Maxillary Hypoplasia and Non-Invasive Ventilation: Literature Review and Proposed New Treatment Protocol
Abstract
1. Introduction
2. Review Section
2.1. Database Search
2.2. Included Studies
- ▪
- In 2000, Li et al. [10] described a 15-year-old child with OSAS and obesity who underwent CPAP with a nasal mask for 10 years, resulting in midface hypoplasia and a Class III skeletal relationship on lateral cephalometric radiography, despite the absence of known syndromic conditions. However, no description of possible treatment for this dento-skeletal alteration was provided.
- ▪
- In 2002, Villa et al. [11] presented the case of a 7-year-old patient affected by CCHS who had been undergoing bi-level positive airway pressure (BiPAP) since the age of 9 months. The authors attributed the development of maxillary retrusion to prolonged pressure exerted by the ventilator interface and headgear on the zygomatico-maxillary region. In this case, a treatment option involving the application of a Delaire mask over the nasal mask was proposed, leading to normalization of the clinical profile picture.
- ▪
- In 2013, Shibata et al. [13] published another case report highlighting the development of maxillary hypoplasia in a 5-year-old patient with CCHS undergoing BiPAP since the age of 2 months. The authors attributed the midface retrusion observed on lateral cephalometric radiography to growth suppression induced by NIV and described the possibility of mitigating, but not correcting, the clinical picture using a Delaire mask.
- ▪
- In 2017, Haviv et al. [15] reported the case of a 29-year-old patient with myasthenia gravis treated with CPAP since the age of 12 years, exhibiting severe Class III malocclusion, attributed to the use of a tight-fitting nasal mask.
- ▪
- In the first study, conducted on 40 children undergoing NIV, global facial flattening was observed in 68% of patients, with maxillary retrusion in 37% of cases, correlated with more than 10 h per day of PAP use.
- ▪
- In the second study, 12 pediatric patients undergoing PAP therapy for at least 6 months and 6 h per day were compared to a control group of 11 patients affected by OSAS not treated with NIV. The authors did not identify associations between midfacial projection and PAP usage in growing patients.
- ▪
- The last study compared 50 children compliant with PAP therapy to 50 non-compliant subjects, analyzing annual cephalometric analyses. The results showed progressive maxillary retrusion in the compliant sample compared to the non-compliant sample.
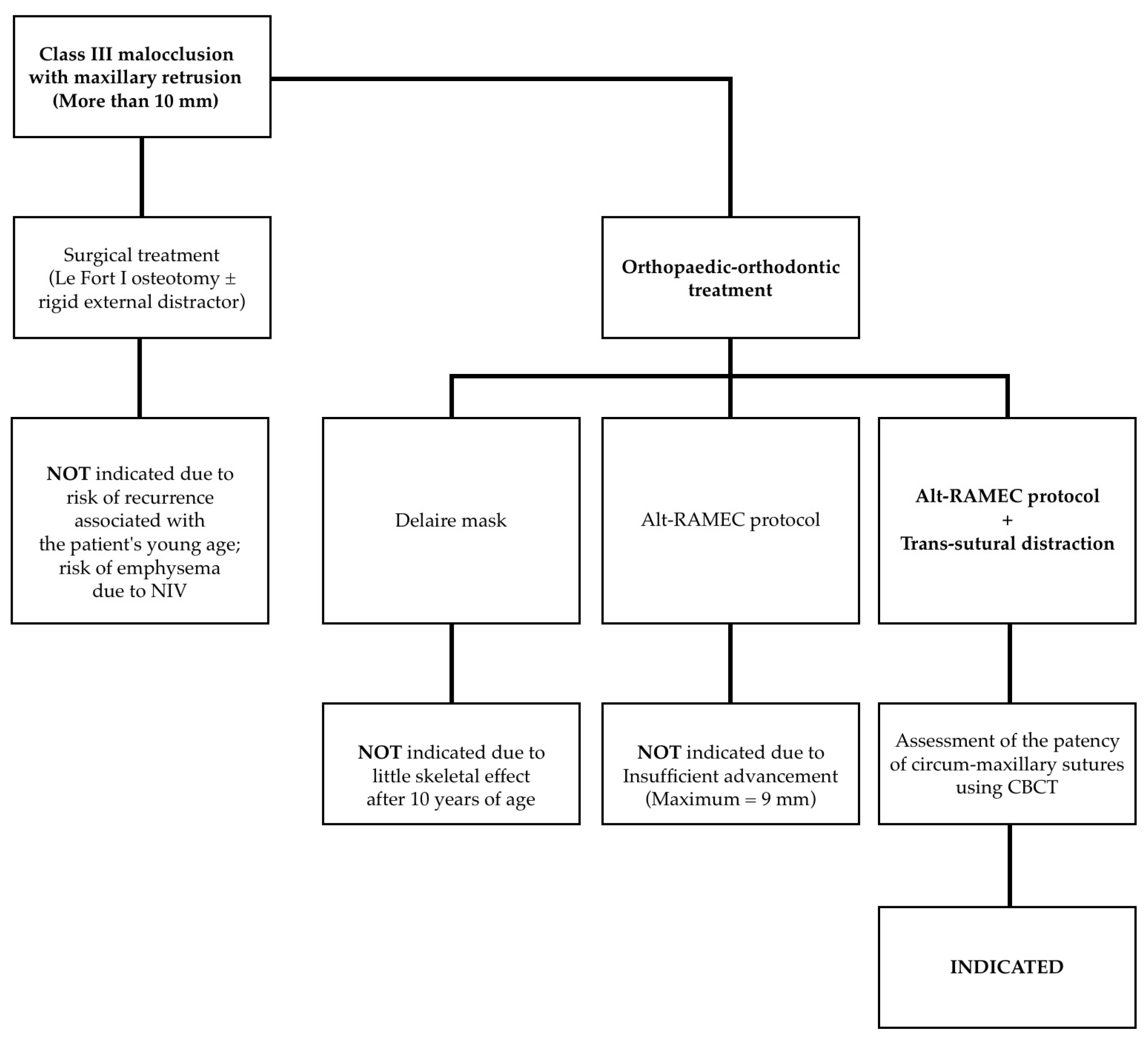
3. Proposal of a New Treatment Protocol
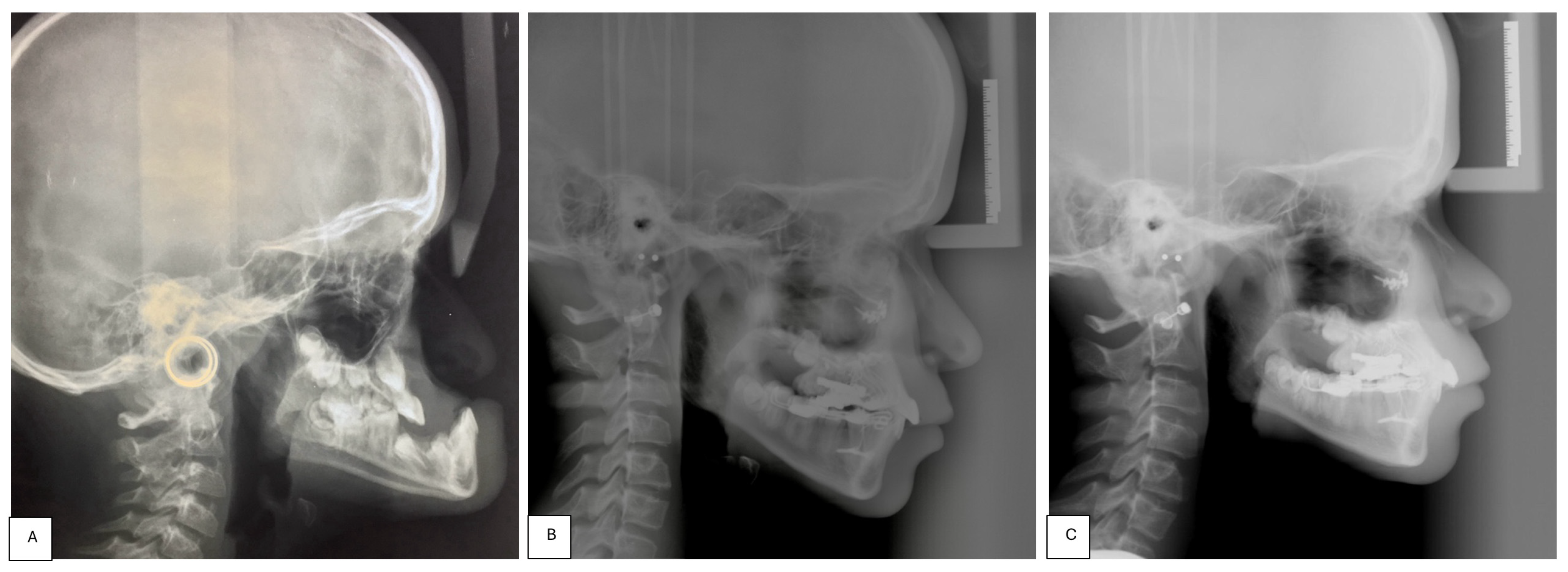
- Perform a CBCT to confirm the patency of the circum-maxillary sutures.
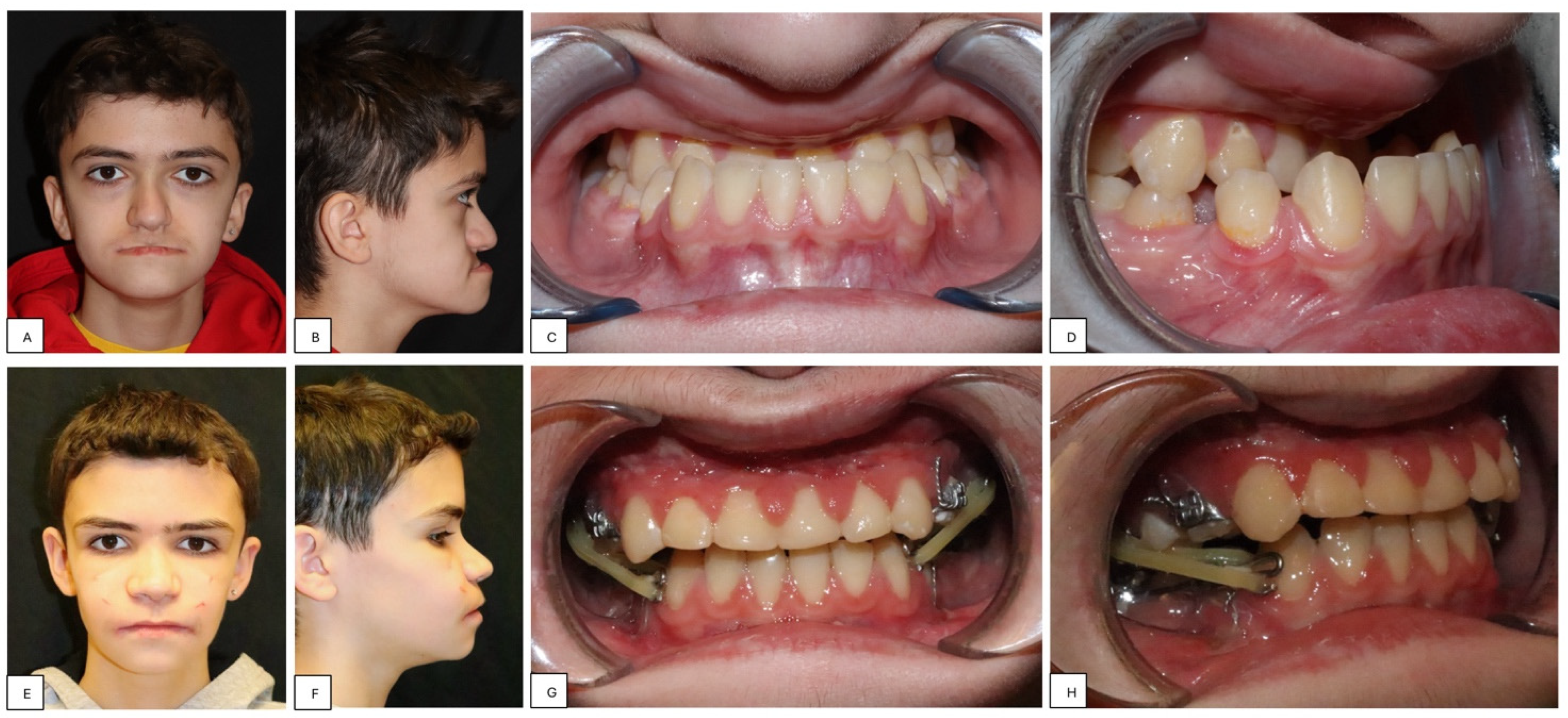
- Placement of a two-hinged rapid maxillary expander (RPE) and expansion of maxilla using a modified Alt-RAMEC (alternate rapid maxillary expansion and constriction) protocol [17,18,19] resulting in loosening of all circum-maxillary sutures without surgery. The treatment protocol consisted of 11 cycles with 7 days of expansion and 7 days of constriction, 1 mm per day, alternatively. After 11 weeks of alternate expansion–constriction, mild mobility of the whole maxilla was felt clinically.
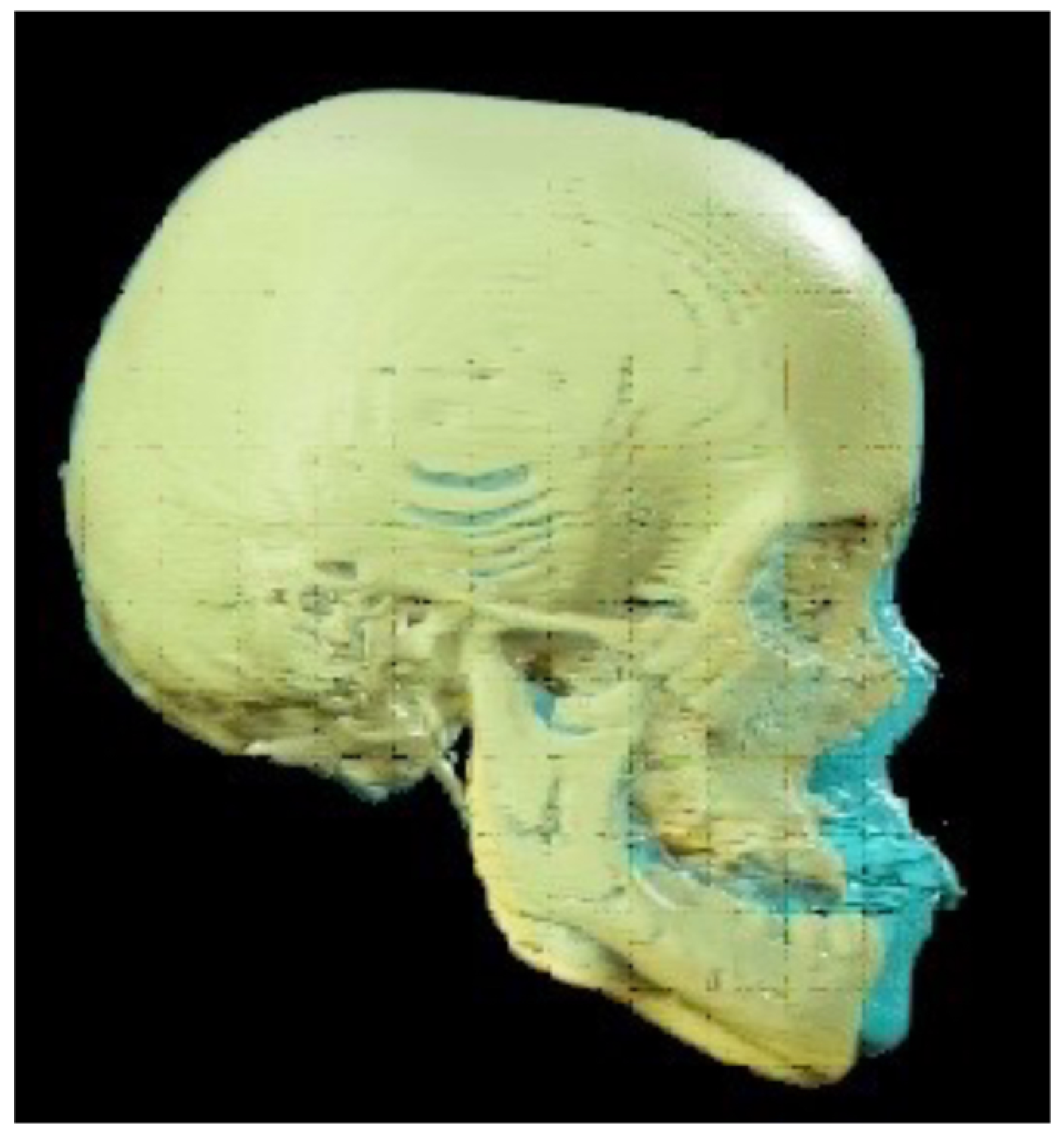
- Placement of an external rigid distractor (RED) during a minor surgery under general anesthesia. The surgical procedure was performed with oro-tracheal intubation through an incision at the level of the upper vestibular fornix with skeletonization of the maxilla. The patency of the zygomatico-maxillary suture was noted. A plate and corresponding screws (Matrix Midface, DePuy Synthes, Raynham, MA, USA) were positioned on each side at the level of the maxillo-malar complex, and two plates, corresponding screws, and percutaneous pins (External Midface Distractor, DePuy Synthes, Raynham, MA, USA) were placed at the level of the lower orbital rim. Percutaneous traction wires were secured to the inferior plates and left protruding at the level of the naso-labial fold. After all intraoral wounds were sutured, the halo was positioned using four cranial screws per side (External Midface Distractor, DePuy Synthes, Raynham, MA, USA). The immediate post-operative period was organized in the Pediatric Intensive Care Unit. As ongoing NIV therapy was critical, the tractions of the RED were positioned in such a way as not to impede the placement of the NIV interface during night (Figure 5).
- Activation of the RED to allow advancement of the midface to overcorrect the dysmorphism. The activation started the day after surgery, 0.5 mm per day. After 4 weeks, the dislodgement of a bone anchorage plate was observed, necessitating an additional intervention to reposition it with a modification of its geometry. The activation continued for 5 additional weeks, for a total of 9 weeks, with an advancement reaching approximately 20 mm at the maxillary level.
- At the end of the distraction phase, after an additional stabilization period of 4 weeks, the RED was removed.
- Continuation of orthopedic–orthodontic treatment for approximately 9 months using intra-oral traction elastics, following the placement of orthodontic anchorage screws in the jaws under local anesthesia.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medical Subject Headings (MeSH). Available online: https://www.ncbi.nlm.nih.gov/mesh/?term=Non+invasive+ventilation (accessed on 26 May 2024).
- Gong, Y.; Sankari, A. Noninvasive Ventilation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Norregaard, O. Noninvasive ventilation in children. Eur. Respir. J. 2002, 20, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- Potchileev, I.; Doroshenko, M.; Mohammed, A.N. Positive Pressure Ventilation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Castro-Codesal, M.L.; Dehaan, K.; Featherstone, R.; Bedi, P.K.; Carrasco, C.M.; Katz, S.L.; Chan, E.Y.; Bendiak, G.N.; Almeida, F.R.; Olmstead, D.L.; et al. Long-term non-invasive ventilation therapies in children: A scoping review. Sleep Med. Rev. 2018, 37, 148–158. [Google Scholar] [CrossRef] [PubMed]
- DeMauro, S.B.; Wei, J.L.; Lin, R.J. Perspectives on neonatal and infant tracheostomy. Semin. Fetal Neonatal Med. 2016, 21, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Korayem, M.M.; Witmans, M.; MacLean, J.; Heo, G.; El-Hakim, H.; Flores-Mir, C.; Major, P.W. Craniofacial morphology in pediatric patients with persistent obstructive sleep apnea with or without positive airway pressure therapy: A cross-sectional cephalometric comparison with controls. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Fauroux, B.; Lavis, J.-F.; Nicot, F.; Picard, A.; Boelle, P.-Y.; Clément, A.; Vazquez, M.-P. Facial side effects during noninvasive positive pressure ventilation in children. Intensiv. Care Med. 2005, 31, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Castro-Codesal, M.L.; Olmstead, D.L.; MacLean, J.E. Mask interfaces for home non-invasive ventilation in infants and children. Paediatr. Respir. Rev. 2019, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Riley, R.W.; Guilleminault, C. An Unreported Risk in the Use of Home Nasal Continuous Positive Airway Pressure and Home Nasal Ventilation in Children: Mid-face hypoplasia. Chest 2000, 117, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.P.; Pagani, J.; Ambrosio, R.; Ronchetti, R.; Bernkopf, E. Mid-face hypoplasia after long-term nasal ventilation. Am. J. Respir. Crit. Care Med. 2002, 166, 1142–1143. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Almeida, F.R.; Tsuda, T.; Moritsuchi, Y.; Lowe, A.A. Craniofacial Changes After 2 Years of Nasal Continuous Positive Airway Pressure Use in Patients with Obstructive Sleep Apnea. Chest 2010, 138, 870–874. [Google Scholar] [CrossRef]
- Shibata, M.; Tanikawa, C.; Yashiro, K.; Shintaku, Y.; Kogo, M.; Yamashiro, T. Early dentofacial orthopedic treatment of a patient with maxillary hypoplasia and congenital central hypoventilation syndrome. Orthod. Waves 2014, 73, 29–33. [Google Scholar] [CrossRef]
- Roberts, S.D.; Kapadia, H.; Greenlee, G.; Chen, M.L. Midfacial and Dental Changes Associated with Nasal Positive Airway Pressure in Children with Obstructive Sleep Apnea and Craniofacial Conditions. J. Clin. Sleep Med. 2016, 12, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Haviv, Y.; Leibovitz, S.; Almoznino, G.; Sharav, Y.; Zilberman, U. Maxillary deformity following CPAP treatment in myasthenia gravis. Cranio 2018, 36, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Bariani, R.C.B.; Guimarães, T.M.; Cappellette, M., Jr.; Moreira, G.; Fujita, R.R. The impact of positive airway pressure on midface growth: A literature review. Braz. J. Otorhinolaryngol. 2020, 86, 647–653. [Google Scholar] [CrossRef]
- Liou, E.J.W.; Tsai, W.C. A New Protocol for Maxillary Protraction in Cleft Patients: Repetitive Weekly Protocol of Alternate Rapid Maxillary Expansions and Constrictions. Cleft Palate-Craniofacial J. 2005, 42, 121–127. [Google Scholar] [CrossRef]
- Meazzini, M.C.; Zappia, L.B.; Tortora, C.; Autelitano, L.; Tintinelli, R. Short- and Long-Term Effects of Late Maxillary Advancement with the Liou-Alt-RAMEC Protocol in Unilateral Cleft Lip and Palate. Cleft Palate-Craniofacial J. 2019, 56, 159–167. [Google Scholar] [CrossRef]
- Meazzini, M.C.; Torre, C.; Cappello, A.; Tintinelli, R.; De Ponti, E.; Mazzoleni, F. Long-term follow-up of late maxillary orthopedic advancement with the Liou-Alternate rapid maxillary expansion-constriction technique in patients with skeletal Class III malocclusion. Am. J. Orthod. Dentofac. Orthop. 2021, 160, 221–230. [Google Scholar] [CrossRef]
- Zaidi, S.; Gandhi, J.; Vatsia, S.; Smith, N.L.; Khan, S.A. Congenital central hypoventilation syndrome: An overview of etiopathogenesis, associated pathologies, clinical presentation, and management. Auton. Neurosci. 2018, 210, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.H.; Robertson, B.R.; Winograd, J.M.; Mullick, T.; Manson, P.N. Successful Distraction Osteogenesis across a Growing Cranial Suture without an Osteotomy. Plast. Reconstr. Surg. 1999, 103, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Staffenberg, D.A.; Wood, R.J.; McCarthy, J.G.; Grayson, B.H.; Glasberg, S.B. Midface Distraction Advancement in the Canine Without Osteotomies. Ann. Plast. Surg. 1995, 34, 512–517. [Google Scholar] [CrossRef]
- Tong, H.; Gao, F.; Yin, J.; Zhang, X.; Zhang, C.; Yin, N.; Zhao, Z. Transsutural Distraction Osteogenesis Applied to Maxillary Complex with New Internalized Distraction Device: Analysis of the Feasibility and Long-Term Osteogenesis Outcome. J. Craniofacial Surg. 2015, 26, 402–407. [Google Scholar] [CrossRef]
- Liu, C.; Hou, M.; Liang, L.; Huang, X.; Zhang, T.; Zhang, H.; Ma, X.; Song, R. Sutural Distraction Osteogenesis (SDO) Versus Osteotomy Distraction Osteogenesis (ODO) for Midfacial Advancement: A New Technique and Primary Clinical Report. J. Craniofacial Surg. 2005, 16, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Gao, F.; Yin, J.; Shi, Z.; Song, T.; Li, H.; Sun, X.; Wang, Y.; Yin, N.; Zhao, Z. Three-dimensional quantitative evaluation of midfacial skeletal changes after trans-sutural distraction osteogenesis for midfacial hypoplasia in growing patients with cleft lip and palate. J. Cranio-Maxillofac. Surg. 2015, 43, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Jin, M.; Bai, Y.; Xue, H.; Zhao, Z. The Effect of Trans-Sutural Distraction Osteogenesis on Nasal Bone, Nasal Septum, and Nasal Airway in the Treatment for Midfacial Hypoplasia in Growing Patients. J. Craniofacial Surg. 2023, 34, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Viana, M.A.; Graber, T.M.; Omerza, F.F.; BeGole, E.A. The effectiveness of protraction face mask therapy: A meta-analysis. Am. J. Orthod. Dentofac. Orthop. 1999, 115, 675–685. [Google Scholar] [CrossRef] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Human clinical studies | Patients with midface hypoplasia due to other known causes |
| Publication from any date to April 2024 | Full text not in English |
| Letters to editors, case reports, case series, opinion articles, descriptive studies, retrospective studies, prospective studies, review articles | |
| Evaluation of the effects of NIV on maxillary growth in pediatric population |
| Authors | Year | Type of Study | Findings | |
|---|---|---|---|---|
| Li et al. | [10] | 2000 | Case Report | Description of a 15-year-old patient affected by OSAS with midface hypoplasia treated with CPAP. |
| Villa et al. | [11] | 2002 | Case Report | Description of a 7-year-old patient affected by CCHS with midface hypoplasia treated with BiPAP. Proposal of a successful treatment option for maxillary hypoplasia involving Delaire mask. |
| Fauroux et al. | [8] | 2005 | Retrospective Study | Clinical evaluation of facial side effects in 40 patients affected by OSAS, neuromuscular disorders, and cystic fibrosis treated with non-invasive positive pressure ventilation (NPPV). Maxillary retrusion was present in 37% of patients and was associated with daily use (>10 h per day). |
| Tsuda et al. | [12] | 2010 | Prospective Study | Serial cephalometric evaluation of 46 patients affected by OSAS treated with CPAP for a minimum of 2 years. The use of CPAP may cause a significant retrusion of anterior maxilla. |
| Korayem et al. | [7] | 2013 | Retrospective Study | Cephalometric evaluation of craniofacial morphology in 12 patients affected by OSAS treated with positive airway pressure (PAP) versus 11 patients affected by OSAS who were not treated with PAP. No association was demonstrated between midface projection and PAP usage in growing patients. |
| Shibata et al. | [13] | 2013 | Case Report | Description of a 5-year-old patient affected by CCHS with midface hypoplasia treated with BiPAP. Proposal of a partially successful treatment option for maxillary hypoplasia involving a Delaire mask. |
| Roberts et al. | [14] | 2015 | Retrospective Study | Serial cephalometric evaluation in 50 children affected by OSAS compliant with PAP therapy and 50 children affected by OSAS non-compliant with PAP therapy. Midface retrusion was highlighted in patients using PAP for more than 20 h per week for more than 6 months. |
| Haviv et al. | [15] | 2017 | Case Report | Description of a 29-year-old patient affected by myasthenia gravis with midface hypoplasia treated with CPAP. |
| Bariani et al. | [16] | 2020 | Systematic Review | Analysis of five studies previously published. Midface hypoplasia is associated with long-term use of PAP during growth. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meazzini, M.C.; Moretti, M.; Canzi, G.; Sozzi, D.; Novelli, G.; Mazzoleni, F. Maxillary Hypoplasia and Non-Invasive Ventilation: Literature Review and Proposed New Treatment Protocol. Children 2024, 11, 720. https://doi.org/10.3390/children11060720
Meazzini MC, Moretti M, Canzi G, Sozzi D, Novelli G, Mazzoleni F. Maxillary Hypoplasia and Non-Invasive Ventilation: Literature Review and Proposed New Treatment Protocol. Children. 2024; 11(6):720. https://doi.org/10.3390/children11060720
Chicago/Turabian StyleMeazzini, Maria Costanza, Mattia Moretti, Gabriele Canzi, Davide Sozzi, Giorgio Novelli, and Fabio Mazzoleni. 2024. "Maxillary Hypoplasia and Non-Invasive Ventilation: Literature Review and Proposed New Treatment Protocol" Children 11, no. 6: 720. https://doi.org/10.3390/children11060720
APA StyleMeazzini, M. C., Moretti, M., Canzi, G., Sozzi, D., Novelli, G., & Mazzoleni, F. (2024). Maxillary Hypoplasia and Non-Invasive Ventilation: Literature Review and Proposed New Treatment Protocol. Children, 11(6), 720. https://doi.org/10.3390/children11060720







