Nonlinear Relationship Between Coronary Perfusion Pressure and In-Hospital Outcomes After Infant Congenital Heart Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Clinical Data
2.3. Statistical Analysis
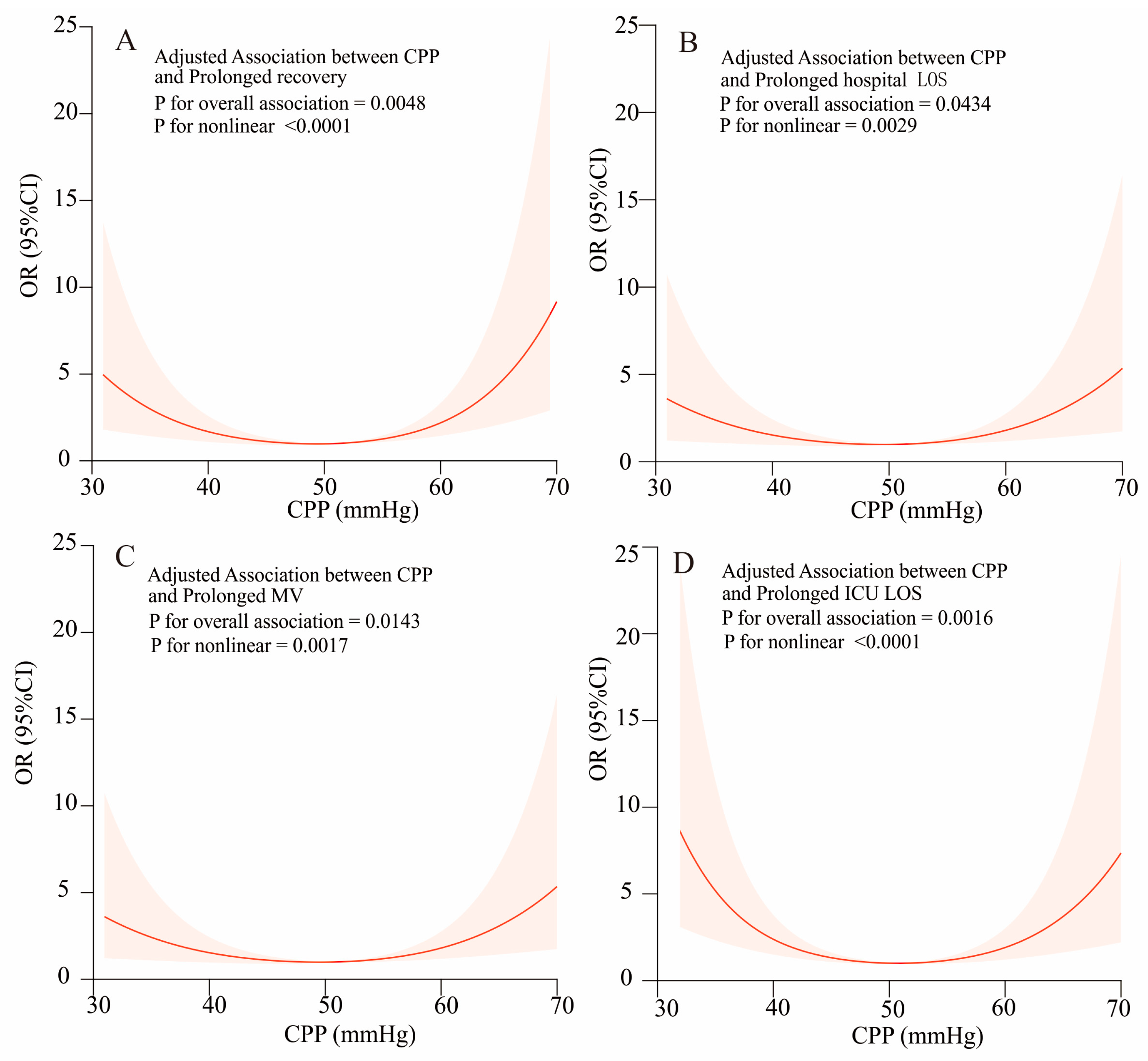
3. Results
3.1. Baseline Patient Characteristics
3.2. Univariate Analyses of the Associations Between Risk Factors and Poor In-Hospital Outcomes
3.3. Association of the Cpp Level with In-Hospital Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, Z.; Yuan, X.; Rao, K.; Zheng, Z.; Hu, S. National trend in congenital heart disease mortality in China during 2003 to 2010: A population-based study. J. Thorac. Cardiovasc. Surg. 2014, 148, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Scherer, B.; Moser, E.A.; Brown, J.W.; Rodefeld, M.D.; Turrentine, M.W.; Mastropietro, C.W. Vasoactive-ventilation-renal score reliably predicts hospital length of stay after surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2016, 152, 1423–1429.e1. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; An, J.; Lin, R.; Dong, C.; Zheng, A.; Li, J.; Duan, H.; Shu, Q.; Li, H. Prediction of complications after paediatric cardiac surgery. Eur. J. Cardiothorac. Surg. 2020, 57, 350–358. [Google Scholar] [CrossRef]
- Green, M.D.; Parker, D.M.; Everett, A.D.; Vricella, L.; Jacobs, M.L.; Jacobs, J.P.; Brown, J.R. Cardiac Biomarkers Associated With Hospital Length of Stay After Pediatric Congenital Heart Surgery. Ann. Thorac. Surg. 2021, 112, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.M.; Mongé, M.C.; Hill, K.D.; Kim, S.; Pasquali, S.K.; Yerokun, B.A.; Jacobs, J.P.; Backer, C.L.; Mazwi, M.L.; Jacobs, M.L. Associations Between Unplanned Cardiac Reinterventions and Outcomes After Pediatric Cardiac Operations. Ann. Thorac. Surg. 2018, 105, 1255–1263. [Google Scholar] [CrossRef]
- Gorlin, R. Regulation of coronary blood flow. Br. Heart J. 1971, 33, 9–14. [Google Scholar] [CrossRef]
- Otlewski, M.P.; Geddes, L.A.; Pargett, M.; Babbs, C.F. Methods for calculating coronary perfusion pressure during CPR. Cardiovasc. Eng. 2009, 9, 98–103. [Google Scholar] [CrossRef]
- Paradis, N.A.; Martin, G.B.; Rivers, E.P.; Goetting, M.G.; Appleton, T.J.; Feingold, M.; Nowak, R.M. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA 1990, 263, 1106–1113. [Google Scholar] [CrossRef]
- McEvoy, J.W.; Chen, Y.; Rawlings, A.; Hoogeveen, R.C.; Ballantyne, C.M.; Blumenthal, R.S.; Coresh, J.; Selvin, E. Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J. Am. Coll. Cardiol. 2016, 68, 1713–1722. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Kajio, H. Low diastolic blood pressure and adverse outcomes in heart failure with preserved ejection fraction. Int. J. Cardiol. 2018, 263, 69–74. [Google Scholar] [CrossRef]
- Rodríguez, R.; Tamayo, E.; Alvarez, F.J.; Castrodeza, J.; Lajo, C.; Flórez, S. Central venous pressure, rewarming time, and total fluid replacement volume are predictors of mortality and complications after cardiac surgery. Rev. Esp. Anestesiol. Reanim. 2008, 55, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Fincke, R.; Hochman, J.S.; Lowe, A.M.; Menon, V.; Slater, J.N.; Webb, J.G.; LeJemtel, T.H.; Cotter, G. Shock Investigators. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial registry. J. Am. Coll. Cardiol. 2004, 44, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Mazimba, S.; Jeukeng, C.; Ondigi, O.; Mwansa, H.; Johnson, A.E.; Elumogo, C.; Breathett, K.; Kwon, Y.; Mubanga, M.; Mwansa, V.; et al. Coronary perfusion pressure is associated with adverse outcomes in advanced heart failure. Perfusion 2022, 38, 1492–1500. [Google Scholar] [CrossRef]
- Buchanan, K.D.; Kolm, P.; Iantorno, M.; Gajanana, D.; Rogers, T.; Gai, J.; Torguson, R.; Ben-Dor, I.; Suddath, W.O.; Satler, L.F.; et al. Coronary perfusion pressure and left ventricular hemodynamics as predictors of cardiovascular collapse following percutaneous coronary intervention. Cardiovasc. Revascularization Med. 2018, 20, 11–15. [Google Scholar] [CrossRef]
- Gaies, M.G.; Jeffries, H.E.; Niebler, R.A.; Pasquali, S.K.; Donohue, J.E.; Yu, S.; Gall, C.; Rice, T.B.; Thiagarajan, R.R. Vasoactive-inotropic score is associated with outcome after infant cardiac surgery: An analysis from the Pediatric Cardiac Critical Care Consortium and Virtual PICU System Registries. Pediatr. Crit. Care Med. 2014, 15, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Gaies, M.G.; Jeffries, H.E.; Niebler, R.A.; Pasquali, S.K.; Donohue, J.E.; Yu, S.; Gall, C.; Rice, T.B.; Thiagarajan, R.R. Consensus-based method for risk adjustment for surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2002, 123, 110–118. [Google Scholar]
- Lacour-Gayet, F.; Clarke, D.; Jacobs, J.P.; Comas, J.; Daebritz, S.; Daenen, W.; Gaynor, W.; Hamilton, L.; Jacobs, M.L.; Maruszsewski, B.; et al. The Aristotle score: A complexity-adjusted method to evaluate surgical results. Eur. J. Cardiothorac. Surg. 2004, 25, 911–924. [Google Scholar] [CrossRef]
- Koponen, T.; Karttunen, J.; Musialowicz, T.; Pietiläinen, L.; Uusaro, A.; Lahtinen, P. Vasoactive-inotropic score and the prediction of morbidity and mortality after cardiac surgery. Br. J. Anaesth. 2019, 122, 428–436. [Google Scholar] [CrossRef]
- Epting, C.L.; McBride, M.E.; Wald, E.L.; Costello, J.M. Pathophysiology of Post-Operative Low Cardiac Output Syndrome. Curr. Vasc. Pharmacol. 2016, 14, 14–23. [Google Scholar] [CrossRef]
- Naim, M.Y.; Sutton, R.M.; Friess, S.H.; Bratinov, G.; Bhalala, U.; Kilbaugh, T.J.; Lampe, J.W.; Nadkarni, V.M.; Becker, L.B.; Berg, R.A. Blood Pressure- and Coronary Perfusion Pressure-Targeted Cardiopulmonary Resuscitation Improves 24-Hour Survival From Ventricular Fibrillation Cardiac Arrest. Crit. Care Med. 2016, 44, e1111–e1117. [Google Scholar] [CrossRef]
- Hsieh, M.J.; Chen, C.C.; Chen, D.Y.; Lee, C.H.; Ho, M.Y.; Yeh, J.K.; Huang, Y.C.; Lu, Y.Y.; Chang, C.Y.; Wang, C.Y.; et al. Risk Stratification by Coronary Perfusion Pressure in Left Ventricular Systolic Dysfunction Patients Undergoing Revascularization: A Propensity Score Matching Analysis. Front. Cardiovasc. Med. 2022, 9, 860346. [Google Scholar] [CrossRef]
- Jeukeng, C.; Mazimba, S.; Bilchick, K.C.; Breathett, K.; Ibrahim, S.; Mwansa, V.; Mehta, N.; Kwon, Y.; Elumogo, C.; Olusola, L. Coronary Perfusion Pressure Gradient Is Associated With Increased Adverse Outcomes In Patients With Advanced Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1050. [Google Scholar] [CrossRef]
- Hagel, J.A.; Sperotto, F.; Laussen, P.C.; Salvin, J.W.; Bachu, A.; Kheir, J.N. Shock Index, Coronary Perfusion Pressure, and Rate Pressure Product As Predictors of Adverse Outcome After Pediatric Cardiac Surgery. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2021, 22, e67–e78. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Ferreira, J.P.; Mahfoud, F.; Duarte, K.; Pitt, B.; Zannad, F.; Rossignol, P. Myocardial reperfusion reverses the J-curve association of cardiovascular risk and diastolic blood pressure in patients with left ventricular dysfunction and heart failure after myocardial infarction: Insights from the EPHESUS trial. Eur. Heart. J. 2020, 41, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Hamud, A.; Brezins, M.; Shturman, A.; Abramovich, A.; Dragu, R. Right coronary artery diastolic perfusion pressure on outcome of patients with left heart failure and pulmonary hypertension. ESC Heart Fail. 2021, 8, 4086–4092. [Google Scholar] [CrossRef]
- Ho, K.M.; Leonard, A.D. Concentration-dependent effect of hypocalcaemia on mortality of patients with critical bleeding requiring massive transfusion: A cohort study. Anaesth. Intensive Care 2011, 39, 46–54. [Google Scholar] [CrossRef]
- Qin, X.; Cen, J.; Hu, H.; Chen, X.; Wei, Z.; Wan, Q.; Cao, R. Non-linear relationship between albumin-corrected calcium and 30-day in-hospital mortality in ICU patients: A multicenter retrospective cohort study. Front. Endocrinol. 2022, 13, 1059201. [Google Scholar] [CrossRef]
- Lee, S.E.; Lee, H.Y.; Cho, H.J.; Choe, W.S.; Kim, H.; Choi, J.O.; Jeon, E.S.; Kim, M.S.; Hwang, K.K.; Chae, S.C.; et al. Reverse J-Curve Relationship Between On-Treatment Blood Pressure and Mortality in Patients With Heart Failure. JACC: Heart Fail. 2017, 5, 810–819. [Google Scholar] [CrossRef]
- Chilian, W.M.; Layne, S.M. Coronary microvascular responses to reductions in perfusion pressure. Evid. Persistent Arter. Vasomotor Tone Dur. Coron. Hypoperfusion. Circ. Res. 1990, 66, 1227–1238. [Google Scholar]
- Mosher, P.; Ross, J., Jr.; McFate, P.A.; Shaw, R.F. Control of coronary blood flow by an autoregulatory mechanism. Circ. Res. 1964, 14, 250–259. [Google Scholar] [CrossRef]
- Arnold, G.; Kosche, F.; Miessner, E.; Neitzert, A.; Lochner, W. The importance of the perfusion pressure in the coronary arteries for the contractility and the oxygen consumption of the heart. Pflug. Arch. Gesamte. Physiol. Menschen. Tiere. 1968, 299, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.J.; Iwamoto, T.; Williams, A.G.; Fan, W.L.; Downey, H.F. Coronary pressure-flow autoregulation protects myocardium from pressure-induced changes in oxygen consumption. Am. J. Physiol. 1994, 266, H2359–H2368. [Google Scholar] [CrossRef] [PubMed]
| Unadjusted Model OR (95%CI) p | Minimally Adjusted Model OR (95%CI) p | Fully Adjusted Model OR (95%CI) p | |
|---|---|---|---|
| Prolonged recovery | |||
| CPP | 0.99 (0.97–1.01, p = 0.290) | 0.99 (0.97–1.01, p = 0.289) | 0.99 (0.97–1.01, p = 0.290) |
| CPP (tertiles) | |||
| moderate | 1(ref) | 1(ref) | 1(ref) |
| low | 4.28 (1.94–9.46, p < 0.001) | 3.66 (1.59–8.42, p = 0.002) | 3.72 (1.48–9.35, p = 0.005) |
| high | 3.39 (1.52–7.58, p = 0.003) | 4.00 (1.70–9.37, p = 0.002) | 6.04 (2.32–15.72, p < 0.001) |
| Prolonged MV | |||
| CPP | 0.98 (0.95–1.01) p = 0.117 | 1.00 (0.97–1.03) p = 0.801 | 1.01 (0.98–1.04) p = 0.610 |
| CPP (tertiles) | |||
| moderate | 1(ref) | 1(ref) | 1(ref) |
| low | 4.17 (1.66–10.45) p = 0.002 | 3.44 (1.29–9.12) p = 0.013 | 3.77 (1.26–11.32) p = 0.018 |
| high | 2.67 (1.03–6.91) p = 0.043 | 3.39 (1.24–9.28) p = 0.018 | 5.34 (1.73–16.49) p = 0.004 |
| Prolonged hospital LOS | |||
| CPP | 0.97 (0.94–1.00) p = 0.025 | 0.98 (0.95–1.00) p = 0.094 | 0.98 (0.95–1.01) p = 0.259 |
| CPP (tertiles) | |||
| moderate | 1(ref) | 1(ref) | 1(ref) |
| low | 5.88 (2.25–15.39) p < 0.001 | 5.20 (1.94–13.89) p = 0.001 | 3.77 (1.26–11.32) p = 0.018 |
| high | 2.94 (1.08–8.01) p = 0.035 | 2.98 (1.07–8.30) p = 0.037 | 5.34 (1.73–16.49) p = 0.004 |
| Prolonged ICU LOS | |||
| CPP | 0.97 (0.94–0.99) p = 0.013 | 0.98 (0.95–1.00) p = 0.100 | 0.98 (0.95–1.02) p = 0.342 |
| CPP (tertiles) | |||
| moderate | 1(ref) | 1(ref) | 1(ref) |
| low | 7.60 (2.73–21.17) p < 0.001 | 6.76 (2.35–19.41) p < 0.001 | 7.25 (2.28–23.04) p < 0.001 |
| high | 3.59 (1.24–10.40) p = 0.019 | 4.08 (1.36–12.23) p = 0.012 | 5.68 (1.67–19.29) p = 0.005 |
| CPP Groups | Low Tertile | Moderate Tertile | High Tertile | p |
|---|---|---|---|---|
| (19~45 mmHg) | (45~55 mmHg) | (56~83 mmHg) | ||
| Baseline characteristics | ||||
| N | 75 | 62 | 71 | |
| Male (%) | 39 (52.0%) | 35 (56.5%) | 42 (59.2%) | 0.679 |
| Age (day) | 151.0 (78.5;227.5) | 148.5 (75.0;209.0) | 185.0 (119.5;230.5) | 0.226 |
| Height (cm) | 61.0 (55.5;66.5) | 60.5(55.0;67.0) | 63.0 (58.0;67.5) | 0.241 |
| Weight (kg) | 5.3 (4.3; 7.0) | 5.5 (4.5; 6.5) | 6.5 (5.0; 7.5) | 0.059 |
| Gestation (weeks) | 40.0 (38.0;40.0) | 40.0 (38.0;40.0) | 39.0 (38.0;40.0) | 0.410 |
| Preoperative hemoglobin (g/L) | 114.0 (105.0;127.5) | 112.5 (105.0;124.0) | 109.0 (103.5;118.5) | 0.190 |
| Preoperative platelet(109/L) | 357.0 (304.5;456.5) | 377.0 (316.0;452.0) | 374.0 (323.0;456.5) | 0.531 |
| Preoperative ALT (U/L) | 22.0 (16.0;30.5) | 27.0 (20.0;34.0) | 24.0 (18.0;31.0) | 0.083 |
| Preoperative creatinine (µmol/L) | 18.9 (15.6;24.3) | 17.1 (15.0;22.6) | 17.7 (15.0;21.2) | 0.071 |
| RACHS-1 score(%) | 0.014 | |||
| 1 | 6 (8.0%) | 7 (11.3%) | 6 (8.5%) | |
| 2 | 49 (65.3%) | 47 (75.8%) | 62 (87.3%) | |
| 3 | 18 (24.0%) | 7 (11.3%) | 2 (2.8%) | |
| 4 | 2 ( 2.7%) | 1 (1.6%) | 1 (1.4%) | |
| Transfusion of RBCs (U) | 2.0 (1.0; 2.8) | 2.5 (1.5; 3.0) | 2.0 (1.0; 3.0) | 0.14 |
| Cardiopulmonary bypass time (min) | 70.0 (60.5;94.5) | 77.0 (58.0;89.0) | 73.0 (58.5;101.0) | 0.729 |
| Aortic cross clamp time (min) | 40.0 (31.0;55.5) | 43.5 (32.0;54.0) | 40.0 (29.0;60.0) | 0.793 |
| Heart rate (beats/min) | 144.1 ± 19.3 | 132.0 ± 19.2 | 132.5 ± 21.5 | <0.001 |
| Systolic blood pressure (mmHg) | 76.8 ± 15.7 | 96.6 ± 12.5 | 110.7 ± 13.1 | <0.001 |
| Central venous pressure (mmHg) | 6.0 (5.0; 8.0) | 6.0 (5.0; 8.0) | 6.0 (4.0; 7.0) | 0.024 |
| Coronary perfusion pressure (mmHg) | 36.0 (33.0;41.0) | 51.0 (48.0;53.0) | 62.0 (58.0;67.0) | <0.001 |
| Vasoactive-inotrope score | 5 (3.0;5.2) | 5 (3.0;8.0) | 5 (0;8.0) | 0.85 |
| Diastolic blood pressure (mmHg) | 43.1 ± 5.8 | 57.1 ± 4.0 | 69.0 ± 5.9 | <0.001 |
| Outcomes | ||||
| ICU LOS (hours) | 290.0 (81.5;540.5) | 134.0 (51.0;259.0) | 236.0 (94.5;398.5) | 0.001 |
| Hospital LOS (days) | 12.1 (7.0;17.1) | 7.1 (5.9; 9.2) | 10.1 (6.8;14.8) | <0.001 |
| Mechanical ventilation (hours) Prolonged recovery n (%) | 70.0 (13.0;140.5) 36 (48.0%) | 16.5 (6.0;52.0) 11 (17.7.4%) | 23.0 (7.5;97.5) 30 (42.3%) | <0.001 0.001 |
| Prolonged hospital LOS n (%) | 29 (38.7%) | 6 (9.7%) | 17 (23.9%) | <0.001 |
| Prolonged MV n (%) | 26 (34.7%) | 7 (11.3%) | 18 (25.4%) | 0.007 |
| Prolonged ICU LOS n (%) | 30 (40.0%) | 5 (8.1%) | 18 (25.4%) | <0.001 |
| Mortality (%) n (%) | 1(1.3%) | 1 (1.6%) | 0 (0.0%) | 0.584 |
| Name | Univariable Analyses |
|---|---|
| Prolonged Recovery | |
| Variables | (OR, 95%CI, p) |
| CPP groups | |
| Moderate | 1(ref) |
| Low | 4.28 (1.94–9.46) p < 0.001 |
| High | 3.39 (1.52–7.58) p = 0.003 |
| Height (cm) | 0.98 (0.94–1.02) p = 0.36 |
| Weight (kg) | 0.91 (0.77–1.07) p = 0.24 |
| Male (%) | 0.85 (0.48–1.50) p = 0.57 |
| Age (days) | 1.00 (0.99–1.00) p = 0.21 |
| Gestation (weeks) | 0.97 (0.86–1.09) p = 0.60 |
| RACHS_1 score | |
| 1 | 1(ref) |
| 2 | 0.97 (0.35–2.71) p = 0.96 |
| 3 | 5.15 (1.44–18.36) p = 0.01 |
| 4 | 6.50 (0.55–76.18) p = 0.14 |
| Transfusion of RBC (u) | 1.14 (0.96–1.36) p = 0.13 |
| Cardiopulmonary bypass time (min) | 1.00 (0.99–1.00) p = 0.34 |
| Aortic cross clamp time (min) | 0.99 (0.98–1.00) p = 0.19 |
| Preoperative hemoglobin (g/L) | 1.01 (0.99–1.02) p = 0.51 |
| Preoperative platelet (109/L) | 1.00 (1.00–1.00) p = 0.56 |
| Preoperative ALT (U/L) | 0.99 (0.97–1.00) p = 0.11 |
| Preoperative creatinine (µmol/L) | 1.08 (1.03–1.13) p = 0.001 |
| Heart rate (beats/min) | 1.02 (1.01–1.03) p = 0.007 |
| Central venous pressure (mmHg) Systolic blood pressure (mmHg) Diastolic blood pressure (mmHg) Vasoactive inotrope score | 1.01 (0.90–1.13) p = 0.84 0.98 (0.96–0.99) p = 0.001 0.99 (0.96–1.01) p = 0.29 1.01 (0.99–1.03) p = 0.44 |
| Prolonged Recovery | Prolonged Hospital LOS | Prolonged MV | Prolonged ICU LOS | |
|---|---|---|---|---|
| OR (95%CI) p | OR (95%CI) p | OR (95%CI) p | OR (95%CI) p | |
| Model I | 1.02 (0.99,1.05) 0.28 | 0.99 (0.96, 1.02) 0.61 | 1.01 (0.98, 1.05) 0.45 | 0.99 (0.96, 1.02) 0.52 |
| Model II | ||||
| Turning point (K) | 47 | 52 | 50 | 48 |
| <K effect 1 | 0.87(0.81,0.94) < 0.001 | 0.92 (0.87, 0.97) 0.002 | 0.92 (0.87, 0.99) 0.015 | 0.84 (0.78, 0.91) < 0.001 |
| >K effect 2 | 1.13(1.07,1.20) <0.001 | 1.10 (1.03, 1.17) 0.004 | 1.11 (1.04, 1.19) 0.001 | 1.13 (1.06, 1.21) <0.001 |
| p for log-likelihood ratio test | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, T.; Luo, D.; Wang, Q.; Chen, J.; Huang, H.; Zhang, C. Nonlinear Relationship Between Coronary Perfusion Pressure and In-Hospital Outcomes After Infant Congenital Heart Surgery. Children 2024, 11, 1419. https://doi.org/10.3390/children11121419
Ge T, Luo D, Wang Q, Chen J, Huang H, Zhang C. Nonlinear Relationship Between Coronary Perfusion Pressure and In-Hospital Outcomes After Infant Congenital Heart Surgery. Children. 2024; 11(12):1419. https://doi.org/10.3390/children11121419
Chicago/Turabian StyleGe, Tongkai, Dandong Luo, Qiuji Wang, Jimei Chen, Huanlei Huang, and Chongjian Zhang. 2024. "Nonlinear Relationship Between Coronary Perfusion Pressure and In-Hospital Outcomes After Infant Congenital Heart Surgery" Children 11, no. 12: 1419. https://doi.org/10.3390/children11121419
APA StyleGe, T., Luo, D., Wang, Q., Chen, J., Huang, H., & Zhang, C. (2024). Nonlinear Relationship Between Coronary Perfusion Pressure and In-Hospital Outcomes After Infant Congenital Heart Surgery. Children, 11(12), 1419. https://doi.org/10.3390/children11121419







