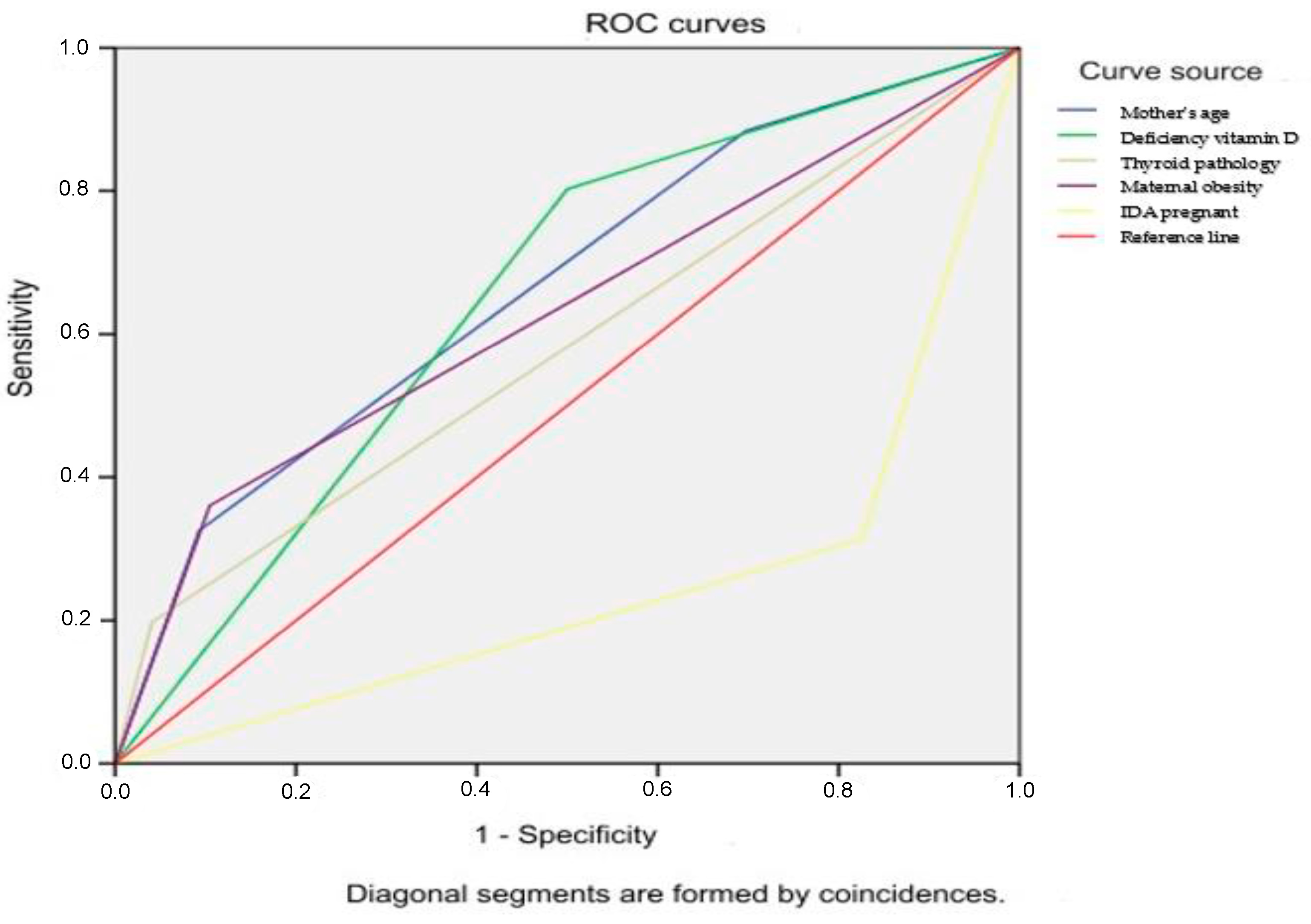
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yadav, H.; Lee, N. Factors influencing macrosomia in pregnant women in a tertiary care hospital in Malaysia. J. Obstet. Gynaecol. Res. 2014, 40, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Mohammadbeigi, A.; Tabatabaee, S.H.; Mohammadsalehi, N.; Yazdani, M.A. Gestational diabetes and its association with un-pleasant outcomes of pregnancy. Pak. J. Med. Sci. 2008, 4, 566–570. [Google Scholar]
- Yang, S.; Zhou, A.; Xiong, C.; Yang, R.; Bassig, B.A.; Hu, R.; Zhang, Y.; Yao, C.; Zhang, Y.; Qiu, L.; et al. Parental Body Mass Index, Gestational Weight Gain, and Risk of Macrosomia: A Population-Based Case–Control Study in China. Paediatr. Périnat. Epidemiol. 2015, 29, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Black, M.H.; Sacks, D.A.; Xiang, A.H.; Lawrence, J.M. The relative contribution of prepregnancy overweight and obesity, gestational weight gain, and IADPSG-defined gestational diabetes mellitus to fetal overgrowth. Diabetes Care 2013, 36, 56–62. [Google Scholar] [CrossRef]
- Zonana-Nacach, A.; Baldenebro-Preciado, R.; Ruiz-Dorado, M.A. The effect of gestational weight gain on maternal and neonatal outcomes. Salud Publica Mex. 2010, 52, 220–225. [Google Scholar]
- Levy, A.; Wiznitzer, A.; Holcberg, G.; Mazor, M.; Sheiner, E. Family history of diabetes mellitus as an independent risk factor for macrosomia and cesarean delivery. J. Matern. Neonatal Med. 2010, 23, 148–152. [Google Scholar] [CrossRef]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Ho, A.; Flynn, A.C.; Pasupathy, D. Nutrition in pregnancy. Obstet. Gynaecol. Reprod. Med. 2020, 26, 259–264. [Google Scholar] [CrossRef]
- Urrutia-Pereira, M.; Solé, D. Vitamin D deficiency in pregnancy and its impact on the fetus, the newborn and in childhood. Rev. Paul. Pediatrician. 2015, 33, 104–113. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharm. 2012, 3, 118–126. [Google Scholar]
- Palacios, C.; De-Regil, L.M.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation during pregnancy: Updated meta-analysis on maternal outcomes. J. Steroid Biochem. Mol. Biol. 2016, 164, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Thorne-Lyman, A.; Fawzi, W.W. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: A systematic review and meta-analysis. Paediatr. Perinat Epidemiol. 2012, 26, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Khazai, N.; Judd, S.E.; Tangpricha, V. Calcium and vitamin D: Skeletal and extraskeletal health. Curr. Rheumatol. Rep. 2008, 10, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, M.L.; Felton, S.K.; Riek, A.E.; Bernal-Mizrachi, C. Implications of vitamin D deficiency in pregnancy and lactation. Am. J. Obstet. Gynecol. 2010, 202, 429.e1–429.e9. [Google Scholar] [CrossRef]
- Karras, S.N.; Fakhoury, H.; Muscogiuri, G.; Grant, W.B.; Ouweland, J.M.V.D.; Colao, A.M.; Kotsa, K. Maternal vitamin D levels during pregnancy and neonatal health: Evidence to date and clinical implications. Ther. Adv. Musculoskelet. Dis. 2016, 8, 124–135. [Google Scholar] [CrossRef]
- Hollis, B.; Wagner, C. New insights into the vitamin D requirements during pregnancy. Bone Res. 2017, 5, 17030. [Google Scholar] [CrossRef]
- Morales, E.; Rodriguez, A.; Valvi, D.; Iñiguez, C.; Esplugues, A.; Vioque, J.; Marina, L.S.; Jiménez, A.; Espada, M.; Dehli, C.R.; et al. Deficit of vitamin D in pregnancy and growth and overweight in the offspring. Int. J. Obes. 2015, 39, 61–68. [Google Scholar] [CrossRef]
- Fiamenghi, V.I.; de Mello, E.D. Vitamin D deficiency in children and adolescents with obesity: A meta- analysis. J. Pediatr. 2021, 97, 273–279. [Google Scholar] [CrossRef]
- Amberntsson, A.; Papadopoulou, E.; Winkvist, A.; Lissner, L.; Meltzer, H.M.; Brantsaeter, A.L.; Augustin, H. Maternal vitamin D intake and BMI during pregnancy in relation to child’s growth and weight status from birth to 8 years: A large national cohort study. BMJ Open 2021, 11, e048980. [Google Scholar] [CrossRef]
- Wen, J.; Kang, C.; Wang, J.; Cui, X.; Hong, Q.; Wang, X.; Zhu, L.; Xu, P.; Fu, Z.; You, L.; et al. Association of maternal serum 25-hydroxyvitamin D concentrations in second and third trimester with risk of macrosomia. Sci. Rep. 2018, 8, 6196. [Google Scholar] [CrossRef]
- Munteanu, C.; Schwartz, B. The relationship between nutrition and the immune system. Front. Nutr. 2022, 9, 1082500. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Hu, H.; Zhang, M.; Long, W.; Liu, J.; Jiang, J.; Yu, B. Iron deficiency in late pregnancy and its associations with birth outcomes in Chinese pregnant women: A retrospective cohort study. Nutr. Metab. 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Drukker, L.; Hants, Y.; Farkash, R.; Ruchlemer, R.; Samueloff, A.; Grisaru-Granovsky, S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion 2015, 55, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.calculator.net/sample-size-calculator.html (accessed on 24 February 2023).
- Available online: https://ibl-international.com/en/25-oh-vitamin-d-elisa (accessed on 24 February 2023).
- Trimboli, F.; Rotundo, S.; Armili, S.; Mimmi, S.; Lucia, F.; Montenegro, N.; Antico, G.C.; Cerra, A.; Gaetano, M.; Galato, F.; et al. Serum 25-hydroxyvitamin D measurement: Comparative evaluation of three automated immunoassays. Pract. Lab. Med. 2021, 26, e00251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Olofsson, P. Umbilical cord pH, blood gases, and lactate at birth: Normal values, interpretation, and clinical utility. Am. J. Obstet. Gynecol. 2023, 228, S1222–S1240. [Google Scholar] [CrossRef]
- Mai, X.-M.; Chen, Y.; Camargo, C.A., Jr.; Langhammer, A. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: The HUNT study. Am. J. Epidemiol. 2012, 175, 1029–1036. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A.; Kilbane, M.; McKenna, M.J.; McAuliffe, F.M. The relationship between maternal and fetal vitamin d, insulin resistance, and fetal growth. Reprod. Sci. 2013, 20, 536–541. [Google Scholar] [CrossRef]
- Ariyawatkul, K.; Lersbuasin, P. Prevalence of vitamin D deficiency in cord blood of newborns and the association with maternal vitamin D status. Eur. J. Pediatr. 2018, 177, 1541–1545. [Google Scholar] [CrossRef]
- Kassai, M.S.; Cafeo, F.R.; Affonso-Kaufman, F.A.; Suano-Souza, F.I.; Sarni, R.O.S. Vitamin D plasma concentrations in pregnant women and their preterm newborns. BMC Pregnancy Childbirth 2018, 18, 412. [Google Scholar] [CrossRef]
- Saraf, R.; Morton, S.M.B.; Camargo, C.A., Jr.; Grant, C.C. Global summary of maternal and newborn vitamin D status—A systematic review. Matern. Child. Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hong, Q.; Wang, X.; Zhu, L.; Wu, T.; Xu, P.; Fu, Z.; You, L.; Wang, X.; Ji, C.; et al. The effect of maternal vitamin D deficiency during pregnancy on body fat and adipogenesis in rat offspring. Sci. Rep. 2018, 8, 365. [Google Scholar] [CrossRef]
- Wang, J.; Thingholm, L.B.; Skiecevičienė, J.; Rausch, P.; Kummen, M.; Hov, J.R.; Degenhardt, F.; Heinsen, F.-A.; Rühlemann, M.C.; Szymczak, S.; et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat. Genet. 2016, 48, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Y.; Zeng, Y.; Zhang, Q.; Xiao, X. The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review. Nutrients 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; AP Steegers, E.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Fu, L.; Hao, J.-H.; Yu, Z.; Zhu, P.; Wang, H.; Xu, Y.-Y.; Zhang, C.; Tao, F.-B.; Xu, D.-X. Maternal Vitamin D Deficiency During Pregnancy Elevates the Risks of Small for Gestational Age and Low Birth Weight Infants in Chinese Population. J. Clin. Endocrinol. Metab. 2015, 100, 1912–1919. [Google Scholar] [CrossRef]
- Hacker, A.N.; Fung, E.B.; King, J.C. Role of calcium during pregnancy: Maternal and fetal needs. Nutr. Rev. 2012, 70, 397–409. [Google Scholar] [CrossRef]
- Lewandowska, M. The Role of Maternal Weight in the Hierarchy of Macrosomia Predictors; Overall Effect of Analysis of Three Prediction Indicators. Nutrients 2021, 13, 801. [Google Scholar] [CrossRef]
- Owens, L.A.; O’Sullivan, E.P.; Kirwan, B.; Avalos, G.; Gaffney, G.; Dunne, F.; Collaborators, F.T.A.D. ATLANTIC DIP: The impact of obesity on pregnancy outcome in glucose-tolerant women. Diabetes Care 2010, 33, 577–579. [Google Scholar] [CrossRef]
- Viswanathan, M.; Siega-Riz, A.M.; Moos, M.K.; Deierlein, A.; Mumford, S.; Knaack, J.; Thieda, P.; Lux, L.J.; Lohr, K.N. Outcomes of maternal weight gain. Evid. Rep. Technol. Assess. (Full Rep.) 2008, 168, 1–223. [Google Scholar] [PubMed] [PubMed Central]
- Mathew, M.; Machado, L.; Al-Ghabshi, R.; Al-Haddabi, R. Fetal macrosomia. Risk factor and outcome. Saudi Med. J. 2005, 26, 96–100. [Google Scholar] [PubMed]
- Addo, V.N. Body mass index, weight gain during pregnancy and obstetric outcomes. Ghana Med. J. 2010, 44, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Vrijkotte, T.G.; Hrudey, E.J.; Twickler, M.B. Early Maternal Thyroid Function During Gestation Is Associated With Fetal Growth, Particularly in Male Newborns. J. Clin. Endocrinol. Metab. 2017, 102, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Zhang, Z.; Ma, L.; Zhang, B.; Dang, S.; Yan, H. Association between maternal iron supplementation and newborn birth weight: A quantile regression analysis. Ital. J. Pediatr. 2021, 47, 133. [Google Scholar] [CrossRef]
- Georgieff, M.K. The importance of iron deficiency in pregnancy on fetal, neonatal, and infant neurodevelopmental outcomes. Int. J. Gynecol. Obstet. 2023, 162 (Suppl. 2), 83–88. [Google Scholar] [CrossRef]
| Rate | Main Group | Control | p |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Weight (grams) | 4361 (±301.7) | 3302 (±395.4) | 0.001 |
| Length (centimeters) | 54.4 (±2.44) | 52.9 (±2.41) | 0.001 |
| Head circumference (centimeters) | 36.5 (±1.10) | 35.2 (±1.59) | 0.001 |
| Chest circumference (centimeters) | 36.5 (±1.98) | 34.4 (±2.34) | 0.001 |
| Apgar at the end of 1 min (scores) | 7 (±1.49) | 8 (±0.99) | 0.001 |
| Apgar at the end of the fifth minute (scores) | 8 (±1.49) | 9 (±1.0) | 0.001 |
| Mother’s age (years) | 32.4 (±5.54) | 28.4 (±5.28) | 0.001 |
| Childbirth parity (number) | 4 (±1.53) | 2 (±1.06) | 0.001 |
| Gestational age (weeks) | 38.9 (±1.53) | 38.9 (±1.49) | 0.95 |
| Glucose level (mmol/L) | 3.2 (±1.16) | 3.6 (±0.80) | 0.001 |
| Hemoglobin (g/L) | 194 (±30.6) | 187 (±13.5) | 0.06 |
| Bilirubin (μmol/L) | 27.3 (±16.4) | 21.9 (±14.2) | 0.01 |
| Reticulocytes (%) | 10 (±4.40) | 9 (±3.65) | 0.114 |
| BMI at the time of pregnancy (m/h2) | 27.2 (±9.66) | 22.5 (±4.26) | 0.001 |
| BMI before childbirth (m/h2) | 31.1 (±9.71) | 25.5 (±4.86) | 0.001 |
| Pathology | Main Group | Control | p |
|---|---|---|---|
| Maternal obesity: | |||
| Overweight | 7 (8.1%) | 6 (3.5%) | <0.001 |
| Obesity 1st degree | 11 (12.8%) | 8 (4.7%) | <0.001 |
| Obesity 2 degrees | 9 (10.5%) | 4 (2.3%) | <0.001 |
| Obesity 3 degrees | 4 (4.7%) | 0 (0.0%) | <0.001 |
| Diabetes mellitus: | |||
| T1DM | 5 (5.8%) | 0 (0.0%) | <0.001 |
| T2DM | 5 (5.8%) | 1 (0.6%) | <0.001 |
| Gestational diabetes | 12 (14.0%) | 5 (2.9%) | <0.001 |
| Impaired glucose tolerance | 7 (8.1%) | 3 (1.7%) | <0.001 |
| Maternal hypothyroidism | 23 (26.7%) | 17 (9.9%) | <0.001 |
| Maternal IDA | 59 (68.6%) | 30 (17.4%) | <0.001 |
| Vitamin D Rate | Average Rate | 95% CI | Median | Min | Max | Statistical Significance |
|---|---|---|---|---|---|---|
| Main group n = 86 | 13.2 ± 6.7 | 11.7–14.6 | 11.05 | 1.3 | 35.1 | t = 5.759 p < 0.05 |
| Control group n = 172 | 21.3 ± 12.1 | 19.5–23.1 | 18.8 | 0.5 | 44.2 |
| Vitamin D Level | Main Group | Control | Statistical Significance |
|---|---|---|---|
| Severe deficiency | 35 (40.7%) | 10 (5.8%) | ꭓ2 = 71.788, df = 3, p < 0.001 |
| Deficiency | 31 (36.0%) | 72 (41.9%) | |
| Insufficiency | 15 (17.4%) | 14 (8.1%) | |
| Normal rate | 5 (5.8%) | 76 (44.2%) |
| Variable | Adjusted OR | (95% CI) | SEE | p-Value |
|---|---|---|---|---|
| Diabetes mellitus type 1 | 6.673 | 1.669–26.682 | 1.218 | 0.007 |
| Diabetes mellitus type 2 | 14.298 | 1.636–124.961 | 0.515 | 0.016 |
| Gestational diabetes | 6.863 | 2.316–20.328 | 0.655 | <0.001 |
| Maternal IDA | 10.343 | 5.665–18.886 | 0.307 | <0.001 |
| BMI gain during pregnancy: | ||||
| Overweight and obesity | 4.822 | 2.499–9.309 | 0.335 | <0.001 |
| Maternal hypothyroidism | 4.12 | 1.994–5.516 | 0.370 | <0.001 |
| Mothers’ age: | ||||
| 26–35 years old | 2.4 | 1.124–5.124 | 0.237 | 0.024 |
| Over 36 years old | 9.1 | 3.647–22.692 | 0.507 | <0.001 |
| Vitamin D deficiency in the cord blood | 4.059 | 2.207–7.463 | 0.311 | <0.001 |
| Variable | Adjusted OR | (95% CI) | p-Value |
|---|---|---|---|
| Maternal IDA | 0.086 | 0.041–0.181 | <0.001 |
| Mothers’ age: | |||
| 26–35 years old | 3.631 | 1.467–8.989 | 0.005 |
| Over 36 years old | 19.539 | 6.19–61.682 | <0.001 |
| Maternal hypothyroidism | 9.353 | 2.863–30.569 | <0.001 |
| Vitamin D deficiency in the cord blood | 2.288 | 1.06–4.943 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ailbayeva, N.; Alimbayeva, A.; Yurkovskaya, O.; Faizova, R.; Tanatarov, S.; Taiorazova, G.; Abylgazinova, A.; Orekhov, A.; Jamedinova, U.; Pivina, L. Vitamin D Deficiency and Maternal Diseases as Risk Factors for the Development of Macrosomia in Newborns. Children 2024, 11, 1160. https://doi.org/10.3390/children11101160
Ailbayeva N, Alimbayeva A, Yurkovskaya O, Faizova R, Tanatarov S, Taiorazova G, Abylgazinova A, Orekhov A, Jamedinova U, Pivina L. Vitamin D Deficiency and Maternal Diseases as Risk Factors for the Development of Macrosomia in Newborns. Children. 2024; 11(10):1160. https://doi.org/10.3390/children11101160
Chicago/Turabian StyleAilbayeva, Nazym, Aliya Alimbayeva, Oxana Yurkovskaya, Raida Faizova, Sayat Tanatarov, Gulnara Taiorazova, Aizhan Abylgazinova, Andrey Orekhov, Ulzhan Jamedinova, and Lyudmila Pivina. 2024. "Vitamin D Deficiency and Maternal Diseases as Risk Factors for the Development of Macrosomia in Newborns" Children 11, no. 10: 1160. https://doi.org/10.3390/children11101160
APA StyleAilbayeva, N., Alimbayeva, A., Yurkovskaya, O., Faizova, R., Tanatarov, S., Taiorazova, G., Abylgazinova, A., Orekhov, A., Jamedinova, U., & Pivina, L. (2024). Vitamin D Deficiency and Maternal Diseases as Risk Factors for the Development of Macrosomia in Newborns. Children, 11(10), 1160. https://doi.org/10.3390/children11101160





