Treating Children and Adolescents with Obesity: Predictors of Early Dropout in Pediatric Weight-Management Programs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Evaluation and Laboratory Assessment
2.3. Statistical Analysis
3. Results
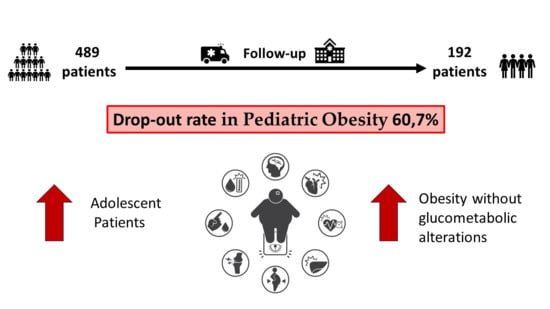
3.1. Dropout Evaluation
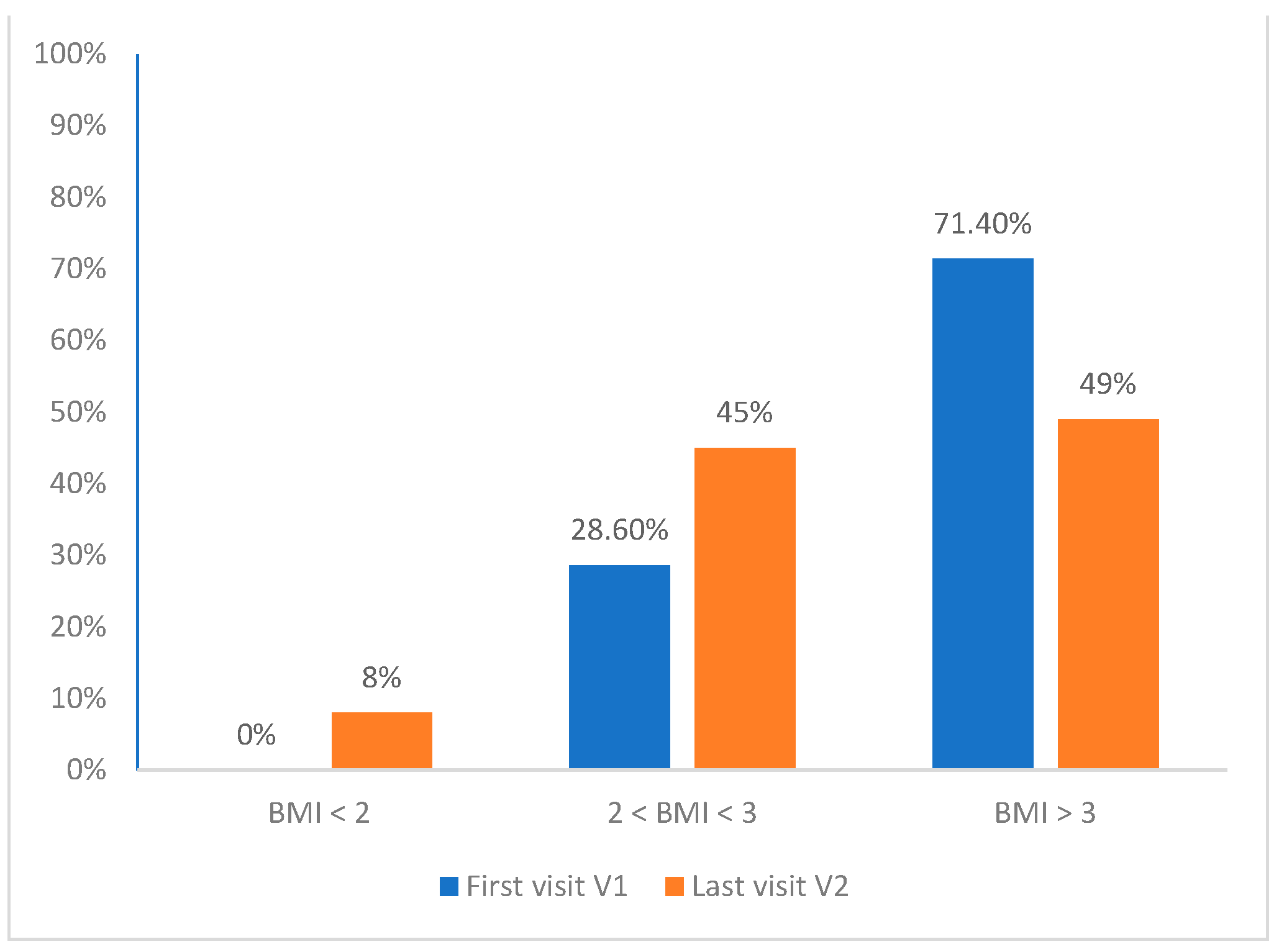
3.2. Weight Loss Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corica, D.; Aversa, T.; Ruggeri, R.M.; Cristani, M.; Alibrandi, A.; Pepe, G.; De Luca, F.; Wasniewska, M. Could AGE/RAGE-Related Oxidative Homeostasis Dysregulation Enhance Susceptibility to Pathogenesis of Cardio-Metabolic Complications in Childhood Obesity? Front. Endocrinol. 2019, 10, 426. [Google Scholar] [CrossRef]
- OECD. The Heavy Burden of Obesity: The Economics of Prevention; OECD Health Policy Studies, OECD Publishing: Paris, France, 2019. [Google Scholar] [CrossRef]
- Corica, D.; Bottari, A.; Aversa, T.; Morabito, L.A.; Curatola, S.; Alibrandi, A.; Ascenti, G.; Wasniewska, M. Prospective assessment of liver stiffness by shear wave elastography in childhood obesity: A pilot study. Endocrine 2022, 75, 59–69. [Google Scholar] [CrossRef]
- Franks, P.W.; Hanson, R.L.; Knowler, W.C.; Sievers, M.L.; Bennett, P.H.; Looker, H.C. Childhood obesity, other cardiovascular risk factors, and premature death. N. Engl. J. Med. 2010, 362, 485–493. [Google Scholar] [CrossRef]
- Skinner, A.C.; Perrin, E.M.; Moss, L.A.; Skelton, J.A. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N. Engl. J. Med. 2015, 373, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Mulder, C.; Twisk, J.W.; van Mechelen, W.; Chinapaw, M.J. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef]
- Scherer, D.P.E. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes 2006, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Kaufman, F.R. Metabolic complications of childhood obesity: Identifying and mitigating the risk. Diabetes Care 2008, 31 (Suppl. S2), S310–S316. [Google Scholar] [CrossRef]
- Valenzise, M.; D’Amico, F.; Cucinotta, U.; Lugarà, C.; Zirilli, G.; Zema, A.; Wasniewska, M.; Pajno, G.B. The lockdown effects on a pediatric obese population in the COVID-19 era. Ital. J. Pediatr. 2021, 47, 209. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Aversa, T.; Valenzise, M.; Messina, M.F.; Alibrandi, A.; De Luca, F.; Wasniewska, M. Does Family History of Obesity, Cardiovascular, and Metabolic Diseases Influence Onset and Severity of Childhood Obesity? Front. Endocrinol. 2018, 9, 187. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Mead, E.; Brown, T.; Rees, K.; Azevedo, L.B.; Whittaker, V.; Jones, D.; Olajide, J.; Mainardi, G.M.; Corpeleijn, E.; O’Malley, C.; et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev. 2017, 6, CD012651. [Google Scholar] [CrossRef]
- Maffeis, C.; Olivieri, F.; Valerio, G.; Verduci, E.; Licenziati, M.R.; Calcaterra, V.; Pelizzo, G.; Salerno, M.; Staiano, A.; Bernasconi, S.; et al. The treatment of obesity in children and adolescents: Consensus position statement of the Italian society of pediatric endocrinology and diabetology, Italian Society of Pediatrics and Italian Society of Pediatric Surgery. Ital. J. Pediatr. 2023, 49, 69. [Google Scholar] [CrossRef]
- Hassan, H.; Snoeck Henkemans, S.; van Teeffelen, J.; Kornelisse, K.; Bindels, P.J.E.; Koes, B.W.; van Middelkoop, M. Determinants of dropout and compliance of children participating in a multidisciplinary intervention programme for overweight and obesity in socially deprived areas. Fam. Pract. 2023, 40, 345–351. [Google Scholar] [CrossRef]
- Ligthart, K.A.M.; Buitendijk, L.; Koes, B.W.; van Middelkoop, M. The association between ethnicity, socioeconomic status and compliance to pediatric weight-management interventions—A systematic review. Obes. Res. Clin. Pract. 2017, 11 (Suppl. S1), 1–51. [Google Scholar] [CrossRef]
- Jensen, C.D.; Aylward, B.S.; Steele, R.G. Predictors of attendance in a practical clinical trial of two pediatric weight management interventions. Obesity 2012, 20, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Atlantis, E.; Barnes, E.H.; Singh, M.A. Efficacy of exercise for treating overweight in children and adolescents: A systematic review. Int. J. Obes. 2006, 30, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Wilfley, D.E.; Stein, R.I.; Saelens, B.E.; Mockus, D.S.; Matt, G.E.; Hayden-Wade, H.A.; Welch, R.R.; Schechtman, K.B.; Thompson, P.A.; Epstein, L.H. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. JAMA 2007, 298, 1661–1673. [Google Scholar] [CrossRef]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef]
- McGovern, L.; Johnson, J.N.; Paulo, R.; Hettinger, A.; Singhal, V.; Kamath, C.; Erwin, P.J.; Montori, V.M. Clinical review: Treatment of pediatric obesity: A systematic review and meta-analysis of randomized trials. J. Clin. Endocrinol. Metab. 2008, 93, 4600–4605. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M. Growth at Adolescence; Blackwell Scientific: Oxford, UK, 1955; p. 212. [Google Scholar]
- Steinbeck, K.S.; Lister, N.B.; Gow, M.L.; Baur, L.A. Treatment of adolescent obesity. Nat. Rev. Endocrinol. 2018, 14, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Oreto, L.; Pepe, G.; Calabrò, M.P.; Longobardo, L.; Morabito, L.; Pajno, G.B.; Alibrandi, A.; Aversa, T.; Wasniewska, M. Precocious preclinical cardiovascular sonographic markers in metabolically healthy and unhealthy childhood obesity. Front. Endocrinol. 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.E.; Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120 (Suppl. S4), S164–S192. [Google Scholar]
- Ammar, A.; Brach, M.; Trabelsi, K.; Chtourou, H.; Boukhris, O.; Masmoudi, L.; Bouaziz, B.; Bentlage, E.; How, D.; Ahmed, M.; et al. Effects of COVID-19 home confinement on eating behaviour and physical activity: Results of the ECLB-COVID19 international online survey. Nutrients 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Hannon, T.S.; Arslanian, S.A. Obesity in Adolescents. N. Engl. J. Med. 2023, 389, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Pepe, G.; Aversa, T.; Currò, M.; Curatola, S.; Li Pomi, A.; Alibrandi, A.; Ientile, R.; Wasniewska, M. Meal-Related Asprosin Serum Levels Are Affected by Insulin Resistance and Impaired Fasting Glucose in Children with Obesity. Front. Endocrinol. 2022, 12, 805700. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, C.D.; Ashton, V.; Campbell, K.J.; Edmunds, L.; Kelly, S.; Waters, E. Interventions for treating obesity in children. Cochrane Database Syst. Rev. 2009, 4, CD001872. [Google Scholar] [CrossRef] [PubMed]
- Corica, D.; Li Pomi, A.; Curatola, S.; Giandalia, A.; Tropeano, A.; Alibrandi, A.; Aversa, T.; Wasniewska, M. Impact of COVID-19 Pandemic on the Effectiveness of Outpatient Counseling in Childhood Obesity Management. Front. Endocrinol. 2022, 13, 879440. [Google Scholar] [CrossRef]
- Pott, W.; Albayrak, O.; Hebebrand, J.; Pauli-Pott, U. Treating childhood obesity: Family background variables and the child’s success in a weight-control intervention. Int. J. Eat. Disord. 2009, 42, 284–289. [Google Scholar] [CrossRef]
- Savoye, M.; Shaw, M.; Dziura, J.; Tamborlane, W.V.; Rose, P.; Guandalini, C.; Goldberg-Gell, R.; Burgert, T.S.; Cali, A.M.; Weiss, R. Effects of a Weight Management Program on Body Composition and Metabolic Parameters in Overweight Children: A Randomized Controlled Trial. JAMA 2007, 297, 2697–2704. [Google Scholar] [CrossRef]
- Munsch, S.; Roth, B.; Michael, T.; Meyer, A.H.; Biedert, E.; Roth, S.; Speck, V.; Zumsteg, U.; Isler, E.; Margraf, J. Randomized controlled comparison of two cognitive behavioral therapies for obese children: Mother versus mother-child cognitive behavioral therapy. Psychother. Psychosom. 2008, 77, 235–246. [Google Scholar] [CrossRef]
- Canoy, D.; Bundred, P. Obesity in children. BMJ Clin. Evid. 2011, 2011, 0325. [Google Scholar]
- Wasniewska, M.; Valenzise, M.; Manganaro, A.; Bombaci, S.; Iudicello, R.; Aversa, T.; De Luca, F.; Lombardo, F. Increased intima media thickness at many arterial sites in obese adolescents with abdominal adiposity, insulin resistance, and high LDL-cholesterol. J. Endocrinol. Investig. 2011, 34, 647–649. [Google Scholar] [CrossRef]
- Aggoun, Y. Obesity, metabolic syndrome, and cardiovascular disease. Pediatr. Res. 2007, 61, 653–659. [Google Scholar] [CrossRef]
- Danielsen, Y.S.; Nordhus, I.H.; Júlíusson, P.B.; Mæhle, M.; Pallesen, S. Effect of a family-based cognitive behavioural intervention on body mass index, self-esteem and symptoms of depression in children with obesity (aged 7–13): A randomised waiting list controlled trial. Obes. Res. Clin. Pract. 2013, 7, e116–e128. [Google Scholar] [CrossRef]
- Lofrano-Prado, M.C.; Donato Junior, J.; Lambertucci, A.C.; Lambertucci, R.H.; Malik, N.; Ritti-Dias, R.M.; Correia, M.A.; Botero, J.P.; Prado, W.L. Recreational Physical Activity Improves Adherence and Dropout in a Non-Intensive Behavioral Intervention for Adolescents with Obesity. Res. Q. Exerc. Sport 2022, 93, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.E.; Ohlemeyer, C.L. Parent reasons for nonreturn to a pediatric weight management program. Clin. Pediatr. 2006, 45, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Zeller, M.; Kirk, S.; Claytor, R.; Khoury, P.; Grieme, J.; Santangelo, M.; Daniels, S. Predictors of attrition from a pediatric weight management program. J. Pediatr. 2004, 144, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, P.; Svensson, V.; Kowalski, J.; Nyberg, G.; Ekblom, O.; Marcus, C. Importance of age for 3-year continuous behavioral obesity treatment success and dropout rate. Obes. Facts 2012, 5, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, A.; Salonen, A.; Jian, C.; Yanover, C.; Antosz, A.; Shmoish, M.; Wasniewska, M.; Bereket, A.; Wudy, S.A.; Hartmann, M.F.; et al. Personalized approach to childhood obesity: Lessons from gut microbiota and omics studies. Narrative review and insights from the 29th European childhood obesity congress. Pediatr. Obes. 2021, 16, e12835. [Google Scholar] [CrossRef] [PubMed]
| Variable | Percent |
|---|---|
| M | 46.8% |
| F | 53.2% |
| 2 ≤ BMI < 3 DS | 28.6% |
| BMI ≥ 3 DS | 71.4% |
| Prepubertal | 57.7% |
| Pubertal | 29.7% |
| Complete pubertal development | 12.7% |
| FH of obesity | 41.3% |
| FH of T2DM | 44.4% |
| Pathological HOMA-IR | 45.1% |
| Total cholesterol > 170 mg/dL | 41.8% |
| Triglycerides > 110 mg/dL | 17.4% |
| Fasting glucose > 100 mg/dL | 11.9% |
| Fasting insulin > 15 uUI/mL | 41.4% |
| Variable | Group A (Mean ± SD) | Group B (Mean ± SD) | p Value |
|---|---|---|---|
| Age V1 (years) | 9.90 ± 2.83 | 9.73 ± 3.08 | >0.05 |
| BMI V1 (kg/m2) | 29.93 ± 4.51 | 29.47± 4.56 | >0.05 |
| BMI SD V1 | 3.798 ± 1.27 | 3.78 ± 1.23 | >0.05 |
| Follow-up duration (months) | 16.10 ± 25.20 | 28.26 ± 17.53 | 0.002 |
| Age V2 (years) | 11.37 ± 2.79 | 12.16 ± 2.93 | 0.003 |
| BMI V2 (kg/m2) | 30.13 ± 5.04 | 29.49 ± 4.75 | >0.05 |
| BMI SD V2 | 3.28 ± 1.01 | 2.97 ± 1.07 | 0.002 |
| Variable | Group A (n 297) | Group B (n 192) | p Value |
|---|---|---|---|
| M | 44.8% | 50% | n.s. |
| F | 55.2% | 50% | n.s. |
| 2 ≤ BMI < 3 SD (V1) | 27.3% | 30.7% | n.s. |
| BMI ≥ 3 SD (V1) | 72.7% | 69.3% | n.s. |
| BMI < 2 SD (V2) | 4.0% | 12.5% | n.s. |
| 2 ≤ BMI < 3 SD (V2) | 42.4% | 47.4% | n.s. |
| BMI ≥ 3 SD (V2) | 53.2% | 40.1% | 0.027 |
| Prepuberal | 56.2% | 59.9% | n.s. |
| Puberal | 28.6% | 31.2% | n.s. |
| Complete pubertal development | 15.2% | 8.9% | n.s. |
| FH of obesity | 43.8% | 37.5% | n.s. |
| FH of T2DM | 38.7% | 39.6% | n.s. |
| Pathological HOMA-IR | 41.3% | 51.1% | 0.036 |
| Total cholesterol > 170 mg/dL | 43.0% | 39.9% | n.s. |
| Triglycerides > 110 mg/dL | 17,4% | 17.4% | n.s. |
| Fasting glucose > 100 mg/dL | 8.9% | 16.5% | 0.012 |
| Fasting Insulin > 15 uUI/mL | 38.2% | 46.3% | n.s. |
| Variables | OR | I.C. 95% | p Value |
|---|---|---|---|
| Advanced pubertal stage | 1.39 | 1.04–1.85 | 0.022 |
| Fasting glucose > 100 mg/dL | 0.52 | 0.29–0.93 | 0.028 |
| Fasting insulin > 15 uUI/mL | 0.64 | 0.93–0.96 | 0.032 |
| Variables | HR | I.C. 95% | p Value |
|---|---|---|---|
| Advanced pubertal stage | 1.43 | 1.21–1.69 | 0.001 |
| Fasting glucose > 100 mg/dL | 0.99 | 0.98–1.01 | 0.281 |
| Fasting insulin > 15 uUI/mL | 0.98 | 0.96–0.99 | 0.003 |
| BMI < 2 DS at V2 | 2 ≤ BMI < 3 at V2 | BMI ≥ 3 DS at V2 | |
|---|---|---|---|
| 2 ≤ BMI < 3 DS at V1 | 21.4% | 70.7% | 7.9% |
| BMI ≥ 3 DS at V1 | 1.7% | 34.1% | 64.2% |
| Variables | OR | I.C.95% | p |
|---|---|---|---|
| Age > 10 anni | 0.57 | 0.39–0.83 | 0.003 |
| Nonpathological HOMA-IR | 1.50 | 1.05–2.21 | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luppino, G.; Wasniewska, M.; Casto, C.; Ferraloro, C.; Li Pomi, A.; Pepe, G.; Morabito, L.A.; Alibrandi, A.; Corica, D.; Aversa, T. Treating Children and Adolescents with Obesity: Predictors of Early Dropout in Pediatric Weight-Management Programs. Children 2024, 11, 205. https://doi.org/10.3390/children11020205
Luppino G, Wasniewska M, Casto C, Ferraloro C, Li Pomi A, Pepe G, Morabito LA, Alibrandi A, Corica D, Aversa T. Treating Children and Adolescents with Obesity: Predictors of Early Dropout in Pediatric Weight-Management Programs. Children. 2024; 11(2):205. https://doi.org/10.3390/children11020205
Chicago/Turabian StyleLuppino, Giovanni, Malgorzata Wasniewska, Celeste Casto, Chiara Ferraloro, Alessandra Li Pomi, Giorgia Pepe, Letteria Anna Morabito, Angela Alibrandi, Domenico Corica, and Tommaso Aversa. 2024. "Treating Children and Adolescents with Obesity: Predictors of Early Dropout in Pediatric Weight-Management Programs" Children 11, no. 2: 205. https://doi.org/10.3390/children11020205
APA StyleLuppino, G., Wasniewska, M., Casto, C., Ferraloro, C., Li Pomi, A., Pepe, G., Morabito, L. A., Alibrandi, A., Corica, D., & Aversa, T. (2024). Treating Children and Adolescents with Obesity: Predictors of Early Dropout in Pediatric Weight-Management Programs. Children, 11(2), 205. https://doi.org/10.3390/children11020205