Metabolic Bone Diseases Affecting Tooth Eruption: A Narrative Review
Abstract
1. Introduction
1.1. Pre-Emergent Eruption
- colony-stimulating factor-1 (CSF-1);
- receptor activator of nuclear factor kB (RANK) as a receptor and receptor activator of nuclear factor kB-ligand (RANKL) to stimulate osteoclast precursors and induce osteoclastogenesis and osteoclast activity, enabling a major osteoclastogenic spurt to occur;
1.2. Theories of Tooth Eruption
1.3. Post-Emergent Eruption
1.4. Bone Metabolism and Tooth Eruption Disorders
2. Materials and Methods
3. Results
3.1. Nonsyndromic Disorders
3.1.1. Hyperthyroidism and Hypothyroidism
3.1.2. Hypoparathyroidism and Pseudohypoparathyroidism
3.1.3. Growth Hormone Deficiency (GHD)
3.1.4. Sickle Cell Anemia
3.2. Syndromic Disorders
3.2.1. Amelogenesis Imperfecta (AI)
3.2.2. Apert Syndrome
3.2.3. Carpenter Syndrome
3.2.4. Cherubism
3.2.5. Cleidocranial Dysostosis
3.2.6. Mucopolysaccharidosis Type I-H (MPS I-H), II (MPS II), and VI (MPS VI)
3.2.7. Ectodermal Dysplasia
3.2.8. GAPO Syndrome
3.2.9. Gardner Syndrome
3.2.10. Gorlin–Goltz Syndrome
3.2.11. McCune–Albright Syndrome (MAS)
3.2.12. Osteoglophonic Dysplasia
3.2.13. Osteopetrosis
3.2.14. Osteogenesis Imperfecta (OI)
3.2.15. Hutchinson–Gilford Syndrome (Progeria)
3.2.16. Sclerosteosis
3.2.17. Hypophosphatemic Rickets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Amelogenesis Imperfecta |
| APC | Adenomatous Polyposis Coli |
| BMPs | Bone Morphogenic Proteins |
| cAMP | 3′-5′ Cyclic Adenosine Monophosphate |
| CNS | Central Nervous System |
| CSF-1 | Colony-Stimulating Factor-1 |
| DF | Dental Follicle |
| DTE | Delayed TE |
| FGF | Fibroblast Growth Factor |
| FGFR | Fibroblast Growth Factor Receptor |
| FTE | Failure Of TE |
| GH | Growth Hormone |
| GHD | Growth Hormone Deficiency |
| HPSCT | Hematopoietic Stem Cell Transplant |
| IL-1α | Interleukin-1α |
| MAS | McCune –Albright Syndrome |
| MBD | Metabolic Bone Diseases |
| MFE | Mechanical Failure of Eruption |
| MCP-1 | Monocyte Chemotactic Protein-1 |
| NGF | Nerve Growth Factor |
| OI | Osteogenesis Imperfecta |
| OPG | Osteoprotegerin |
| PDL | Periodontal Ligament |
| PFE | Primary Failure of Eruption |
| PHEX | Phosphate-Regulating Endopeptidase X-Linked |
| PRR | Preventive Resin Restoration |
| PTH | Parathyroid Hormone |
| PTHrp | Parathyroid Hormone-Related Protein |
| PTCH1 | Protein Patched Homolog 1 |
| RANK | Receptor Activator of Nuclear Factor Kb |
| RANKL | Receptor Activator of Nuclear Factor Kb-Ligand |
| REE | Reduced Enamel Epithelium |
| TE | Tooth Eruption |
| TGF-β | Transforming Growth Factor-Beta |
| TNF-α | Tumor Necrosis Factor-a |
References
- Karadayi, B.; Afsin, H.; Ozaslan, A.; Karadayi, S. Development of dental charts according to tooth development and eruption for Turkish children and young adults. Imaging Sci. Dent. 2014, 44, 103–113. [Google Scholar] [CrossRef][Green Version]
- Wise, G.E. Cellular and molecular basis of tooth eruption. Orthod. Craniofac. Res. 2009, 12, 67–73. [Google Scholar] [CrossRef]
- Suri, L.; Gagari, E.; Vastardis, H. Delayed tooth eruption: Pathogenesis, diagnosis, and treatment. A literature review. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 432–445. [Google Scholar] [CrossRef]
- Oton-Gonzalez, L.; Mazziotta, C.; Iaquinta, M.R.; Mazzoni, E.; Nocini, R.; Trevisiol, L.; D’Agostino, A.; Tognon, M.; Rotondo, J.C.; Martini, F. Genetics and epigenetics of bone remodeling and metabolic bone diseases. Int. J. Mol. Sci. 2022, 23, 1500. [Google Scholar] [CrossRef]
- Dorotheou, D.; Gkantidis, N.; Karamolegkou, M.; Kalyvas, D.; Kiliaridis, S.; Kitraki, E. Tooth eruption: Altered gene expression in the dental follicle of patients with cleidocranial dysplasia. Orthod. Craniofac. Res. 2013, 16, 20–27. [Google Scholar] [CrossRef]
- Dalben Gda, S.; das Neves, L.T.; Gomide, M.R. Oral findings in patients with Apert syndrome. J. Appl. Oral Sci. 2006, 14, 465–469. [Google Scholar] [CrossRef]
- Cardoso, I.L.; Gazelle, A. Gardner Syndrome: Complications/manifestations in the oral cavity and their relationship with oral health. J. Dent. Open Access 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Collins, M.A.; Mauriello, S.M.; Tyndall, D.A.; Wright, J.T. Dental anomalies associated with amelogenesis imperfecta: A radiographic assessment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 358–364. [Google Scholar] [CrossRef]
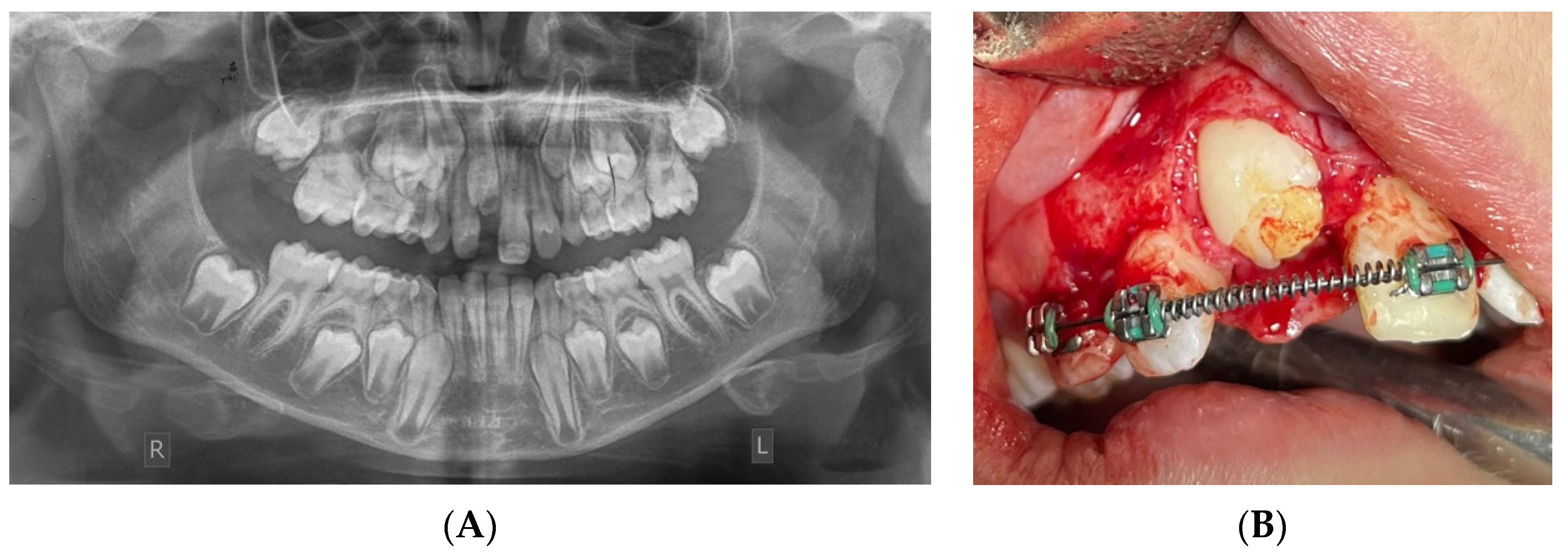
- Hanisch, M.; Hanisch, L.; Kleinheinz, J.; Jung, S. Primary failure of eruption (PFE): A systematic review. Head Face Med. 2018, 14, 5. [Google Scholar] [CrossRef]
- Sharma, G.; Kneafsey, L.; Ashley, P.; Noar, J. Failure of eruption of permanent molars: A diagnostic dilemma. Int. J. Paediatr. Dent. 2016, 26, 91–99. [Google Scholar] [CrossRef]
- Eşian, D.; Bica, C.I.; Stoica, O.E.; Dako, T.; Vlasa, A.; Bud, E.S.; Salcudean, D.; Beresescu, L. Prevalence and Manifestations of Dental Ankylosis in Primary Molars Using Panoramic X-rays: A Cross-Sectional Study. Children 2022, 9, 1188. [Google Scholar] [CrossRef]
- Proffit, W.R.; Frazier-Bowers, S.A. Mechanism and control of tooth eruption: Overview and clinical implications. Orthod. Craniofac. Res. 2009, 12, 59–66. [Google Scholar] [CrossRef]
- Bastos, V.C.; Gomez, R.S.; Gomes, C.C. Revisiting the Human Dental Follicle: From Tooth Development to Its Association with Unerupted or Impacted Teeth and Pathological Changes. Dev. Dyn. 2022, 251, 408–423. [Google Scholar] [CrossRef]
- Marks, S.C.; Schroeder, H.E. Tooth eruption: Theories and facts. Anat. Rec. 1996, 245, 374–393. [Google Scholar] [CrossRef]
- Fukushima, H.; Kajiya, H.; Takada, K.; Okamoto, F.; Okabe, K. Expression and role of RANKL in periodontal ligament cells during physiological root-resorption in human deciduous teeth. Eur. J. Oral Sci. 2003, 111, 346–352. [Google Scholar] [CrossRef]
- Anderson, D.M.; Maraskovsky, E.; Billingsley, W.L.; Dougall, W.C.; Tometsko, M.E.; Roux, E.R.; Teepe, M.C.; DuBose, R.F.; Cosman, D.; Galibert, L. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 1997, 390, 175–179. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.-i.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Ten Cate, A.R.; Nanci, A. Ten Cate’s Oral Histology: Development, Structure, and Function; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Yamaguchi, T.; Hosomichi, K.; Shirota, T.; Miyamoto, Y.; Ono, W.; Ono, N. Primary Failure of Tooth Eruption: Etiology and Management. Jpn. Dent. Sci. Rev. 2022, 58, 258–267. [Google Scholar] [CrossRef]
- Nel, S.; Hendrik, H.; Boy, S.; Raubenheimer, E. Recent perspectives vis-à-vis the biological basis of tooth eruption: Clinical review. S. Afr. Dent. J. 2015, 70, 238–241. [Google Scholar]
- Bhaskar, S.N.; Kumar, G.S. Orban’s Oral Histology & Embryology-E-Book, 14th ed.; Elsevier: New Delhi, India, 2015. [Google Scholar]
- Kv, S.; Sangeetha, R.; Santana, N.; Priya, G.; Kumari, M.; Murali, P.; Gayathri, V.S. The Tooth Eruption and Its Abnormalities—A Narrative Review. SRM J. Res. Dent. Sci. 2022, 13, 109. [Google Scholar] [CrossRef]
- Risinger, R.K.; Proffit, W.R. Continuous overnight observation of human premolar eruption. Arch. Oral Biol. 1996, 41, 779–789. [Google Scholar] [CrossRef]
- Shimada, A.; Komatsu, K.; Chiba, M. Effects of local injections of vasoactive drugs on eruption rate of incisor teeth in anaesthetized rats. Arch. Oral Biol. 2006, 51, 449–456. [Google Scholar] [CrossRef]
- Cheek, C.C.; Paterson, R.L.; Proffit, W.R. Response of erupting human second premolars to blood flow changes. Arch. Oral Biol. 2002, 47, 851–858. [Google Scholar] [CrossRef]
- Shimada, A.; Shibata, T.; Komatsu, K. Relationship between the tooth eruption and regional blood flow in angiotensin II-induced hypertensive rats. Arch. Oral Biol. 2004, 49, 427–433. [Google Scholar] [CrossRef]
- Wise, G.; King, G. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008, 87, 414–434. [Google Scholar] [CrossRef]
- Kim, T.; Bae, C.; Lee, J.; Ko, S.; Yang, X.; Jiang, R.; Cho, E. β-catenin is required in odontoblasts for tooth root formation. J. Dent. Res. 2013, 92, 215–221. [Google Scholar] [CrossRef]
- Shapira, Y.; Kuftinec, M.M. Rootless eruption of a mandibular permanent canine. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 563–566. [Google Scholar] [CrossRef]
- Wang, X.P. Tooth eruption without roots. J. Dent. Res. 2013, 92, 212–214. [Google Scholar]
- Berkovitz, B.K.B.; Moxham, B.J.; Newman, H.N. The Periodontal Ligament in Health and Disease, 2nd ed.; Mosby-Wolfe: London, UK, 1995. [Google Scholar]
- McCulloch, C.A.G.; Lekic, P.; McKee, M.D. Role of Physical Forces in Regulating the Form and Function of the Periodontal Ligament. Periodontology 2000, 24, 56–72. [Google Scholar] [CrossRef]
- Kook, S.H.; Hwang, J.M.; Park, J.S.; Kim, E.M.; Heo, J.S.; Jeon, Y.M.; Lee, J.C. Mechanical Force Induces Type I Collagen Expression in Human Periodontal Ligament Fibroblasts through Activation of ERK/JNK and AP-1. J. Cell. Biochem. 2009, 106, 1060–1067. [Google Scholar] [CrossRef]
- Weinreb, M.; Gal, D.; Weinreb, M.; Pitaru, S. Changes in the shape and orientation of periodontal ligament fibroblasts in the continuously erupting rat incisor following removal of the occlusal load. J. Dent. Res. 1997, 76, 1660–1666. [Google Scholar] [CrossRef]
- Marks, S., Jr.; Larson, E.; Wise, G.; Gorski, J. Collagen metabolism and tooth eruption: The effects of sodium morrhuate infusions on premolar eruption in dogs. Schweiz. Monatsschrift Fur Zahnmed. = Rev. Mens. Suisse D’odonto-Stomatol. = Riv. Mens. Svizz. Di Odontol. E Stomatol. 1995, 105, 1029–1032. [Google Scholar]
- Vaahtokari, A.; Vainio, S.; Thesleff, I. Associations between transforming growth factor β1 RNA expression and epithelial–mesenchymal interactions during tooth morphogenesis. Development 1991, 113, 985–994. [Google Scholar] [CrossRef]
- Wise, G.; Frazier-Bowers, S.; D’souza, R. Cellular, molecular, and genetic determinants of tooth eruption. Crit. Rev. Oral Biol. Med. 2002, 13, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Wise, G.E.; Marks, S.C., Jr.; Zhao, L. Effect of CSF-1 on in vivo expression of c-fos in the dental follicle during tooth eruption. Eur. J. Oral Sci. 1998, 106, 397–400. [Google Scholar] [CrossRef]
- Wise, G.; Zhao, L.; Grier IV, R. Localization and expression of CSF-1 receptor in rat dental follicle cells. J. Dent. Res. 1997, 76, 1244–1249. [Google Scholar] [CrossRef]
- Wise, G.; Lin, F.; Zhao, L. Transcription and translation of CSF-1 in the dental follicle. J. Dent. Res. 1995, 74, 1551–1557. [Google Scholar] [CrossRef]
- Wise, G.E.; Que, B.G.; Huang, H.; Lumpkin, S.J. Enhancement of gene expression in rat dental follicle cells by parathyroid hormone-related protein. Arch. Oral Biol. 2000, 45, 903–909. [Google Scholar] [CrossRef]
- Yao, S.; Prpic, V.; Pan, F.; Wise, G.E. TNF-α Upregulates Expression of BMP-2 and BMP-3 Genes in the Rat Dental Follicle—Implications for Tooth Eruption. Connect. Tissue Res. 2010, 51, 59–66. [Google Scholar] [CrossRef]
- Roulias, P.; Kalantzis, N.; Doukaki, D.; Pachiou, A.; Karamesinis, K.; Damanakis, G.; Gizani, S.; Tsolakis, A.I. Teeth Eruption Disorders: A Critical Review. Children 2022, 9, 771. [Google Scholar] [CrossRef]
- Malmgren, B.; Andersson, K.; Lindahl, K.; Kindmark, A.; Grigelioniene, G.; Zachariadis, V.; Dahllof, G.; Astrom, E. Tooth agenesis in osteogenesis imperfecta related to mutations in the collagen type I genes. Oral Dis. 2017, 23, 42–49. [Google Scholar] [CrossRef]
- Klein, O.D.; Oberoi, S.; Huysseune, A.; Hovorakova, M.; Peterka, M.; Peterkova, R. Developmental Disorders of the Dentition: An Update. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163C, 318–332. [Google Scholar] [CrossRef]
- Crawford, P.; Aldred, M. Anomalies of tooth formation and eruption. Paediatr. Dent. 2012, 295–318, 4th ed. [Google Scholar]
- Rasmussen, P.; Kotsaki, A. Inherited retarded eruption in the permanent dentition. J. Clin. Pediatr. Dent. 1997, 21, 205–211. [Google Scholar]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Brief communication: The London atlas of human tooth development and eruption. Am. J. Phys. Anthropol. 2010, 142, 481–490. [Google Scholar] [CrossRef]
- Doubleday, A.R.; Sippel, R.S. Hyperthyroidism. Gland. Surg. 2020, 9, 124–135. [Google Scholar] [CrossRef]
- Little, J.W. Thyroid disorders. Part II: Hypothyroidism and thyroiditis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 102, 148–153. [Google Scholar] [CrossRef]
- Clarke, B.L.; Brown, E.M.; Collins, M.T.; Jüppner, H.; Lakatos, P.; Levine, M.A.; Mannstadt, M.M.; Bilezikian, J.P.; Romanischen, A.F.; Thakker, R.V. Epidemiology and Diagnosis of Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2016, 101, 2284–2299. [Google Scholar] [CrossRef]
- Ucciferro, P.; Anastasopoulou, C. Pseudohypoparathyroidism. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Sizonenko, P.C.; Clayton, P.E.; Cohen, P.; Hintz, R.L.; Tanaka, T.; Laron, Z. Diagnosis and management of growth hormone deficiency in childhood and adolescence. Part 1: Diagnosis of growth hormone deficiency. Growth Horm. IGF Res. 2001, 11, 137–165. [Google Scholar] [CrossRef]
- Thomson, A.M.; McHugh, T.A.; Oron, A.P.; Teply, C.; Lonberg, N.; Vilchis Tella, V.; Wilner, L.B.; Fuller, K.; Hagins, H.; Aboagye, R.G.; et al. Global, regional, and national prevalence and mortality burden of sickle cell disease, 2000–2021: A systematic analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- Bochukova, E.; Schoenmakers, N.; Agostini, M.; Schoenmakers, E.; Rajanayagam, O.; Keogh, J.M.; Henning, E.; Reinemund, J.; Gevers, E.; Sarri, M. A mutation in the thyroid hormone receptor alpha gene. N. Engl. J. Med. 2012, 366, 243–249. [Google Scholar] [CrossRef]
- Vitalle, M.S.d.S.; Weiler, R.M.E.; Niskier, S.R.; Braga, J.A.P. Delayed tooth eruption in an adolescent with hypothyroidism. Rev. Paul. Pediatr. 2012, 30, 613–616. [Google Scholar] [CrossRef][Green Version]
- Little, J.W. Thyroid disorders. Part I: Hyperthyroidism. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 276–284. [Google Scholar] [CrossRef]
- Carlos, L.; Jimenez Soriano, Y.; Sarrion Perez, M.G. Dental management of patients with endocrine disorders. J. Clin. Exp. Dent. 2010, 2, e196–e203. [Google Scholar] [CrossRef]
- Pinto, A.; Glick, M. Management of patients with thyroid disease: Oral health considerations. J. Am. Dent. Assoc. 2002, 133, 849–858. [Google Scholar] [CrossRef]
- Kamarthi, N.; Venkatraman, S.; Patil, P.B. Dental findings in the diagnosis of idiopathic hypoparathyroidism. Ann. Saudi Med. 2013, 33, 411–413. [Google Scholar] [CrossRef]
- Reis, M.T.A.; Matias, D.T.; de Faria, M.E.J.; Martin, R.M. Failure of tooth eruption and brachydactyly in pseudohypoparathyroidism are not related to plasma parathyroid hormone-related protein levels. Bone 2016, 85, 138–141. [Google Scholar] [CrossRef]
- Hejlesen, J.; Underbjerg, L.; Gjørup, H.; Bloch-Zupan, A.; Sikjaer, T. Dental findings in patients with non-surgical hypoparathyroidism and pseudohypoparathyroidism: A systematic review. Front. Physiol. 2018, 9, 376009. [Google Scholar] [CrossRef]
- Ayuk, J.; Sheppard, M.C. Growth hormone and its disorders. Postgrad. Med. J. 2006, 82, 24–30. [Google Scholar] [CrossRef]
- Torlińska-Walkowiak, N.; Majewska, K.A.; Kędzia, A.; Opydo-Szymaczek, J. Clinical Implications of Growth Hormone Deficiency for Oral Health in Children: A Systematic Review. J. Clin. Med. 2021, 10, 3733. [Google Scholar] [CrossRef]
- Atreja, G.; Atreja, S.H.; Jain, N.; Sukhija, U. Oral manifestations in growth hormone disorders. Indian J. Endocrinol. Metab. 2012, 16, 381–383. [Google Scholar] [CrossRef]
- Partyka, M.; Chałas, R.; Dunin-Wilczyńska, I.; Drohomyretska, M.; Klatka, M. Influence of Growth Hormone Therapy on Selected Dental and Skeletal System Parameters. Ann. Agric. Environ. Med. 2018, 25, 60–65. [Google Scholar] [CrossRef]
- Girgis, S.; Cheng, L.; Tsitsikas, D.; Sproat, C. Orofacial manifestations of sickle cell disease: Implications for dental clinicians. Br. Dent. J. 2021, 230, 143–147. [Google Scholar] [CrossRef]
- Kelleher, M.; Bishop, K.; Briggs, P. Oral complications associated with sickle cell anemia: A review and case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1996, 82, 225–228. [Google Scholar] [CrossRef]
- Bin Saleh, S.S. Etiology, Classification, and Restorative Management of Amelogenesis Imperfecta Among Children and Young Adults: A Scoping Review. Cureus 2023, 15, e49968. [Google Scholar] [CrossRef]
- Conrady, C.D.; Patel, B.C.; Sharma, S. Apert Syndrome. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bouaré, F.; Noureldine, M.H.A.; Hajhouji, F.; Ghannane, H.; Jallo, G.I.; Ait Benali, S. Complex craniosynostosis in the context of Carpenter’s syndrome. Childs Nerv. Syst. 2022, 38, 831–835. [Google Scholar] [CrossRef]
- Kannu, P.; Baskin, B.; Bowdin, S. Cherubism; University of Washington: Seattle, WA, USA, 2018. [Google Scholar]
- Kutilek, S.; Machytka, R.; Munzar, P. Cleidocranial dysplasia. Sudan. J. Paediatr. 2019, 19, 165–168. [Google Scholar] [CrossRef]
- Sakuru, R.; Bollu, P.C. Hurler syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Çelik, B.; Tomatsu, S.C.; Tomatsu, S.; Khan, S.A. Epidemiology of Mucopolysaccharidoses Update. Diagnostics 2021, 11, 273. [Google Scholar] [CrossRef]
- D’Avanzo, F.; Zanetti, A.; De Filippis, C.; Tomanin, R. Mucopolysaccharidosis Type VI, an Updated Overview of the Disease. Int. J. Mol. Sci. 2021, 22, 13456. [Google Scholar] [CrossRef]
- Majmundar, V.D.; Baxi, K. Ectodermal Dysplasia; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Falcone, M.M.; Chang, Y.H.; Lidov, H.; Stagner, A.M.; Dagi, L.R. Two siblings with GAPO syndrome: Ophthalmic presentation and histopathologic findings. Ophthalmic Genet. 2023, 44, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Charifa, A.; Jamil, R.T.; Sathe, N.C.; Zhang, X. Gardner Syndrome. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Spiker, A.M.; Troxell, T.; Ramsey, M.L. Gorlin Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2017. [Google Scholar]
- Dumitrescu, C.E.; Collins, M.T. McCune-Albright syndrome. Orphanet J. Rare Dis. 2008, 3, 12. [Google Scholar] [CrossRef]
- Bailey, J.R.; Tapscott, D.C. Osteopetrosis. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Marini, J.C.; Forlino, A.; Bachinger, H.P.; Bishop, N.J.; Byers, P.H.; Paepe, A.; Fassier, F.; Fratzl-Zelman, N.; Kozloff, K.M.; Krakow, D.; et al. Osteogenesis imperfecta. Nat. Rev. Dis. Primers 2017, 3, 17052. [Google Scholar] [CrossRef]
- Gordon, L.B.; Rothman, F.G.; López-Otín, C.; Misteli, T. Progeria: A paradigm for translational medicine. Cell 2014, 156, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; Barnard, A.; Hamersma, H.; van der Wouden, A. The syndromic status of sclerosteosis and van Buchem disease. Clin. Genet. 1984, 25, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sandy, J.L.; Nunez, C.; Wheeler, B.J.; Jefferies, C.; Morris, A.; Siafarikas, A.; Rodda, C.P.; Simm, P.; Biggin, A.; Aum, S.; et al. Prevalence and characteristics of paediatric X-linked hypophosphataemia in Australia and New Zealand: Results from the Australian and the New Zealand Paediatric Surveillance Units survey. Bone 2023, 173, 116791. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, L.; Pereira, A.M.; Jahangiri, L.; Choi, M. Management of amelogenesis imperfecta in adolescent patients: Clinical report. J. Prosthodont. 2019, 28, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Aren, G.; Ozdemir, D.; Firatli, S.; Uygur, C.; Sepet, E.; Firatli, E. Evaluation of oral and systemic manifestations in an amelogenesis imperfecta population. J. Dent. 2003, 31, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.K.; Smales, R.J. Oral rehabilitation of young adults with amelogenesis imperfecta. Int. J. Prosthodont. 2003, 16, 345–349. [Google Scholar]
- Atasu, M.; Biren, S.; Mumcu, G. Hypocalcification type amelogenesis imperfecta in permanent dentition in association with heavily worn primary teeth, gingival hyperplasia, hypodontia and impacted teeth. J. Clin. Pediatr. Dent. 1999, 23, 117–121. [Google Scholar]
- Ray, J.G.; Dutta, S.; Sarangi, S.; Yadav, P. Noneruption of Teeth in Amelogenesis Imperfecta: A Report of Two Cases and Review. J. Oral Maxillofac. Pathol. 2022, 26, 254–258. [Google Scholar] [CrossRef]
- Kaloust, S.; Ishii, K.; Vargervik, K. Dental Development in Apert Syndrome. Cleft Palate Craniofac. J. 1997, 34, 117–121. [Google Scholar] [CrossRef]
- Hohoff, A.; Joos, U.; Meyer, U.; Ehmer, U.; Stamm, T. The spectrum of Apert syndrome: Phenotype, particularities in orthodontic treatment, and characteristics of orthognathic surgery. Head Face Med. 2007, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Renier, D.; Lajeunie, E.; Arnaud, E.; Marchac, D. Management of craniosynostoses. Child’s Nerv. Syst. 2000, 16, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Droubi, L.; Laflouf, M.; Tolibah, Y.A.; Comisi, J.C. Apert Syndrome: Dental Management Considerations and Objectives. J. Oral Biol. Craniofac. Res. 2022, 12, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Shimo, T.; Irie, K. Sonic Hedgehog Signaling and Tooth Development. Int. J. Mol. Sci. 2020, 21, 1587. [Google Scholar] [CrossRef] [PubMed]
- Blankenstein, R.; Brook, A.H.; Smith, R.N.; Patrick, D.; Russell, J.M. Oral findings in Carpenter Syndrome. Int. J. Paediatr. Dent. 2001, 11, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, S.; Helman, S.; Healy, N.; Saman, M.; Wood-Smith, D. Carpenter Syndrome: A Review for the Craniofacial Surgeon. J. Craniofac. Surg. 2014, 25, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Mundlos, S. Cleidocranial dysplasia: Clinical and molecular genetics. J. Med. Genet. 1999, 36, 177–182. [Google Scholar] [PubMed]
- Kolokitha, O.-E.G.; Papadopoulou, A.K. Cleidocranial dysplasia: Etiology, clinical characteristics, diagnostic information and treatment approach. Hell. Orthod. Rev. 2008, 11, 21–33. [Google Scholar]
- Mohamed, S.; He, Q.Q.; Singh, A.A.; Ferro, V. Mucopolysaccharidosis type II (Hunter syndrome): Clinical and biochemical aspects of the disease and approaches to its diagnosis and treatment. Adv. Carbohydr. Chem. Biochem. 2020, 77, 71–117. [Google Scholar] [CrossRef]
- Hirst, L.; Mubeen, S.; Abou-Ameira, G.; Chakrapani, A. Mucopolysaccharidosis (MPS): Review of the literature and case series of five pediatric dental patients. Clin. Case Rep. 2021, 9, 1704–1710. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Barros, C.; Ferrao, J.; Machado, M.D.C.; Fernandes, A.; Proenca, F. Hurler Syndrome: Orofacial Clinical Findings. Cureus 2023, 15, e33313. [Google Scholar] [CrossRef] [PubMed]
- Balaji, M.S.; Sundaram, R.K.; Karthik, P.; Asokan, K. Pycnodysostosis: A bone dysplasia with unusual oral manifestation. Indian J. Dent. 2014, 5, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Hekmatfar, S.; Jafari, K.; Meshki, R.; Badakhsh, S. Dental management of ectodermal dysplasia: Two clinical case reports. J. Dent. Res. Dent. Clin. Dent. Prospect. 2012, 6, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kearns, G.; Sharma, A.; Perrott, D.; Schmidt, B.; Kaban, L.; Vargervik, K. Placement of endosseous implants in children and adolescents with hereditary ectodermal dysplasia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 1999, 88, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, G. GAPO syndrome: A new case. Am. J. Med. Genet. 1995, 58, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Ng Cw, B.; Zhu, H.; Liu, J.; Lin, Y. Bone and dental abnormalities as first signs of familial Gardner’s syndrome in a Chinese family: A literature review and a case report. Med. Sci. 2018, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Nilesh, K.; Tewary, S.; Zope, S.; Patel, J.; Vande, A. Dental, dermatological and radiographic findings in a case of Gorlin-Goltz Syndrome: Report and review. Pan Afr. Med. J. 2017, 27, 96. [Google Scholar] [CrossRef] [PubMed]
- Machado Cde, V.; Knop, L.A.; da Rocha, M.C.; Telles, P.D. Impacted permanent incisors associated with compound odontoma. BMJ Case Rep. 2015, 2015, bcr2014208201. [Google Scholar] [CrossRef] [PubMed]
- Aravinda, K.; Ratnakar, P.; Srinivas, K. Oral manifestations of McCune-Albright syndrome. Indian J. Endocrinol. Metab. 2013, 17, 170–173. [Google Scholar] [CrossRef]
- Akintoye, S.O.; Boyce, A.M.; Collins, M.T. Dental perspectives in fibrous dysplasia and McCune-Albright syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e149–e155. [Google Scholar] [CrossRef]
- Xavier, S.P.; Ribeiro, M.C.; Sicchieri, L.G.; Brentegani, L.G.; Lacerda, S.A. Clinical, microscopic and imaging findings associated to McCune-Albright syndrome: Report of two cases. Braz. Dent. J. 2008, 19, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.S.; Stephen, L.; Beighton, P. Osteoglophonic dysplasia: Dental and orthodontic implications. Orthod. Craniofac. Res. 2006, 9, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Marzin, P.; Baujat, G.; Gensburger, D.; Huber, C.; Bole, C.; Panuel, M.; Finidori, G.; De la Dure, M.; Cormier-Daire, V. Heterozygous FGFR1 mutation may be responsible for an incomplete form of osteoglophonic dysplasia, characterized only by radiolucent bone lesions and teeth retentions. Eur. J. Med. Genet. 2020, 63, 103729. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.K.; Sándor, G.K.; Holmes, H.I.; Carmichael, R.P.; Clokie, C.M. Marble bone disease: A review of osteopetrosis and its oral health implications for dentists. J. Can. Dent. Assoc. 2007, 73, 839–844. [Google Scholar] [PubMed]
- Stark, Z.; Savarirayan, R. Osteopetrosis. Orphanet J. Rare Dis. 2009, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Rabenhorst, B.M.; Morello, R. Management of endocrine disease: Osteogenesis imperfecta: An update on clinical features and therapies. Eur. J. Endocrinol. 2020, 183, R95–R106. [Google Scholar] [CrossRef]
- Sillence, D.O.; Rimoin, D.L.; Danks, D.M. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig. Artic. Ser. 1979, 15, 113–129. [Google Scholar] [PubMed]
- Malmgren, B.; Norgren, S. Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol. Scand. 2002, 60, 65–71. [Google Scholar] [CrossRef]
- Cantero, N.D.R.; Martínez, M.R.M.; Cardelús, B.S.; García, J.M.D.N. Influence of zoledronic acid and pamidronate on tooth eruption in children with osteogenesis imperfecta. Bone 2024, 182, 117069. [Google Scholar] [CrossRef]
- Gordon, L.B.; Brown, W.T.; Collins, F.S. Hutchinson-Gilford Progeria Syndrome; University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Pollex, R.; Hegele, R.A. Hutchinson–Gilford progeria syndrome. Clin. Genet. 2004, 66, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Hazan-Molina, H.; Dror, A.D. Treatment considerations in Hutchinson-Gilford progeria syndrome: A case report. J. Clin. Pediatr. Dent. 2015, 39, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Stephen, L.; Hamersma, H.; Gardner, J.; Beighton, P. Dental and oral manifestations of sclerosteosis. Int. Dent. J. 2001, 51, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.C.; van Bezooijen, R.L.; Mervis, B.; Hamdy, N.A.; Lowik, C.W.; Hamersma, H.; Beighton, P.; Papapoulos, S.E. Bone mineral density in sclerosteosis; affected individuals and gene carriers. J. Clin. Endocrinol. Metab. 2005, 90, 6392–6395. [Google Scholar] [CrossRef]
- Rabbani, A.; Rahmani, P.; Ziaee, V.; Ghodoosi, S. Dental problems in hypophosphatemic rickets, a cross-sectional study. Iran. J. Pediatr. 2012, 22, 531. [Google Scholar]
- Murayama, T.; Iwatsubo, R.; Akiyama, S.; Amano, A.; Morisaki, I. Familial hypophosphatemic vitamin D-resistant rickets: Dental findings and histologic study of teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2000, 90, 310–316. [Google Scholar] [CrossRef]



| Nonsyndromic Disorders | Incidence (Births) | Tooth Eruption Disorder |
|---|---|---|
| Hyperthyroidism | 1:50–200 [48] | Accelerated TE |
| Hypothyroidism | 1:4000–10,000 [49] | DTE |
| Hypoparathyroidism | 2.3–3:100,000 [50] | DTE |
| Pseudohypoparathyroidism | 0.3–1.1:100,000 [51] | DTE |
| Growth Hormone Deficiency | 1:4000–10,000 [52] | DTE |
| Sickle Cell Anemia | 515,000 (425,000–614,000) births in 2021 [53] | DTE |
| Syndromic Disorders | Incidence (Births) | Tooth Eruption Disorder |
|---|---|---|
| Amelogenesis Imperfecta | 1:700–14,000 [69] | FTE, DTE |
| Apert Syndrome | 1:65,000–200,000 [70] | DTE |
| Carpenter Syndrome | 1:1,000,000 [71] | DTE |
| Cherubism | ~300 cases [72] | FTE, DTE |
| Cleidocranial Dysostosis | 1:1,000,000 [73] | DTE |
| MPS I-H | 1:100,000 [74] | DTE |
| MPS II | 1:100,000 [75] | DTE |
| MPS VI | 0.36–1.3:100,000 [76] | DTE |
| Ectodermal Dysplasia | 7:10,000 [77] | DTE |
| GAPO Syndrome | ~60 cases [78] | FTE |
| Gardner Syndrome | 1:7000–30,000 [79] | DTE |
| Gorlin–Goltz Syndrome | 1:40,000–60,000 [80] | DTE |
| McCune–Albright Syndrome | 1:100,000–1,000,000 [81] | DTE |
| Osteoglophonic Dysplasia | Unknown | FTE, DTE |
| Osteopetrosis | 1:20,000–250,000 [82] | FTE, DTE |
| Osteogenesis Imperfecta | 0.2–0.7:10,000 [83] | DTE |
| Progeria | 1:20,000,000 [84] | DTE |
| Sclerosteosis | 1:60,000 (Afrikaner) [85] | DTE |
| Hypophosphatemic Rickets | 1:20,000–200,000 [86] | DTE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, C.I.; Sifakakis, I.; Tournis, S. Metabolic Bone Diseases Affecting Tooth Eruption: A Narrative Review. Children 2024, 11, 748. https://doi.org/10.3390/children11060748
Papadopoulou CI, Sifakakis I, Tournis S. Metabolic Bone Diseases Affecting Tooth Eruption: A Narrative Review. Children. 2024; 11(6):748. https://doi.org/10.3390/children11060748
Chicago/Turabian StylePapadopoulou, Christianna Iris, Iosif Sifakakis, and Symeon Tournis. 2024. "Metabolic Bone Diseases Affecting Tooth Eruption: A Narrative Review" Children 11, no. 6: 748. https://doi.org/10.3390/children11060748
APA StylePapadopoulou, C. I., Sifakakis, I., & Tournis, S. (2024). Metabolic Bone Diseases Affecting Tooth Eruption: A Narrative Review. Children, 11(6), 748. https://doi.org/10.3390/children11060748






