Intertemporal Improvement in Physicians’ Perceptions of the Short-Term Adverse Outcomes of Neonatal Pain: Results of a Two-Time-Point National Survey
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Population
2.3. Methodology
2.4. Data Analysis
3. Results
3.1. Demographic Data
3.2. Physicians’ Perceptions
3.3. Multiple Regression Analysis Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzgerald, M.; Millard, C.; MacIntosh, N. Hyperalgesia in Premature Infants. Lancet 1988, 1, 292. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Tan, Z.; Chen, J.; Weidig, T.; Xu, W.; Cong, X.S. Neonatal Pain: Perceptions and Current Practice. Crit. Care Nurs. Clin. 2018, 30, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Fitri, S.Y.R.; Lusmilasari, L.; Juffrie, M.; Rakhmawati, W. Pain in Neonates: A Concept Analysis. Anesth. Pain Med. 2019, 9, e92455. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, L.A. Neonatal Pain: What’s Age Got to Do with It? Surg. Neurol. Int. 2014, 5, S479–S489. [Google Scholar] [CrossRef]
- Allinson, L.G.; Denehy, L.; Doyle, L.W.; Eeles, A.L.; Dawson, J.A.; Lee, K.J.; Spittle, A.J. Physiological Stress Responses in Infants at 29-32 Weeks’ Postmenstrual Age during Clustered Nursing Cares and Standardised Neurobehavioural Assessments. BMJ Paediatr. Open 2017, 1, e000025. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, L.A.; Murphy, N.; Karp, K.; Polomano, R.C. A Systematic Review of Behavioral and Environmental Interventions for Procedural Pain Management in Preterm Infants. J. Pediatr. Nurs. 2019, 44, 22–30. [Google Scholar] [CrossRef]
- Committee on fetus and newborn and section on anesthesiology and pain medicine prevention and management of procedural pain in the neonate: An update. Pediatrics 2016, 137, e20154271. [CrossRef]
- Balice-Bourgois, C.; Zumstein-Shaha, M.; Vanoni, F.; Jaques, C.; Newman, C.J.; Simonetti, G.D. A Systematic Review of Clinical Practice Guidelines for Acute Procedural Pain on Neonates. Clin. J. Pain 2020, 36, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.D.; Greve-Isdahl, M.; Jylli, L. The Opinions of Clinical Staff Regarding Neonatal Procedural Pain in Two Norwegian Neonatal Intensive Care Units. Acta Paediatr. 2007, 96, 1000–1003. [Google Scholar] [CrossRef]
- Agakidou, E.; Tsoni, K.; Stathopoulou, T.; Thomaidou, A.; Farini, M.; Kontou, A.; Karagianni, P.; Sarafidis, K. Changes in Physicians’ Perceptions and Practices on Neonatal Pain Management Over the Past 20 Years. A Survey Conducted at Two Time-Points. Front. Pediatr. 2021, 9, 667806. [Google Scholar] [CrossRef]
- Akuma, A.O.; Jordan, S. Pain Management in Neonates: A Survey of Nurses and Doctors. J. Adv. Nurs. 2012, 68, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Delaney, C.; Vazquez, V. Neonatal Nurses’ Perceptions of Pain Assessment and Management in NICUs: A National Survey. Adv. Neonatal. Care 2013, 13, 353–360. [Google Scholar] [CrossRef]
- Carlsen Misic, M.; Andersen, R.D.; Strand, S.; Eriksson, M.; Olsson, E. Nurses’ Perception, Knowledge, and Use of Neonatal Pain Assessment. Paediatr. Neonatal. Pain 2021, 3, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.; Loughran-Fowlds, A.; Spence, K. Neonatal Pain: A Comparison of the Beliefs and Practices of Junior Doctors and Current Best Evidence. J. Paediatr. Child Health 2010, 46, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sneyers, B.; Laterre, P.-F.; Perreault, M.M.; Wouters, D.; Spinewine, A. Current Practices and Barriers Impairing Physicians’ and Nurses’ Adherence to Analgo-Sedation Recommendations in the Intensive Care Unit—A National Survey. Crit. Care 2014, 18, 655. [Google Scholar] [CrossRef]
- Byrd, P.J.; Gonzales, I.; Parsons, V. Exploring Barriers to Pain Management in Newborn Intensive Care Units: A Pilot Survey of NICU Nurses. Adv. Neonatal. Care 2009, 9, 299–306. [Google Scholar] [CrossRef]
- Anand, K.J. Pain, Plasticity, and Premature Birth: A Prescription for Permanent Suffering? Nat. Med. 2000, 6, 971–973. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.; McGrath, P.; Ballantyne, M.; Yamada, J.; Dupuis, A.; Gibbins, S.; Franck, L.; Allen Finley, G.; Howlett, A.; Johnston, C.; et al. Influence of Risk of Neurological Impairment and Procedure Invasiveness on Health Professionals’ Management of Procedural Pain in Neonates. Eur. J. Pain 2010, 14, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Valeri, B.O.; Ranger, M.; Chau, C.M.Y.; Cepeda, I.L.; Synnes, A.; Linhares, M.B.M.; Grunau, R.E. Neonatal Invasive Procedures Predict Pain Intensity at School Age in Children Born Very Preterm. Clin. J. Pain 2016, 32, 1086–1093. [Google Scholar] [CrossRef]
- Melchior, M.; Kuhn, P.; Poisbeau, P. The Burden of Early Life Stress on the Nociceptive System Development and Pain Responses. Eur. J. Neurosci. 2022, 55, 2216–2241. [Google Scholar] [CrossRef]
- Ozdemir, H.; Bilgen, H.; Gokulu, G.; Memisoglu, A.; Ozek, E. Does Repeated Painful Stimuli Change Cerebral Near-Infrared Spectroscopy Response in Healthy, Term, Large for Gestational Age Newborns? Clin. J. Pain 2020, 36, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Banga, S.; Datta, V.; Rehan, H.S.; Bhakhri, B.K. Effect of Sucrose Analgesia, for Repeated Painful Procedures, on Short-Term Neurobehavioral Outcome of Preterm Neonates: A Randomized Controlled Trial. J. Trop. Pediatr. 2016, 62, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lindh, V.; Wiklund, U.; Håkansson, S. Assessment of the Effect of EMLA during Venipuncture in the Newborn by Analysis of Heart Rate Variability. Pain 2000, 86, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Gibbins, S.; Stevens, B.; McGrath, P.J.; Yamada, J.; Beyene, J.; Breau, L.; Camfield, C.; Finley, A.; Franck, L.; Johnston, C.; et al. Comparison of Pain Responses in Infants of Different Gestational Ages. Neonatology 2008, 93, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ramaekers, V.T.; Casaer, P.; Daniels, H. Cerebral Hyperperfusion Following Episodes of Bradycardia in the Preterm Infant. Early Hum. Dev. 1993, 34, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, M.; Gao, H.; Xu, G.; Li, F.; Zhou, J.; Zou, Y.; Jiang, H. Effect of Non-Nutritive Sucking and Sucrose Alone and in Combination for Repeated Procedural Pain in Preterm Infants: A Randomized Controlled Trial. Int. J. Nurs. Stud. 2018, 83, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Giesinger, R.E.; More, K.; Odame, J.; Jain, A.; Jankov, R.P.; McNamara, P.J. Controversies in the Identification and Management of Acute Pulmonary Hypertension in Preterm Neonates. Pediatr. Res. 2017, 82, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Vinall, J.; Miller, S.P.; Chau, V.; Brummelte, S.; Synnes, A.R.; Grunau, R.E. Neonatal Pain in Relation to Postnatal Growth in Infants Born Very Preterm. Pain 2012, 153, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.J.; Sippell, W.G.; Schofield, N.M.; Aynsley-Green, A. Does Halothane Anaesthesia Decrease the Metabolic and Endocrine Stress Responses of Newborn Infants Undergoing Operation? Br. Med. J. (Clin. Res. Ed.) 1988, 296, 668–672. [Google Scholar] [CrossRef]
- Grunau, R.E.; Weinberg, J.; Whitfield, M.F. Neonatal Procedural Pain and Preterm Infant Cortisol Response to Novelty at 8 Months. Pediatrics 2004, 114, e77–e84. [Google Scholar] [CrossRef]
- Grunau, R.E.; Haley, D.W.; Whitfield, M.F.; Weinberg, J.; Yu, W.; Thiessen, P. Altered Basal Cortisol Levels at 3, 6, 8 and 18 Months in Infants Born at Extremely Low Gestational Age. J. Pediatr. 2007, 150, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, Z.; Vlaikou, A.-M.; Theodoridou, D.; Markopoulos, G.S.; Tsoni, K.; Agakidou, E.; Drosou-Agakidou, V.; Turck, C.W.; Filiou, M.D.; Syrrou, M. Stressful Newborn Memories: Pre-Conceptual, In Utero, and Postnatal Events. Front. Psychiatry 2019, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Butkevich, I.P.; Mikhailenko, V.A.; Vershinina, E.A.; Barr, G.A. The Long-Term Effects of Neonatal Inflammatory Pain on Cognitive Function and Stress Hormones Depend on the Heterogeneity of the Adolescent Period of Development in Male and Female Rats. Front. Behav. Neurosci. 2021, 15, 691578. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, E.E.; Lönnerdal, B.; Griffin, I.J. Effects of Postnatal Growth Restriction and Subsequent Catch-up Growth on Neurodevelopment and Glucose Homeostasis in Rats. BMC Physiol. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Salekin, M.S.; Mouton, P.R.; Zamzmi, G.; Patel, R.; Goldgof, D.; Kneusel, M.; Elkins, S.L.; Murray, E.; Coughlin, M.E.; Maguire, D.; et al. Future Roles of Artificial Intelligence in Early Pain Management of Newborns. Paediatr. Neonatal. Pain 2021, 3, 134–145. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, D.; Philpotts, L.L.; Turner, D.P.; Houle, T.T.; Chen, L.; Zhang, M.; Yang, J.; Zhang, W.; Deng, H. Current State of Science in Machine Learning Methods for Automatic Infant Pain Evaluation Using Facial Expression Information: Study Protocol of a Systematic Review and Meta-Analysis. BMJ Open 2019, 9, e030482. [Google Scholar] [CrossRef]
| Total | 2000 | 2019 | p * | |
|---|---|---|---|---|
| No | 217 | 104 | 113 | |
| Sex | 0.373 | |||
| Male (n, %) | 64 (29.6) | 34 (32.7) | 30 (26.8) | |
| Female (n, %) | 152 (70.4) | 70 (67.3) | 82 (73.2) | |
| Type of NICU | 0.846 | |||
| Public (n, %) | 186 (86.1) | 89 (85.6) | 97 (86.6) | |
| Private (n, %) | 30 (13.9) | 15 (14.4) | 15 (13.4) | |
| Expertise level | 0.942 | |||
| Neonatologists (n, %) | 162 (75.0) | 77 (74.0) | 85 (75.9) | |
| Pediatricians (n, %) | 15 (6.9) | 8 (7.7) | 7 (6.3) | |
| Trainees (n, %) | 39 (18.1) | 19 (18.3) | 20 (17.9) | |
| Working years in NICU ** | 10.5 (14.5) | 12 (14.0) | 9 (15.0) | 0.899 # |
| Year of Survey | Type of NICU | Sex | Expertise Level | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain Scores | 2000 | 2019 | p * | Public | Private | p * | Male | Female | p * | Neonatologists/ Pediatricians | Trainees | p * |
| Years of work in NICU | 12 (14) | 9 (15) | 0.377 | 10 (13) | 14 (15) | 0.164 | 7 (12) | 12 (14) | 0.059 | 14 (13) | 1.5 (1) | <0.001 |
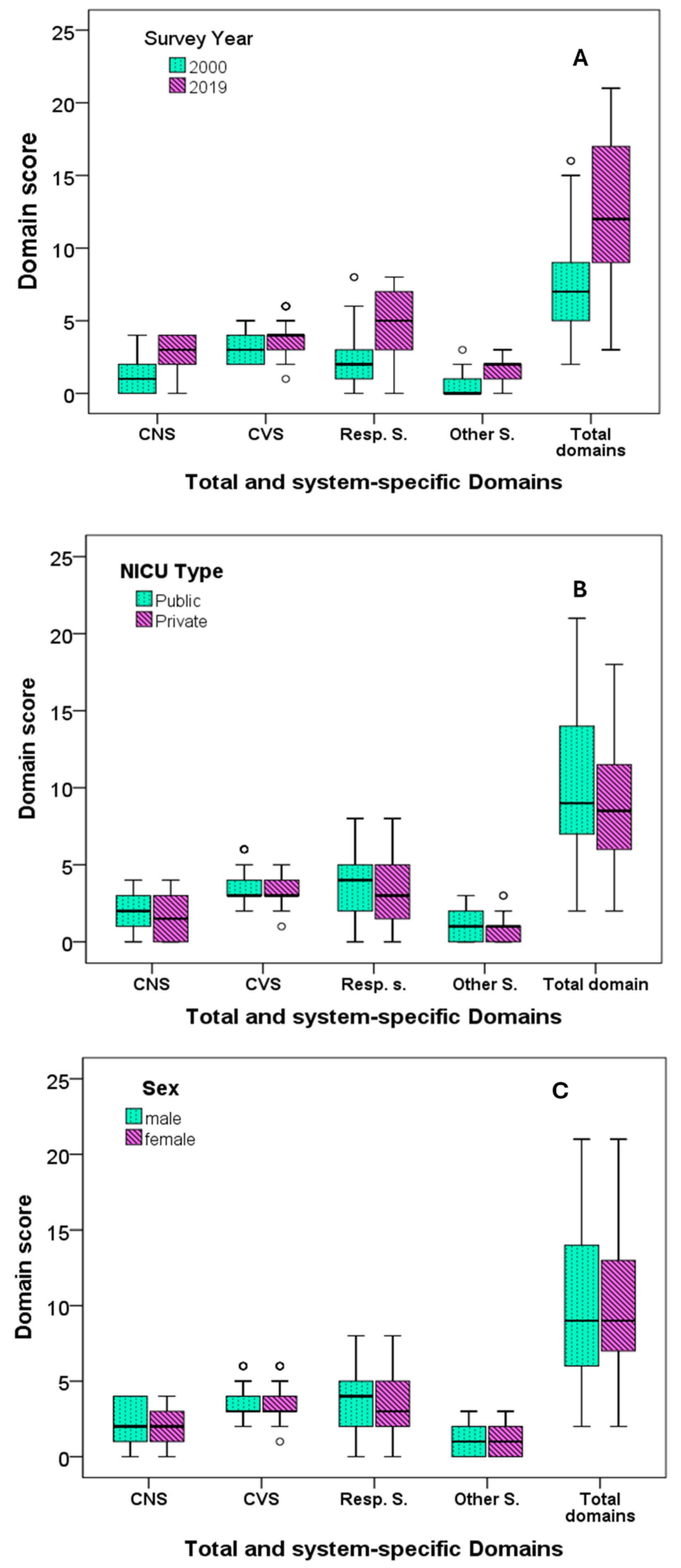
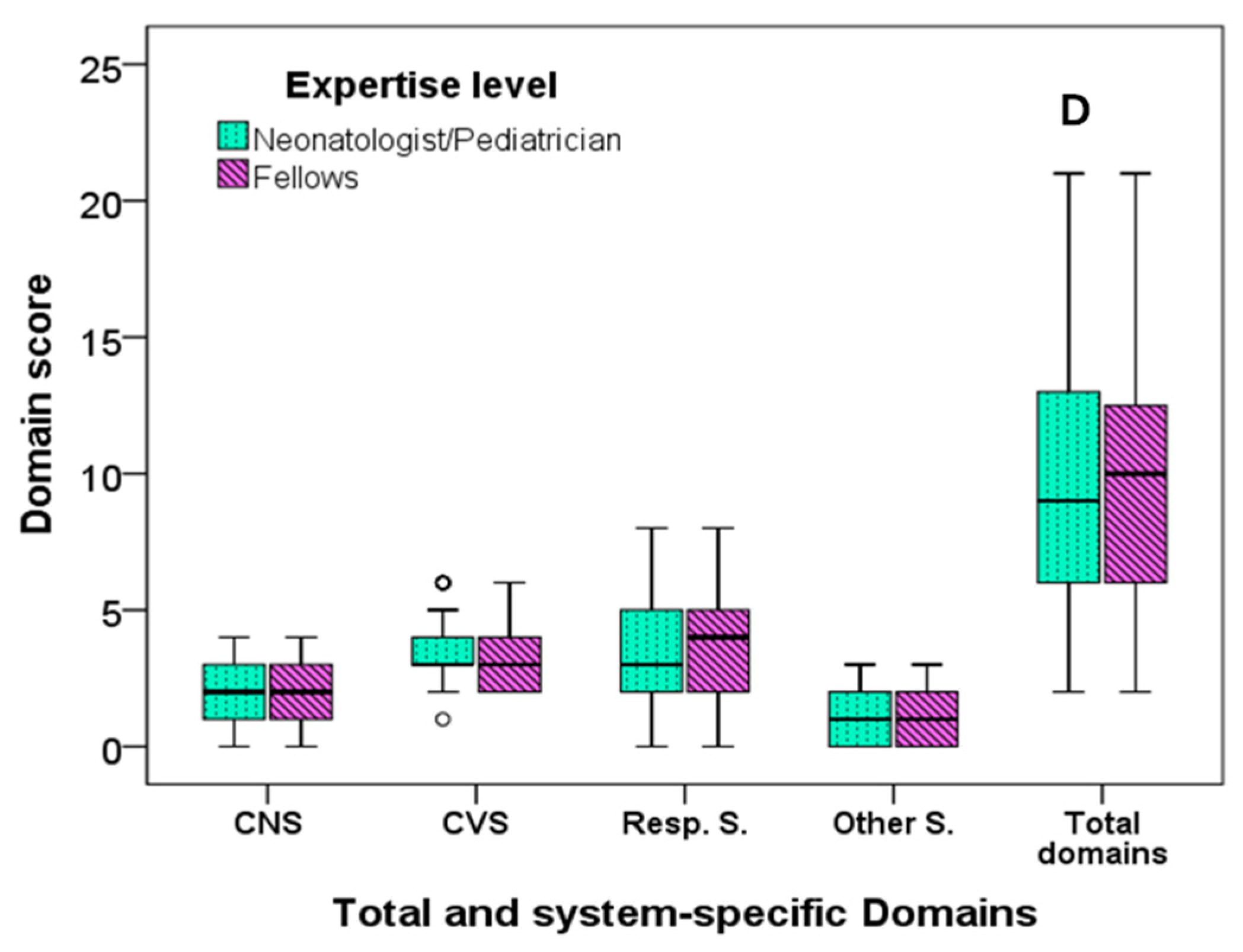
| Total score | 7 (4) | 12 (8) | <0.001 | 9 (7) | 8 (6) | 0.149 | 9 (8) | 9 (7) | 0.887 | 9 (7) | 10 (7) | 0.671 |
| Domain scores | ||||||||||||
| Central nervous system | 1 (2) | 3 (2) | <0.001 | 2 (2) | 1.5 (3) | 0.097 | 2 (3) | 2 (2) | 0.507 | 2 (2) | 2 (2) | 0.728 |
| Cardiovascular system | 3 (2) | 3 (2) | <0.001 | 3 (0) | 3 (1) | 0.334 | 3 (1) | 3 (1) | 0.891 | 3 (1) | 3 (2) | 0.183 |
| Respiratory system | 2 (2) | 5 (4) | <0.001 | 4 (3) | 3 (4) | 0.260 | 4 (3) | 3 (3) | 0.963 | 3 (3) | 4 (3) | 0.377 |
| Other systems | 0 (1) | 2 (1) | <0.001 | 1 (2) | 1 (1) | 0.494 | 1 (2) | 1 (2) | 0.939 | 1 (2) | 1 (2) | 0.608 |
| No of Domain/Item | Domains and Items | Group T1 | Group T2 | p a | OR | 95% CI | |
|---|---|---|---|---|---|---|---|
| (n = 104) | (n = 113) | Lower | Upper | ||||
| 1 | Total CNS | 61 (55) | 104 (90) | <0.001 | 0.157 | 0.075 | 0.329 |
| 1.1 | Change in cerebral blood flow | 25 (24) | 92 (80) | <0.001 | 12.320 | 6.487 | 23.414 |
| 1.2 | Cerebral hemorrhage | 14 (14) | 53 (46) | <0.001 | 5.373 | 2.749 | 10.529 |
| 1.3 | Increased intracranial pressure | 35 (34) | 64 (56) | 0.002 | 2.402 | 1.386 | 4.163 |
| 1.4 | Increased stress responses | 57 (56) | 96 (83) | <0.001 | 3.989 | 2.128 | 7.478 |
| 2 | Total cardiovascular | 80 (78) | 109 (94) | 0.001 | 0.200 | 0.078 | 0.516 |
| 2.1 | Hypertension | 30 (29) | 97 (84) | <0.001 | 12.933 | 6.691 | 25.001 |
| 2.2 | Tachycardia | 76 (74) | 98 (85) | 0.060 | 1.972 | 0.998 | 3.896 |
| 2.3 | Bradycardia | 30 (29) | 59 (51) | 0.001 | 2.529 | 1.442 | 4.433 |
| 2.4 | Heart rate fluctuation | 51 (50) | 105 (91) | <0.001 | 0.095 | 0.045 | 0.203 |
| 2.5 | Pulmonary hypertension | 13 (13) | 32 (28) | 0.007 | 2.639 | 1.297 | 5.372 |
| 2.6 | Circulatory collapse | 7 (7) | 19 (16) | 0.036 | 2.686 | 1.079 | 6.685 |
| 3 | Total respiratory | 76 (73.8) | 113 (100) | <0.001 | 0.050 | 0.012 | 0.216 |
| 3.1 | Irregular breathing pattern | 11 (11) | 73 (63) | <0.001 | 14.537 | 6.995 | 30.210 |
| 3.2 | Apneic spells | 36 (35) | 83 (72) | <0.001 | 4.827 | 2.717 | 8.577 |
| 3.3 | Respiratory distress | 7 (7) | 43 (37) | <0.001 | 8.190 | 3.482 | 19.264 |
| 3.4 | Oxygen desaturations | 61 (59) | 96 (83) | <0.001 | 3.270 | 1.756 | 6.092 |
| 3.5 | Hypoxemia | 39 (38) | 86 (75) | <0.001 | 4.896 | 2.726 | 8.686 |
| 3.6 | Ventilator asynchrony | 54 (52) | 85 (74) | 0.001 | 2.570 | 2.571 | 4.538 |
| 3.7 | Increased duration of mechanical ventilation | 10 (10) | 44 (38) | <0.001 | 5.763 | 2.715 | 12.237 |
| 3.8 | Pneumothorax | 17 (16) | 47 (41) | <0.001 | 3.497 | 1.845 | 6.627 |
| 4 | Total other systems | 31 (30) | 84 (73) | <0.001 | 0.159 | 0.088 | 0.286 |
| 4.1 | Deterioration of the clinical condition | 20 (19) | 76 (66) | <0.001 | 8.087 | 4.340 | 15.070 |
| 4.2 | Hormonal and metabolic derangement | 18 (17) | 65 (56) | <0.001 | 6.139 | 3.276 | 11.505 |
| 4.3 | Slow growth rate | 6 (6) | 47 (41) | <0.001 | 11.174 | 4.523 | 27.607 |
| % Changes | |
|---|---|
| Total score | 100 (151) |
| CNS score | 33 (100) |
| Cardiovascular score | 33 (50) |
| Respiratory score | 67 (166) |
| Other system score | 25 (125) |
| Dependent Variables (Domain Scores) | Significant Independent Variables | B | Sig. | 95% Confidence Interval | Observed Power | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| CNS | Survey year | −1.518 | <0.001 | −1.885 | −1.151 | 1.000 |
| NICU type | 0.553 | 0.036 | 0.035 | 1.070 | 0.554 | |
| Sex | 0.423 | 0.045 | 0.009 | 0.837 | 0.519 | |
| CVS | Survey year | −0.760 | <0.001 | 2.583 | 3.671 | 1.000 |
| Expertise level | 0.578 | 0.015 | 0.114 | 1.043 | 0.685 | |
| Respiratory system | Survey year | −2.802 | <0.001 | 3.228 | 5.246 | 1.000 |
| Other systems * | Survey year | −1.258 | <0.001 | 0.783 | 1.719 | 0.999 |
| Total domain sum score | Survey year | −6.337 | <0.001 | 8.560 | 12.569 | 1.000 |
| NICU type | 1.748 | 0.037 | 0.110 | 3.386 | 0.554 | |
| Expertise level | 1.607 | 0.066 | −0.106 | 3.321 | 0.453 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agakidou, E.; Kontou, A.; Stathopoulou, T.; Farini, M.; Thomaidou, A.; Tsoni, K.; Chotas, W.; Sarafidis, K. Intertemporal Improvement in Physicians’ Perceptions of the Short-Term Adverse Outcomes of Neonatal Pain: Results of a Two-Time-Point National Survey. Children 2024, 11, 471. https://doi.org/10.3390/children11040471
Agakidou E, Kontou A, Stathopoulou T, Farini M, Thomaidou A, Tsoni K, Chotas W, Sarafidis K. Intertemporal Improvement in Physicians’ Perceptions of the Short-Term Adverse Outcomes of Neonatal Pain: Results of a Two-Time-Point National Survey. Children. 2024; 11(4):471. https://doi.org/10.3390/children11040471
Chicago/Turabian StyleAgakidou, Eleni, Angeliki Kontou, Theodora Stathopoulou, Maria Farini, Agathi Thomaidou, Konstantina Tsoni, William Chotas, and Kosmas Sarafidis. 2024. "Intertemporal Improvement in Physicians’ Perceptions of the Short-Term Adverse Outcomes of Neonatal Pain: Results of a Two-Time-Point National Survey" Children 11, no. 4: 471. https://doi.org/10.3390/children11040471






