An International Collaborative Initiative to Establish a Quality-of-Life Questionnaire for Children and Adolescents with Repair of Esophageal Atresia in 14 Countries
Abstract
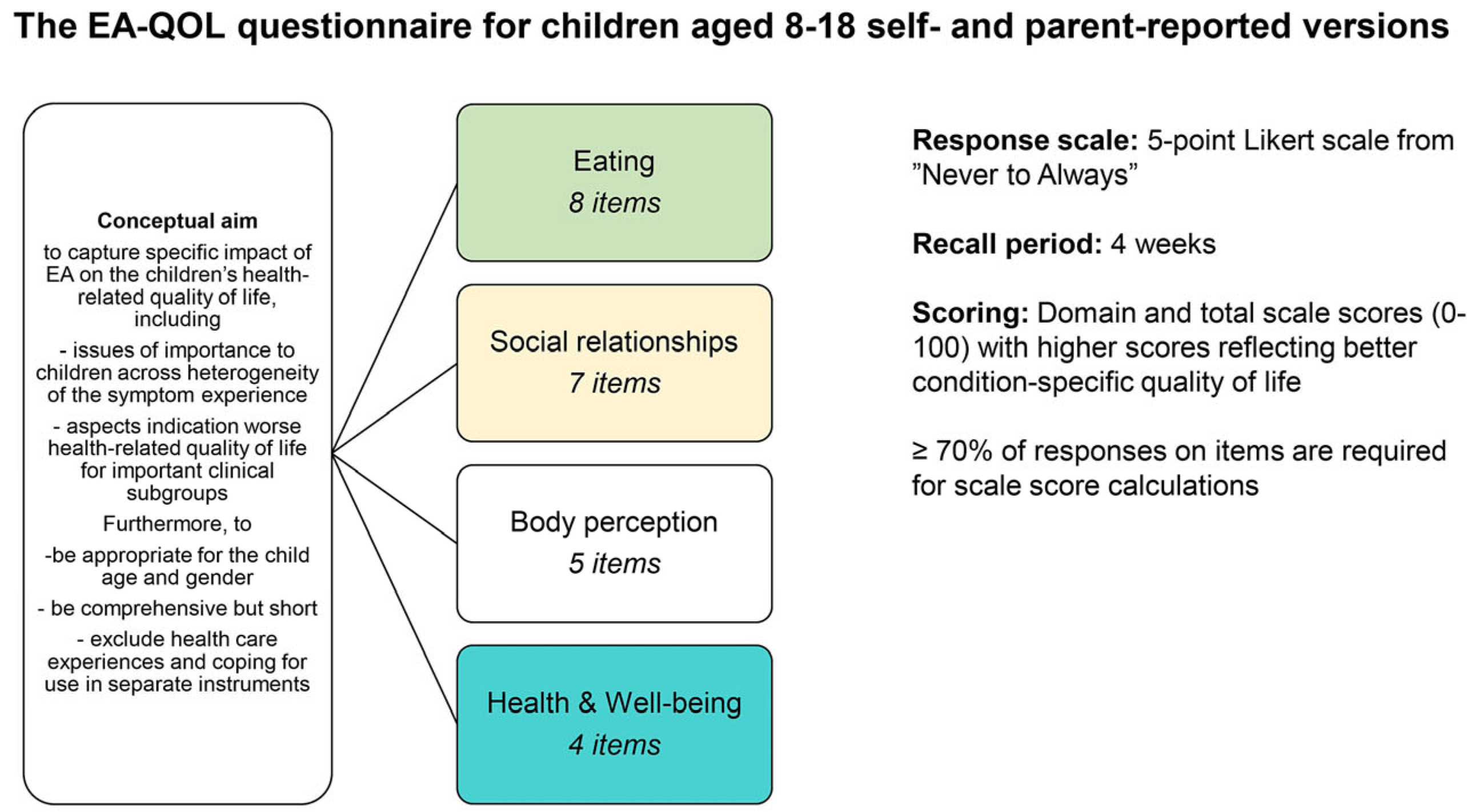
1. Introduction
2. Materials and Methods
2.1. Framework
2.2. Countries/Languages
2.3. Translation Procedure
2.4. Cognitive Debriefing
2.4.1. Study Participants
2.4.2. Data Collection
2.4.3. Data Analysis
2.5. Harmonization between Different Language Versions of the EA-QOL Questionnaire
2.6. Deciscion on the Need to Change the Translations
3. Results
3.1. Item Clarity
3.2. Item Sensitive/Uncomfortable to Answer
3.3. Item Feasibility
3.4. Children’s and Parents’s Comments
3.4.1. Categories of Children’s and Parents’ Comments
- Unclear/ambiguous wording: across 2–4 countries, four items within the eating domain asking about the child’s experiences of food getting stuck (item 1), choking (item 5–6), and vomiting (item 8) received comments from ≥5 children and/or parents that considered unclear/ambiguous wording. These comments gave explanations as to why parents in Norway and the UK rated some items “not easy to understand”. Parents in China wished for clarification of the idea that food gets stuck in the esophagus, not the throat (item 1). Parents in three countries (China, the UK and Hungary), also asked about the definition of “choke” (item 5–6), to ensure it referred to “cough caused by inhalation of food into the trachea while eating” [45]. The parents from the UK described the fact that two original items needed clarification as to whether the child was bothered by the symptom or only if the symptom was present in the child (items 1 and 8), and asked if vomiting (item 8) included regurgitation;
- The category in which it was difficult to answer the item without current experience of the situation mainly concerned the child’s communication about EA with other people, and these comments were mentioned by five children from three countries (item 10) and five children from five countries (item 15);
- Emotive question/strong expression: in agreement with the rating of items as sensitive/uncomfortable to answer, parents from the UK commented that items asking about the child’s social relationship (item 14, in which the translation originally used the word “nasty” instead of “unkind”) and self-perception due to surgical scars (item 19, in which the translation originally used the word “feeling less perfect”) were emotive in the English language;
- Difficult to answer due to young child age: these comments regarded four items (items 9–12) given by one child from Croatia, and four items (items 21–24) commented on by one parent from Croatia.
3.4.2. Item Comprehensiveness
3.4.3. Questionnaire Instructions
3.4.4. Response Scale
3.5. Harmonization
4. Discussion
4.1. Translation
4.2. Cognitive Debriefing
4.3. Study Strengths and Weaknesses
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Affiliations
- 1
- The Department of Pediatrics, Institute of Clinical Sciences, University of Gothenburg, 40530 Gothenburg, Sweden
- 2
- The Department of Pediatric Surgery, Queen Silvia Children’s Hospital, 41685 Gothenburg, Sweden
- 3
- Department of Women’s and Children’s Health, Karolinska Institutet, 17177, Stockholm, Sweden
- 4
- Department of Medical Psychology, University Medical Center Hamburg-Eppendorf, 20251 Hamburg, Germany
- 5
- UCL Great Ormond Street Institute of Child Health, 30 Guilford Street, London WC1N1EH, UK
- 6
- Department of Pediatric Surgery, University Hospital La Paz, 28046 Madrid, Spain
- 7
- Department of Nursing and Obstetrics, Division of Family and Pediatric Nursing, Wroclaw Medical University, 50367 Wroclaw, Poland
- 8
- Division of Pediatric Surgery, Department of Surgery, University Hospital Centre Zagreb, 10000 Zagreb, Croatia
- 9
- Centre for Rare Disorders, Oslo University Hospital, Rikshospitalet, 0372 Oslo, Norway
- 10
- Esophageal and Airway Treatment Center, Department of Pediatric General Surgery, Boston Children’s Hospital, Harvard Medical School, Boston, MA 02115, USA
- 11
- Heim Pál National Pediatric Institute, 1089 Budapest, Hungary
- 12
- Institute for Translational Medicine, Faculty of Medicine, University of Pécs, 7622 Pecs, Hungary
- 13
- Doctoral School of Clinical Medicine, University of Debrecen, 4032 Debrecen, Hungary
- 14
- Department of Neonatal Surgery, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing 100045, China
- 15
- Tygerberg Hospital, Stellenbosch University, Stellenbosch 7602, South Africa
- 16
- Department of Pediatric Surgery, Faculty of Medicine, Hacettepe University, 06100 Ankara, Turkey
- 17
- Surgical Division, Hospital para El Niño Poblano, Puebla 72000, Mexico
- 18
- Centre Hospitalier Universitaire Hôpital Jeanne de Flandre Pôle Enfant 2, Avenue Oscar Lambret, 59037 Lille CEDEX, France
- 19
- EAT (Esophageal Atresia Global Support Groups), Sommerrainstr. 61, 70374 Stuttgart, Germany
- 20
- Department of Paediatric Surgery, Oslo University Hospital, 0450 Oslo, Norway
- 21
- Department of Paediatric Surgery and Urology, Wrocław Medical University, Borowska 213, 50556 Wrocław, Poland
- 22
- Department of Pediatric Surgery, Faculty of Medicine, Istanbul Medeniyet University, 34682 Istanbul, Turkey
- 23
- Department of Neonatal Surgery, The Affiliated Children’s Hospital of Nanchang University, Nanchang 330006, China
- 24
- Pediatric Clinic and Institute, University of Debrecen, 4032 Debrecen, Hungary
- 25
- Department of Psychology, University of Bath, Building 10 West, Bath BA2 7AY, UK
- 26
- Centre of Pediatric Surgery, Hannover Medical School, 30625 Hannover, Germany
- 27
- Hochschule für Angewandte Wissenschaften Hamburg (HAW Hamburg), 20999 Hamburg, Germany
- *
- Correspondence: michaela.m.blom@vgregion.se
- ‡
- These authors contributed equally to this work.
Appendix A.2. Author Contributions
References
- van Lennep, M.; Singendonk, M.M.J.; Dall’Oglio, L.; Gottrand, F.; Krishnan, U.; Terheggen-Lagro, S.W.J.; Omari, T.I.; Benninga, M.A.; van Wijk, M.P. Oesophageal atresia. Nat. Rev. Dis. Primers 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Stoll, C.; Alembik, Y.; Dott, B.; Roth, M.P. Associated malformations in patients with esophageal atresia. Eur. J. Med. Genet. 2009, 52, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Chittmittrapap, S.; Spitz, L.; Kiely, E.M.; Brereton, R.J. Oesophageal atresia and associated anomalies. Arch. Dis. Child. 1989, 64, 364–368. [Google Scholar] [CrossRef]
- Haight, C. Congenital atresia of the esophagus with tracheoesophageal fistula: Reconstruction of esophageal continuity by primary anastomosis. Ann. Surg. 1944, 120, 623–655. [Google Scholar] [CrossRef]
- Zimmer, J.; Eaton, S.; Murchison, L.E.; De Coppi, P.; Ure, B.M.; Dingemann, C. State of Play: Eeght decades of surgery for esophageal atresia. Eur. J. Pediatr. Surg. 2019, 29, 39–48. [Google Scholar] [CrossRef]
- Global PaedSurg Research Collaboration. Mortality from gastrointestinal congenital anomalies at 264 hospitals in 74 low-income, middle-income, and high-income countries: A multicentre, international, prospective cohort study. Lancet 2021, 398, 325–339. [Google Scholar] [CrossRef]
- Coppens, C.H.; van den Engel-Hoek, L.; Scharbatke, H.; de Groot, S.A.F.; Draaisma, J.M.T. Dysphagia in children with repaired oesophageal atresia. Eur. J. Pediatr. 2016, 175, 1209–1217. [Google Scholar] [CrossRef]
- Vergouwe, F.W.T.; Vlot, J.; IJsselstijn, H.; Spaander, M.C.W.; van Rosmalen, J.; Oomen, M.W.N.; Hulscher, J.B.F.; Dirix, M.; Bruno, M.J.; Schurink, M.; et al. Risk factors for refractory anastomotic strictures after oesophageal atresia repair: A multicentre study. Arch. Dis. Child. 2019, 104, 152–157. [Google Scholar] [CrossRef]
- Vergouwe, F.W.T.; van Wijk, M.P.; Spaander, M.C.W.; Bruno, M.J.; Wijnen, R.M.H.; Schnater, J.M.; IJsselstijn, H. Evaluation of gastroesophageal reflux in children Born with esophageal atresia using pH and Impedance Monitoring. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ax, S.Ö.; Abrahamsson, K.; Gatzinsky, V.; Jönsson, L.; Dellenmark-Blom, M. Parent-Reported feeding difficulties among children born with esophageal atresia: Prevalence and early Risk Factors. Eur. J. Pediatr. Surg. 2021, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Koumbourlis, A.C.; Belessis, Y.; Cataletto, M.; Cutrera, R.; DeBoer, E.; Kazachkov, M.; Laberge, S.; Popler, J.; Porcaro, F.; Kovesi, T. Care recommendations for the respiratory complications of esophageal atresia-tracheoesophageal fistula. Pediatr. Pulmonol. 2020, 55, 2713–2729. [Google Scholar] [CrossRef]
- Olbers, J.; Gatzinsky, V.; Jönsson, L.; Friberg, L.G.; Abrahamsson, K.; Sillén, U.; Gustafsson, P. Physiological studies at 7 years of age in children born with esophageal atresia. Eur. J. Pediatr. Surg. 2015, 25, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Traini, I.; Menzies, J.; Hughes, J.; Leach, S.T.; Krishnan, U. Oesophageal atresia: The growth gap. World J. Gastroenterol. 2020, 26, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Vergouwe, F.W.T.; Spoel, M.; van Beelen, N.W.G.; Gischler, S.J.; Wijnen, R.M.H.; van Rosmalen, J.; IJsselstijn, H. Longitudinal evaluation of growth in oesophageal atresia patients up to 12 years. Arch. Dis. Child. Fetal Neonatal Ed. 2017, 102, F417–F422. [Google Scholar] [CrossRef]
- Krishnan, U.; Mousa, H.; Dall’Oglio, L.; Homaira, N.; Rosen, R.; Faure, C.; Gottrand, F. ESPGHAN-NASPGHAN Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children with Esophageal Atresia-Tracheoesophageal Fistula. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 550–570. [Google Scholar] [CrossRef]
- Dingemann, C.; Eaton, S.; Aksnes, G.; Bagolan, P.; Cross, K.M.; De Coppi, P.; Fruithof, J.; Gamba, P.; Husby, S.; Koivusalo, A.; et al. ERNICA Consensus Conference on the Management of Patients with Esophageal Atresia and Tracheoesophageal Fistula: Follow-up and Framework. Eur. J. Pediatr. Surg. 2020, 30, 475–482. [Google Scholar] [CrossRef]
- Bullinger, M. Assessing health related quality of life in medicine. An overview over concepts, methods and applications in international research. Restor. Neurol. Neurosci. 2002, 20, 93–101. [Google Scholar] [PubMed]
- Dellenmark-Blom, M.; Chaplin, J.E.; Gatzinsky, V.; Jönsson, L.; Abrahamson, K. Health-related quality of life among children, young people and adults with esophageal atresia: A review of the literature and recommendations for future research. Qual. Life Res. 2015, 24, 2433–2445. [Google Scholar] [CrossRef] [PubMed]
- Dellenmark-Blom, M.; Quitmann, J.; Dingemann, C. Health-Related Quality of Life in Patients after repair of esophageal atresia: A review of current Literature. Eur. J. Pediatr. Surg. 2020, 30, 239–250. [Google Scholar] [CrossRef]
- Mikkelsen, A.; Boye, B.; Diseth, T.H.; Malt, U.; Mørkrid, L.; IJsselstijn, H.; Emblem, R. Traumatic stress, mental health, and quality of life in adolescents with esophageal atresia. J. Pediatr. Surg. 2022, 57, 1423–1431. [Google Scholar] [CrossRef]
- Gallo, G.; van Tuyll van Serooskerken, E.S.; Tytgat, S.; van der Zee, D.C.; Keyzer-Dekker, C.M.G.; Zwaveling, S.; Hulscher, J.B.F.; Groen, H.; Lindeboom, M.Y.A. Quality of life after esophageal replacement in children. J. Pediatr. Surg. 2021, 56, 239–244. [Google Scholar] [CrossRef]
- Ten Kate, C.A.; Rietman, A.B.; van de Wijngaert, Y.; van Gils-Frijters, A.; Gischler, S.J.; Keyzer-Dekker, C.M.G.; Wijnen, R.M.H.; IJsselstijn, H. Longitudinal health status and quality of life after esophageal atresia repair. J. Pediatr. Gastroenterol. Nutr. 2021, 73, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Dingemann, C.; Meyer, A.; Kircher, G.; Boemers, T.M.; Vaske, B.; Till, H.; Ure, B.M. Long-term health-related quality of life after complex and/or complicated esophageal atresia in adults and children registered in a German patient support group. J. Pediatr. Surg. 2014, 49, 631–638. [Google Scholar] [CrossRef] [PubMed]
- di Natale, A.; Brestel, J.; Mauracher, A.A.; Tharakan, S.J.; Meuli, M.; Möhrlen, U.; Subotic, U. Long-Term outcomes and health-related quality of life in a Swiss patient group with esophageal atresia. Eur. J. Pediatr. Surg. 2022, 32, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Legrand, C.; Michaud, L.; Salleron, J.; Neut, D.; Sfeir, R.; Thumerelle, C.; Bonnevalle, M.; Turck, D.; Gottrand, F. Long-term outcome of children with oesophageal atresia type III. Arch. Dis. Child. 2012, 97, 808–811. [Google Scholar] [CrossRef]
- Flieder, S.; Dellenmark-Blom, M.; Witt, S.; Dingemann, C.; Quitmann, J.H.; Jönsson, L.; Gatzinsky, V.; Chaplin, J.E.; Dammeier, B.G.; Bullinger, M.; et al. Generic health-related quality of life after repair of esophageal atresia and its determinants within a German-Swedish Cohort. Eur. J. Pediatr. Surg. 2019, 29, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Knezevich, M.; Lingongo, M.; Szabo, A.; Yin, Z.; Oldham, K.T.; Calkins, C.M.; Sato, T.T.; Arca, M.J. Long-term quality of life in neonatal surgical disease. Ann. Surg. 2018, 268, 497–505. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER); Center for Devices and Radiological Health (CDRH). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2009; pp. 1–39.
- Matza, L.S.; Patrick, D.L.; Riley, A.W.; Alexander, J.J.; Rajmil, L.; Pleil, A.M.; Bullinger, M. Pediatric patient-reported outcome instruments for research to support medical product labeling: Report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health 2013, 16, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Patrick, D.L.; Burke, L.B.; Gwaltney, C.J.; Leidy, N.K.; Martin, M.L.; Molsen, E.; Ring, L. Content validity—Establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: Part 1—Eliciting concepts for a new PRO instrument. Value Health 2011, 14, 967–977. [Google Scholar] [CrossRef]
- Patrick, D.L.; Burke, L.B.; Gwaltney, C.J.; Leidy, N.K.; Martin, M.L.; Molsen, E.; Ring, L. Content validity—Establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: Part 2—Assessing respondent understanding. Value Health 2011, 14, 978–988. [Google Scholar] [CrossRef]
- Dellenmark-Blom, M.; Chaplin, J.E.; Gatzinsky, V.; Jönsson, L.; Wigert, H.; Apell, J.; Sillén, U.; Abrahamsson, K. Health-related quality of life experiences among children and adolescents born with esophageal atresia: Development of a condition-specific questionnaire for pediatric patients. J. Pediatr. Surg. 2016, 51, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Dellenmark-Blom, M.; Abrahamsson, K.; Quitmann, J.H.; Sommer, R.; Witt, S.; Dingemann, J.; Flieder, S.; Jonsson, L.; Gatzinsky, V.; Bullinger, M.; et al. Development and pilot-testing of a condition-specific instrument to assess the quality-of-life in children and adolescents born with esophageal atresia. Dis. Esophagus 2017, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dellenmark-Blom, M.; Dingemann, J.; Witt, S.; Quitmann, J.H.; Jonsson, L.; Gatzinsky, V.; Chaplin, J.E.; Bullinger, M.; Flieder, S.; Ure, B.M.; et al. The Esophageal-atresia-Quality-of-life questionnaires: Feasibility, validity and reliability in Sweden and Germany. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Bullinger, M. Current issues in cross-cultural quality of life instrument development. Arch. Phys. Med. Rehabil. 2003, 84 (Suppl. 2), S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Kankaraš, M.; Moors, G. Researching Measurement Equivalence in Cross-Cultural Studies. Psihologija 2010, 43, 121–136. [Google Scholar] [CrossRef]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Eremenco, S.; Mear, I.; Martin, M.; Houchin, C.; Gawlicki, M.; Hareendran, A.; Wiklund, I.; Chong, L.Y.; von Maltzahn, R.; et al. Multinational trials-recommendations on the translations required, approaches to using the same language in different countries, and the approaches to support pooling the data: The ISPOR Patient-Reported Outcomes Translation and Linguistic Validation Good Research Practices Task Force report. Value Health 2009, 12, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Soyer, T.; Arslan, U.E.; Ulukaya Durakbaşa, Ç.; Aydöner, S.; Boybeyi-Türer, Ö.; Quitmann, J.H.; Dingemann, J.; Dellenmark-Blom, M. Feasibility, reliability, and validity of the Turkish Version of the Esophageal-Atresia-Quality-of-Life Questionnaires to assess condition-specific quality of life in children and adolescents born with esophageal atresia. Turk. J. Gastroenterol. 2021, 32, 640–650. [Google Scholar] [CrossRef]
- Rozensztrauch, A.; Śmigiel, R.; Patkowski, D.; Gerus, S.; Kłaniewska, M.; Quitmann, J.H.; Dellenmark-Blom, M. Reliability and validity of the Polish Version of the Esophageal-Atresia-Quality-of-Life Questionnaires to assess condition-Specific quality of life in children and adolescents born with esophageal atresia. Int. J. Environ. Res. Public Health 2022, 19, 8047. [Google Scholar] [CrossRef]
- Ten Kate, C.A.; IJsselstijn, H.; Dellenmark-Blom, M.; van Tuyll van Serooskerken, E.S.; Joosten, M.; Wijnen, R.M.H.; van Wijk, M.P.; On Behalf Of The Dcea Study Group. Psychometric Performance of a condition-specific quality-of-life instrument for Dutch children born with esophageal atresia. Children 2022, 9, 1508. [Google Scholar] [CrossRef]
- Besner, A.S.; Ferreira, J.L.; Ow, N.; Gaffar, R.; Guadagno, E.; Emil, S.; Poenaru, D. Patient-reported outcome measures in pediatric surgery—A systematic review. J. Pediatr. Surg. 2022, 57, 798–812. [Google Scholar] [CrossRef]
- The International EA-QOL Group. Establishment of a condition-specific quality-of-life questionnaire for children born with esophageal atresia aged 2–7 across 14 countries. Front. Pediatr. 2023, 11, 1253892. [Google Scholar] [CrossRef] [PubMed]
- DeMuro, C.J.; Lewis, S.A.; DiBenedetti, D.B.; Price, M.A.; Fehnel, S.E. Successful implementation of cognitive interviews in special populations. Expert Rev. Pharmacoecon. Outcomes Res. 2012, 12, 181–187. [Google Scholar] [CrossRef]
- Li, S.; Dellenmark-Blom, M.; Zhao, Y.; Gu, Y.; Li, S.; Yang, S.; Quitmann, J.H.; Huang, J. The Chinese Mandarin Version of the Esophageal-Atresia-Quality-of-Life Questionnaires for Children and Adolescents: Evaluation of Linguistic and Content Validity. Int. J. Environ. Res. Public Health 2022, 19, 14923. [Google Scholar] [CrossRef]
- Bengtsson, M. How to plan and perform a qualitative study using content analysis. NursingPlus Open 2016, 2, 8–14. [Google Scholar] [CrossRef]
- Swedish Institute. Key Facts about Sweden. Available online: https://sweden.se/life/society/key-facts-about-sweden (accessed on 17 January 2024).
- Tripathy, S.; Myatra, S.N. Are the instruments for quality of life assessment comparable between cultures? No. Intensive Care Med. 2020, 46, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Ahmed, S.; Graham, C.; Kind, P.; Sun, Y.N.; Yu, C.H. Similarities and differences in health-related quality-of-life concepts between the East and the West: A Qualitative Analysis of the Content of Health-Related Quality-of-Life Measures. Value Health Reg. Issues 2021, 24, 96–106. [Google Scholar] [CrossRef]
- Yu, D.S.; Lee, D.T.; Woo, J. Issues and challenges of instrument translation. West. J. Nurs. Res. 2004, 26, 307–320. [Google Scholar] [CrossRef]
- Tsangaris, E.; Wong Riff, K.W.Y.; Goodacre, T.; Forrest, C.R.; Dreise, M.; Sykes, J.; de Chalain, T.; Harman, K.; O’Mahony, A.; Pusic, A.L.; et al. Establishing Content Validity of the CLEFT-Q: A New Patient-reported Outcome Instrument for Cleft Lip/Palate. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1305. [Google Scholar] [CrossRef]
- Chassany, O.; Shaheen, N.J.; Karlsson, M.; Hughes, N.; Rydén, A. Systematic review: Symptom assessment using patient-reported outcomes in gastroesophageal reflux disease and dyspepsia. Scand. J. Gastroenterol. 2012, 47, 1412–1421. [Google Scholar] [CrossRef]
- Witt, S.; Dellenmark-Blom, M.; Flieder, S.; Dingemann, J.; Abrahamsson, K.; Jonsson, L.; Gatzinsky, V.; Chaplin, J.E.; Ure, B.; Dingemann, C.; et al. Health-related quality of life experiences in children and adolescents born with esophageal atresia: A Swedish–German focus group study. Child Care Health Dev. 2019, 45, 79–88. [Google Scholar] [CrossRef]
- Bele, S.; Chugh, A.; Mohamed, B.; Teela, L.; Haverman, L.; Santana, M.J. Patient-Reported Outcome Measures in routine pediatric clinical care: A Systematic Review. Front. Pediatr. 2020, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- de Vos, C.; Dellenmark-Blom, M.; Sikwete, F.M.; Sidler, D.; van Wyk, L.; Goussard, P. Disease Specific Quality of Life as Part of the Long-term Follow-up for Children Born with Esophageal Atresia in an Academic Unit in South Africa—A Pilot Study. Pediatr. Surg. Int. 2023, preprint. [Google Scholar] [CrossRef]
- Klassen, A.F.; Dalton, L.; Goodacre, T.E.E.; Harman, K.E.; Slator, R.; Tsangaris, E.; Courtemanche, D.J.; Goldstein, J.; Allen, G.C.; Mahony, A.O.; et al. Impact of Completing CLEFT-Q Scales That Ask About Appearance on Children and Young Adults: An International Study. Cleft Palate-Craniofacial J. 2020, 57, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, D.d.; Huisman, M. Prevention and Treatment of Item Nonresponse. J. Off. Stat. 2003, 19, 153. [Google Scholar]
- Krosnick, J.A. Response strategies for coping with the cognitive demands of attitude measures in surveys. Appl. Cogn. Psychol. 1991, 5, 213–236. [Google Scholar] [CrossRef]
- Feick, L.F. Latent class analysis of survey questions that include don’t know responses. Public Opin. Q. 1989, 53, 525–547. [Google Scholar] [CrossRef]
- Fayers, P.M.; Machin, D. Quality of Life: The Assessment, Analysis and Reporting of Patient-Reported Outcomes; John Wiley & Sons, Incorporated: Hoboken, NJ, USA, 2016. [Google Scholar]
- Oppenheim, A.N. Questionnaire Design, Interviewing, and Attitude Measurement; Pinter: London, UK, 1992. [Google Scholar]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Klassen, A.F.; Riff, K.W.W.; Longmire, N.M.; Albert, A.; Allen, G.C.; Aydin, M.A.; Baker, S.B.; Cano, S.J.; Chan, A.J.; Courtemanche, D.J.; et al. Psychometric findings and normative values for the CLEFT-Q based on 2434 children and young adult patients with cleft lip and/or palate from 12 countries. CMAJ 2018, 190, E455–E462. [Google Scholar] [CrossRef]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; de Vet, H.C. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiol. 2007, 60, 34–42. [Google Scholar] [CrossRef]
- Thoyre, S.M.; Pados, B.F.; Park, J.; Estrem, H.; Hodges, E.A.; McComish, C.; Van Riper, M.; Murdoch, K. Development and content validation of the Pediatric Eating Assessment Tool (Pedi-EAT). Am. J. Speech-Lang. Pathol. 2014, 23, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Thoyre, S.M.; Pados, B.F.; Park, J.; Estrem, H.; McComish, C.; Hodges, E.A. The Pediatric Eating Assessment Tool: Factor Structure and Psychometric Properties. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Maddison, J.; Taylor, J.; O’Neill, M.; Cade, J.; Hewitt, C.; Horridge, K.; McCarter, A.; Fraser, L.K.; Beresford, B. Outcomes for gastrostomy-fed children and their parents: Qualitative findings from the ‘Your Tube’ study. Dev. Med. Child Neurol. 2021, 63, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, J.E.; Koopman, H.M.; Schmidt, S.; The DISABKIDS Group. DISABKIDS smiley questionnaire: The TAKE 6 assisted health-related quality of life measure for 4 to 7-year-olds. Clin. Psychol. Psychother. 2008, 15, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, M.C.; Schmidt, S.; Muehlan, H.; Debensason, D.; Bullinger, M.; DISABKIDS Group. Field testing of a European quality of life instrument for children and adolescents with chronic conditions: The 37-item DISABKIDS Chronic Generic Module. Qual. Life Res. 2007, 16, 881–893. [Google Scholar] [CrossRef]
- Baars, R.M.; Atherton, C.I.; Koopman, H.M.; Bullinger, M.; Power, M.; The DISABKIDS group. The European DISABKIDS project: Development of seven condition-specific modules to measure health related quality of life in children and adolescents. Health Qual. Life Outcomes 2005, 3, 70. [Google Scholar] [CrossRef]
- Tsangaris, E.; Riff, K.; Vargas, F.; Aguilera, M.P.; Alarcón, M.M.; Cazalla, A.A.; Thabane, L.; Thoma, A.; Klassen, A.F. Translation and cultural adaptation of the CLEFT-Q for use in Colombia, Chile, and Spain. Health Qual. Life Outcomes 2017, 15, 228. [Google Scholar] [CrossRef]
- Drosatou, C.; Vlachopapadopoulou, E.A.; Bullinger, M.; Quitmann, J.; Silva, N.; Salemi, G.; Pavlopoulou, I.; Michalacos, S.; Tsoumakas, K. Validation of the Greek version of the Quality of Life in Short Stature Youth (QoLISSY) questionnaire. J. Pediatr. Endocrinol. Metab. 2019, 32, 215–224. [Google Scholar] [CrossRef]
- Quitmann, J.; Giammarco, A.; Maghnie, M.; Napoli, F.; Di Giovanni, I.; Carducci, C.; Mohn, A.; Bullinger, M.; Sommer, R. Validation of the Italian Quality of Life in Short Stature Youth (QoLISSY) questionnaire. J. Endocrinol. Investig. 2017, 40, 1077–1084. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Herdman, M.; Devine, J.; Otto, C.; Bullinger, M.; Rose, M.; Klasen, F. The European KIDSCREEN approach to measure quality of life and well-being in children: Development, current application, and future advances. Qual. Life Res. 2014, 23, 791–803. [Google Scholar] [CrossRef]
- Pope, C.; Ziebland, S.; Mays, N. Qualitative Research in Health Care: Analysing Qualitative Data. BMJ 2000, 320, 114–116. [Google Scholar] [CrossRef] [PubMed]





| n (%), Self-Report (n = 82) | n (%), Parent-Proxy Report (n = 86) | |
|---|---|---|
| Child male sex | 51 (62.2) | 55 (64.0) |
| Gross type a | ||
| A, isolated EA | 13 (16.0) | 14 (16.7) |
| C, EA with distal TEF | 65 (80.2) | 67 (79.8) |
| D, EA with prximal and distal TEF | 2 (2.5) | 2 (2.4) |
| E, only TEF | 1 (1.2) | 1 (1.2) |
| Child age (median/min–max) | 12 (8–18) | 11 (8–18) |
| Country of residence | ||
| United Kingdom | 10 (12.2) | 10 (11.6) |
| Spain | 9 (11.0) | 9 (10.5) |
| Poland | 9 (11.0) | 9 (10.5) |
| Croatia | 9 (11.0) | 9 (10.5) |
| Norway | 8 (9.8) | 8 (9.3) |
| Hungary | 6 (7.3) | 6 (7.0) |
| USA | 6 (7.3) | 6 (7.0) |
| China | 6 (7.3) | 6 (7.0) |
| South Africa | 6 (7.3) | 6 (7.0) |
| Turkey | 5 (6.1) | 7 (8.1) |
| Mexico | 4 (4.9) | 5 (5.8) |
| France | 4 (4.9) | 5 (5.8) |
| Parent-proxy mother | 70 (81.4) | |
| Parent-proxy age (median/min–max) | 43 (26–66) b |
| Easy to Understand a | Sensitive/Uncomfortable to Answer a | Missing Item Responses b | |||||
|---|---|---|---|---|---|---|---|
| Self-Report | Parent-Proxy Report | Self-Report | Parent-Proxy Report | Self-Report | Parent-Proxy Report | ||
| Items—Eating | |||||||
| 1. | I feel distressed that food gets stuck in my throat when I eat | 81 (98.8) | 81 (94.2) | 3 (3.7) | 0 | 0 | 0 |
| 2 | My health condition restricts me from eating certain foods’ | 79 (96.3) | 83 (96.5) | 1 (1.2) | 1 (1.2) | 1 (1.4) c | 1 (1.4) d |
| 3 | It hurts when I eat because of my health condition (e.g., when food sticks, heartburn, tummy ache) | 81 (98.8) | 84 (97.4) | 1 (1.2) | 1 (1.2) | 0 | 2 (2.7) d |
| 4. | I have to remind myself to drink liquids when I eat | 80 (97.6) | 80 (93.0) | 2 (2.4) | 2 (2.3) | 0 | 0 |
| 5 | I am afraid when I choke while eating | 79 (96.3) | 81 (94.2) | 2 (2.4) | 2 (2.3) | 0 | 0 |
| 6. | I feel that my experiences of choking make it difficult for me to eat | 79 (96.3) | 81 (94.2) | 1 (1.2) | 1 (1.2) | 1 (1.4) c | 0 |
| 7 | I can eat at the same speed/pace as other children my age | 80 (97.6) | 83 (96.5) | 1 (1.2) | 2 (2.3) | 0 | 0 |
| 8. | It bothers me if I vomit after I eat | 79 (96.3) | 74 (86.0) | 1 (1.2) | 3 (3.5) | 0 | 0 |
| Items—Social relationship | |||||||
| 9. | I feel like the only one who was born with esophageal atresia | 79 (96.3) | 85 (98.8) | 5 (6.1) | 6 (7.0) | 0 | 0 |
| 10. | It is complicated to explain to others what esophageal atresia is | 79 (96.3) | 82 (96.5) a | 1 (1.2) | 1 (1.2) | 0 | 0 |
| 11. | People call me names (perhaps because of your size, having an unusual cough, eating slowly, or because you have a surgical scar) | 79 (96.3) | 85 (98.8) | 5 (6.1) | 8 (9.3) | 0 | 0 |
| 12. | I feel that other people are staring at me (e.g., when coughing, choking, dressing in the locker room) | 81 (98.8) | 86 (100) | 6 (7.3) | 2 (2.3) | 0 | 0 |
| 13. | I get tired of people asking about the scar/scars | 81 (98.8) | 86 (100) | 3 (3.7) | 1 (1.2) a | 0 | 0 |
| 14. | Other people say unkind things about me | 79 (96.3) | 81 (94.2) | 2 (2.5) a | 8 (9.3) | 0 | 1 (1.4) d |
| 15. | It feels awkward when other people ask me about esophageal atresia | 78 (95.1) | 84 (97.7) | 2 (2.4) | 3 (3.5) a | 0 | 0 |
| Items—Body Perception | |||||||
| 16. | I feel different because I have scars | 80 (97.6) | 85 (98.8) | 4 (4.9) | 4 (4.7) | 0 | 0 |
| 17. | I am careful about what I wear because of my scar/scars | 78 (96.3) a | 85 (98.8) | 5 (6.2) a | 2 (2.3) | 0 | 0 |
| 18. | I feel awkward when my scar/scars are visible to other people (e.g., new people, boy- or girlfriend, people in the changing room, or in the swimming pool) | 80 (98.8) | 83 (96.5) | 4 (4.9) a | 6 (7.0) | 0 | 1 (1.4) d |
| 19. | I am unhappy with the way I look because I have scars | 78 (97.5) b | 81 (95.3) a | 7 (8.8) b | 10 (11.6) | 1 (1.4) c | 1 (1.4) d |
| 20. | It bothers me that I am smaller than children my age | 80 (98.8) a | 85 (98.8) | 4 (4.9) a | 2 (2.3) | 0 | 0 |
| Items—Health and Well-being | |||||||
| 21. | I am bothered by breathing difficulties if I exercise and play | 80 (98.8) a | 84 (97.7) | 3 (3.7) a | 2 (2.3) | 0 | 0 |
| 22. | I have trouble falling or staying asleep at night because of my health condition (e.g., acid reflux, heartburn, or respiratory problems) | 80 (98.8) a | 82 (95.3) | 1 (1.2) a | 2 (2.3) | 0 | 0 |
| 23. | I am worried about my future because of esophageal atresia (e.g., school, friends, boy- or girlfriend, work) | 80 (98.8) a | 82 (95.3) | 3 (3.8) b | 4 (4.7) | 0 | 1 (1.4) d |
| 24. | Esophageal atresia makes me sad | 81 (100) a | 82 (95.3) | 8 (9.9) a | 3 (3.5) | 0 | 0 |
| Items not Fulfilling the Desired Criteria | Modification/Changes in Item Wording | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Language | Item Clarity a | Item Sensitive to Answer b | Item Feasibility c | No Change | Response Scale | Eating (Items 1–8) | Social Relationship (Items 9–15) | Body Perception (Items 16–20) | Health and Well-Being (Items 21–24) |
| Turkish | X | ||||||||
| Polish | X | ||||||||
| Hungarian | X | ||||||||
| Croatian | P: 22, 23, 24 | X | |||||||
| French | C: 24 | X | |||||||
| Norwegian | P:1, 5 14, 15, 23 | P: 14, 18, 19, 20 | 1, 5, 6 | ||||||
| Chinese | 1, 5, 6 | ||||||||
| UK English | P: 4, 5, 6, 7, 8, 14, 19 | C: 24 | Was seldom changed, to rarely | 1, 2, 4, 5, 6, 7, 8 | 14 | 19 | 22 | ||
| P: 11,14, 18, 19 | |||||||||
| US English | C: 16, 17, 18, 19, 20, 21, 23 | Was seldom changed, to rarely | 1, 2, 4, 5, 6, 7, 8 | 14 | 19 | 22 | |||
| South African English | C: 9, 11, 12, 16, 17, 24 | Was seldom changed, to rarely | 1, 2, 4–8 | 14, 15 | 17, 18, 19, 20 | 22 | |||
| P: 9, 11, 19, 21 | |||||||||
| European Spanish | P: 8 | 1, 3, 4, 5, 6, 8 | 10, 11, 14 | 16, 17, 18 | 21, 23, 24 | ||||
| Mexican Spanish | C: 2 | 1, 2, 3, 4, 5, 6, 8 | 10, 11, 12, 15 | 16, 17, 18, 19, 20 | 21, 22, 23, 24 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
The International EA-QOL Group. An International Collaborative Initiative to Establish a Quality-of-Life Questionnaire for Children and Adolescents with Repair of Esophageal Atresia in 14 Countries. Children 2024, 11, 286. https://doi.org/10.3390/children11030286
The International EA-QOL Group. An International Collaborative Initiative to Establish a Quality-of-Life Questionnaire for Children and Adolescents with Repair of Esophageal Atresia in 14 Countries. Children. 2024; 11(3):286. https://doi.org/10.3390/children11030286
Chicago/Turabian StyleThe International EA-QOL Group. 2024. "An International Collaborative Initiative to Establish a Quality-of-Life Questionnaire for Children and Adolescents with Repair of Esophageal Atresia in 14 Countries" Children 11, no. 3: 286. https://doi.org/10.3390/children11030286
APA StyleThe International EA-QOL Group. (2024). An International Collaborative Initiative to Establish a Quality-of-Life Questionnaire for Children and Adolescents with Repair of Esophageal Atresia in 14 Countries. Children, 11(3), 286. https://doi.org/10.3390/children11030286







