Pediatric Cardio-Oncology: Screening, Risk Stratification, and Prevention of Cardiotoxicity Associated with Anthracyclines
Abstract
:1. Introduction
2. Risk Factors
2.1. Treatment-Related Factors
2.2. Individual-Related Factors
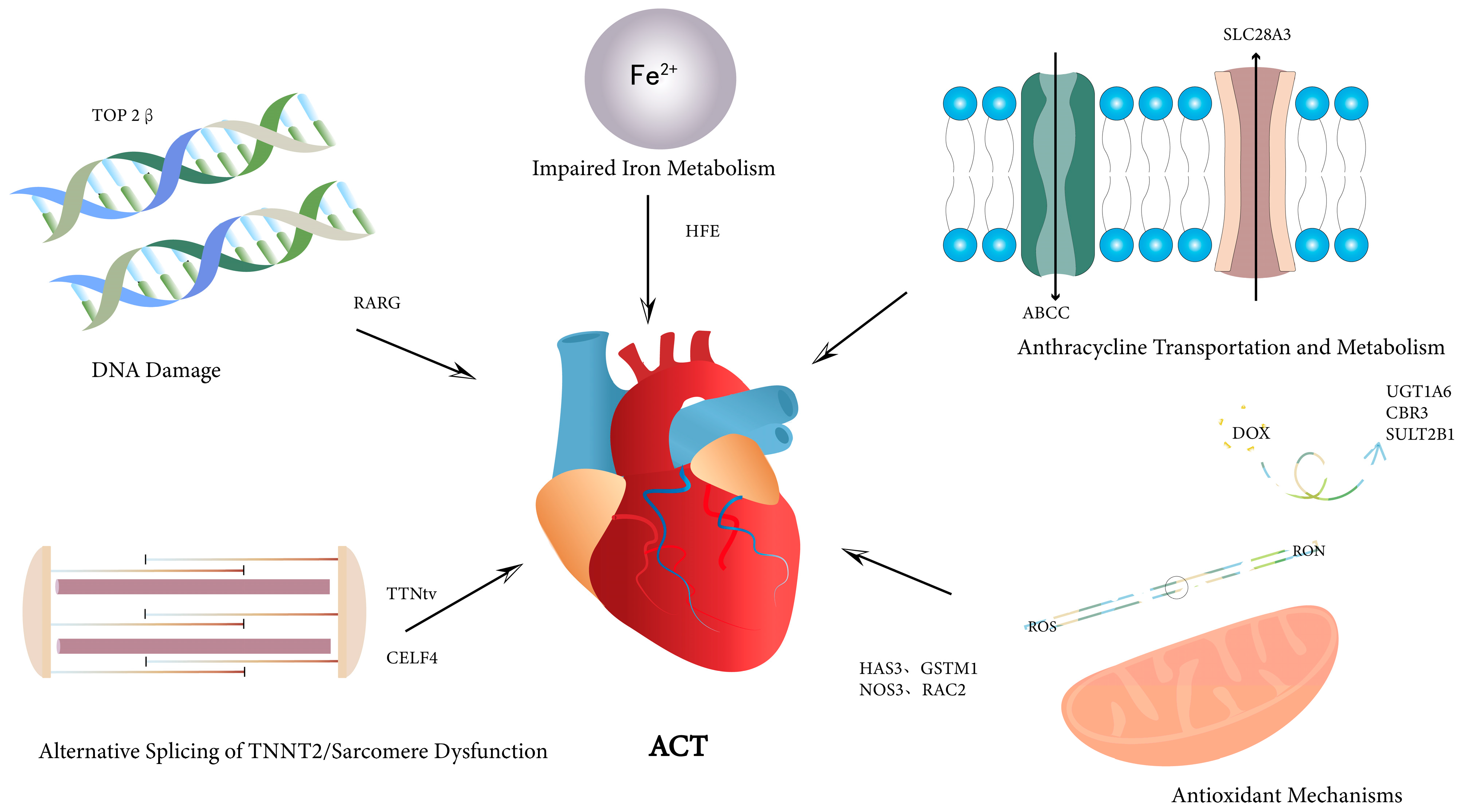
2.3. Genetic Polymorphisms
3. Screening Strategies
3.1. Serum Biomarkers
3.2. Conventional and Novel Electrocardiography Methods
3.3. Echocardiography and Multimodality Imaging
4. Prevention
4.1. Cardioprotective Drugs
4.2. Lifestyle
4.3. Genetic Screening
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunger, S.P.; Mullighan, C.G. Acute Lymphoblastic Leukemia in Children. N. Engl. J. Med. 2015, 373, 1541–1552. [Google Scholar] [CrossRef]
- Martins-Teixeira, M.B.; Carvalho, I. Antitumour Anthracyclines: Progress and Perspectives. ChemMedChem 2020, 15, 933–948. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Adams, M.J.; Colan, S.D.; Constine, L.S.; Herman, E.H.; Hsu, D.T.; Hudson, M.M.; Kremer, L.C.; Landy, D.C.; Miller, T.L.; et al. Long-Term Cardiovascular Toxicity in Children, Adolescents, and Young Adults Who Receive Cancer Therapy: Pathophysiology, Course, Monitoring, Management, Prevention, and Research Directions: A Scientific Statement from the American Heart Association. Circulation 2013, 128, 1927–1995. [Google Scholar] [CrossRef]
- Lenihan, D.J.; Fradley, M.G.; Dent, S.; Brezden-Masley, C.; Carver, J.; Filho, R.K.; Neilan, T.G.; Blaes, A.; Melloni, C.; Herrmann, J.; et al. Proceedings from the Global Cardio-Oncology Summit: The Top 10 Priorities to Actualize for Cardiooncology. Cardio Oncol. 2019, 1, 256–272. [Google Scholar] [CrossRef]
- Bates, J.E.; Howell, R.M.; Liu, Q.; Yasui, Y.; Mulrooney, D.A.; Dhakal, S.; Smith, S.A.; Leisenring, W.M.; Indelicato, D.J.; Gibson, T.M.; et al. Therapy-Related Cardiac Risk in Childhood Cancer Survivors: An Analysis of the Childhood Cancer Survivor Study. J. Clin. Oncol. 2019, 37, 1090–1101. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Armstrong, G.T.; Huang, S.; Ness, K.K.; Ehrhardt, M.J.; Joshi, V.M.; Plana, J.C.; Soliman, E.Z.; Green, D.M.; Srivastava, D.; et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-Sectional Study. Ann. Intern. Med. 2016, 164, 93–101. [Google Scholar] [CrossRef]
- Armenian, S.H.; Hudson, M.M.; Mulder, R.L.; Chen, M.H.; Constine, L.S.; Dwyer, M.; Nathan, P.C.; Tissing, W.J.; Shankar, S.; Sieswerda, E.; et al. Recommendations for Cardiomyopathy Surveillance for Survivors of Childhood Cancer: A Report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015, 16, e123–e136. [Google Scholar] [CrossRef]
- Leger, K.; Slone, T.; Lemler, M.; Leonard, D.; Cochran, C.; Bowman, W.P.; Bashore, L.; Winick, N. Subclinical Cardiotoxicity in Childhood Cancer Survivors Exposed to Very Low Dose Anthracycline Therapy. Pediatr. Blood Cancer 2015, 62, 123–127. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes among Adults Treated for Childhood Cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Mulrooney, D.A.; Yeazel, M.W.; Kawashima, T.; Mertens, A.C.; Mitby, P.; Stovall, M.; Donaldson, S.S.; Green, D.M.; Sklar, C.A.; Robison, L.L.; et al. Cardiac Outcomes in a Cohort of Adult Survivors of Childhood and Adolescent Cancer: Retrospective Analysis of the Childhood Cancer Survivor Study Cohort. BMJ 2009, 339, b4606. [Google Scholar] [CrossRef]
- van der Pal, H.J.; van Dalen, E.C.; van Delden, E.; van Dijk, I.W.; Kok, W.E.; Geskus, R.B.; Sieswerda, E.; Oldenburger, F.; Koning, C.C.; van Leeuwen, F.E.; et al. High Risk of Symptomatic Cardiac Events in Childhood Cancer Survivors. J. Clin. Oncol. 2012, 30, 1429–1437. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Joshi, V.M.; Ness, K.K.; Marwick, T.H.; Zhang, N.; Srivastava, D.; Griffin, B.P.; Grimm, R.A.; Thomas, J.; Phelan, D.; et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac dysfunction in Adult Survivors of childhood Cancer: Results from the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 2015, 65, 2511–2522. [Google Scholar] [CrossRef]
- Merchant, T.E.; Rose, S.R.; Bosley, C.; Wu, S.; Xiong, X.; Lustig, R.H. Growth Hormone Secretion after Conformal Radiation Therapy in Pediatric Patients with Localized Brain Tumors. J. Clin. Oncol. 2011, 29, 4776–4780. [Google Scholar] [CrossRef]
- Bansal, N.; Blanco, J.G.; Sharma, U.C.; Pokharel, S.; Shisler, S.; Lipshultz, S.E. Cardiovascular Diseases in Survivors of Childhood Cancer. Cancer Metastasis Rev. 2020, 39, 55–68. [Google Scholar] [CrossRef]
- Lieke Feijen, E.A.M.; Font-Gonzalez, A.; van der Pal, H.J.H.; Kok, W.E.M.; Geskus, R.B.; Ronckers, C.M.; Bresters, D.; van Dalen, E.C.; van Dulmen-Den Broeder, E.; van den Berg, M.H.; et al. Risk and Temporal Changes of Heart Failure among 5-Year Childhood Cancer Survivors: A DCOG-LATER Study. J. Am. Heart Assoc. 2019, 8, e009122. [Google Scholar] [CrossRef]
- Sledge, G.W.; Neuberg, D.; Bernardo, P.; Ingle, J.N.; Martino, S.; Rowinsky, E.K.; Wood, W.C. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An intergroup trial (E1193). J. Clin. Oncol. 2003, 21, 588–592. [Google Scholar] [CrossRef]
- Kamendi, H.; Zhou, Y.; Crosby, M.; Keirstead, N.; Snow, D.; Bentley, P.; Patel, N.; Barthlow, H.; Luo, W.; Dragan, Y.; et al. Doxorubicin: Comparison between 3-H Continuous and Bolus Intravenous Administration Paradigms on Cardio-Renal Axis, Mitochondrial Sphingolipids and Pathology. Toxicol. Appl. Pharmacol. 2015, 289, 560–572. [Google Scholar] [CrossRef]
- Yang, F.; Lei, Q.; Li, L.; He, J.C.; Zeng, J.; Luo, C.; Yeung, S.J.; Yang, R. Delivery of Epirubicin Via Slow Infusion as a Strategy to Mitigate Chemotherapy-Induced Cardiotoxicity. PLoS ONE 2017, 12, e0188025. [Google Scholar] [CrossRef]
- van Dalen, E.C.; van der Pal, H.J.; Kremer, L.C. Different Dosage Schedules for Reducing Cardiotoxicity in People with Cancer Receiving Anthracycline Chemotherapy. Cochrane Database Syst. Rev. 2016, 3, Cd005008. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Miller, T.L.; Lipsitz, S.R.; Neuberg, D.S.; Dahlberg, S.E.; Colan, S.D.; Silverman, L.B.; Henkel, J.M.; Franco, V.I.; Cushman, L.L.; et al. Continuous Versus Bolus Infusion of Doxorubicin in Children with All: Long-Term Cardiac Outcomes. Pediatrics 2012, 130, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Loeffen, E.A.H.; van Dalen, E.C.; Mulder, R.L.; van de Wetering, M.D.; Kremer, L.C.M.; Tissing, W.J.E. The Duration of Anthracycline Infusion Should Be at Least One Hour in Children with Cancer: A Clinical Practice Guideline. Pediatr. Blood Cancer 2018, 65, e26867. [Google Scholar] [CrossRef] [PubMed]
- Völler, S.; Boos, J.; Krischke, M.; Würthwein, G.; Kontny, N.E.; Boddy, A.V.; Hempel, G. Age-Dependent Pharmacokinetics of Doxorubicin in Children with Cancer. Clin. Pharmacokinet 2015, 54, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Lipshultz, S.E. Pathophysiology of Anthracycline- and Radiation-Associated Cardiomyopathies: Implications for Screening and Prevention. Pediatr. Blood Cancer 2005, 44, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Kelly, M.J.; Must, A. Early Nutrition and Physical Activity Interventions in Childhood Cancer Survivors. Curr. Obes. Rep. 2017, 6, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.T.; Oeffinger, K.C.; Chen, Y.; Kawashima, T.; Yasui, Y.; Leisenring, W.; Stovall, M.; Chow, E.J.; Sklar, C.A.; Mulrooney, D.A.; et al. Modifiable Risk Factors and Major Cardiac Events among Adult Survivors of Childhood Cancer. J. Clin. Oncol. 2013, 31, 3673–3680. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline Cardiovascular Risk Assessment in Cancer Patients Scheduled to Receive Cardiotoxic Cancer Therapies: A Position Statement and New Risk Assessment Tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in Collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Chow, E.J.; Chen, Y.; Kremer, L.C.; Breslow, N.E.; Hudson, M.M.; Armstrong, G.T.; Border, W.L.; Feijen, E.A.; Green, D.M.; Meacham, L.R.; et al. Individual Prediction of Heart Failure among Childhood Cancer Survivors. J. Clin. Oncol. 2015, 33, 394–402. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Liu, Q.; Yasui, Y.; Neglia, J.P.; Leisenring, W.; Robison, L.L.; Mertens, A.C. Late Mortality among 5-Year Survivors of Childhood Cancer: A Summary from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2328–2338. [Google Scholar] [CrossRef]
- Ikeda, Y.; Aihara, K.; Akaike, M.; Sato, T.; Ishikawa, K.; Ise, T.; Yagi, S.; Iwase, T.; Ueda, Y.; Yoshida, S.; et al. Androgen Receptor Counteracts Doxorubicin-Induced Cardiotoxicity in Male Mice. Mol. Endocrinol. 2010, 24, 1338–1348. [Google Scholar] [CrossRef]
- Altieri, P.; Barisione, C.; Lazzarini, E.; Garuti, A.; Bezante, G.P.; Canepa, M.; Spallarossa, P.; Tocchetti, C.G.; Bollini, S.; Brunelli, C.; et al. Testosterone Antagonizes Doxorubicin-Induced Senescence of Cardiomyocytes. J. Am. Heart Assoc. 2016, 5, e002383. [Google Scholar] [CrossRef]
- Wilcox, N.S.; Rotz, S.J.; Mullen, M.; Song, E.J.; Ky Hamilton, B.; Moslehi, J.; Armenian, S.H.; Wu, J.C.; Rhee, J.W.; Ky, B. Sex-Specific Cardiovascular Risks of Cancer and Its Therapies. Circ. Res. 2022, 130, 632–651. [Google Scholar] [CrossRef]
- Aminkeng, F.; Ross, C.J.; Rassekh, S.R.; Hwang, S.; Rieder, M.J.; Bhavsar, A.P.; Smith, A.; Sanatani, S.; Gelmon, K.A.; Bernstein, D.; et al. Recommendations for Genetic Testing to Reduce the Incidence of Anthracycline-Induced Cardiotoxicity. Br. J. Clin. Pharmacol. 2016, 82, 683–695. [Google Scholar] [CrossRef]
- Siemens, A.; Rassekh, S.R.; Ross, C.J.D.; Carleton, B.C. Development of a Dose-Adjusted Polygenic Risk Model for Anthracycline-Induced Cardiotoxicity. Ther. Drug Monit. 2023, 45, 337–344. [Google Scholar] [CrossRef]
- Magdy, T.; Jouni, M.; Kuo, H.H.; Weddle, C.J.; Lyra-Leite, D.; Fonoudi, H.; Romero-Tejeda, M.; Gharib, M.; Javed, H.; Fajardo, G.; et al. Identification of Drug Transporter Genomic Variants and Inhibitors That Protect against Doxorubicin-Induced Cardiotoxicity. Circulation 2022, 145, 279–294. [Google Scholar] [CrossRef]
- Aminkeng, F.; Bhavsar, A.P.; Visscher, H.; Rassekh, S.R.; Li, Y.; Lee, J.W.; Brunham, L.R.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; et al. A Coding Variant in Rarg Confers Susceptibility to Anthracycline-Induced Cardiotoxicity in Childhood Cancer. Nat. Genet. 2015, 47, 1079–1084. [Google Scholar] [CrossRef]
- Visscher, H.; Ross, C.J.; Rassekh, S.R.; Sandor, G.S.; Caron, H.N.; van Dalen, E.C.; Kremer, L.C.; van der Pal, H.J.; Rogers, P.C.; Rieder, M.J.; et al. Validation of Variants in Slc28a3 and Ugt1a6 as Genetic Markers Predictive of Anthracycline-Induced Cardiotoxicity in Children. Pediatr. Blood Cancer 2013, 60, 1375–1381. [Google Scholar] [CrossRef]
- Blanco, J.G.; Sun, C.L.; Landier, W.; Chen, L.; Esparza-Duran, D.; Leisenring, W.; Mays, A.; Friedman, D.L.; Ginsberg, J.P.; Hudson, M.M.; et al. Anthracycline-Related Cardiomyopathy after Childhood Cancer: Role of Polymorphisms in Carbonyl Reductase Genes—A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 1415–1421. [Google Scholar] [CrossRef]
- Krajinovic, M.; Elbared, J.; Drouin, S.; Bertout, L.; Rezgui, A.; Ansari, M.; Raboisson, M.J.; Lipshultz, S.E.; Silverman, L.B.; Sallan, S.E.; et al. Polymorphisms of Abcc5 and Nos3 Genes Influence Doxorubicin Cardiotoxicity in Survivors of Childhood Acute Lymphoblastic Leukemia. Pharmacogenom. J. 2016, 16, 530–535. [Google Scholar] [CrossRef]
- Al-Otaibi, T.K.; Weitzman, B.; Tahir, U.A.; Asnani, A. Genetics of Anthracycline-Associated Cardiotoxicity. Front. Cardiovasc. Med. 2022, 9, 867873. [Google Scholar] [CrossRef]
- Wang, X.; Singh, P.; Zhou, L.; Sharafeldin, N.; Landier, W.; Hageman, L.; Burridge, P.; Yasui, Y.; Sapkota, Y.; Blanco, J.G.; et al. Genome-Wide Association Study Identifies Robo2 as a Novel Susceptibility Gene for Anthracycline-Related Cardiomyopathy in Childhood Cancer Survivors. J. Clin. Oncol. 2022, 41, Jco2201527. [Google Scholar] [CrossRef]
- Chaix, M.A.; Parmar, N.; Kinnear, C.; Lafreniere-Roula, M.; Akinrinade, O.; Yao, R.; Miron, A.; Lam, E.; Meng, G.; Christie, A.; et al. Machine Learning Identifies Clinical and Genetic Factors Associated with Anthracycline Cardiotoxicity in Pediatric Cancer Survivors. JACC CardioOncol 2020, 2, 690–706. [Google Scholar] [CrossRef]
- Lin, X.; Wu, G.; Wang, S.; Huang, J. Bibliometric and Visual Analysis of Doxorubicin-Induced Cardiotoxicity. Front. Pharmacol. 2023, 14, 1255158. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Rifai, N.; Dalton, V.M.; Levy, D.E.; Silverman, L.B.; Lipsitz, S.R.; Colan, S.D.; Asselin, B.L.; Barr, R.D.; Clavell, L.A.; et al. The Effect of Dexrazoxane on Myocardial Injury in Doxorubicin-Treated Children with Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2004, 351, 145–153. [Google Scholar] [CrossRef]
- Cheung, Y.F.; Li, V.W.; Lai, C.T.; Shin, V.Y.; Keung, W.; Cheuk, D.K.; Kwong, A.; Li, R.A.; Chan, G.C. Circulating High-Sensitivity Troponin T and MicroRNAs as Markers of Myocardial Damage During Childhood Leukaemia Treatment. Pediatr. Res. 2021, 89, 1245–1252. [Google Scholar] [CrossRef]
- Wallace, T.W.; Abdullah, S.M.; Drazner, M.H.; Das, S.R.; Khera, A.; McGuire, D.K.; Wians, F.; Sabatine, M.S.; Morrow, D.A.; de Lemos, J.A. Prevalence and Determinants of Troponin T Elevation in the General Population. Circulation 2006, 113, 1958–1965. [Google Scholar] [CrossRef]
- McEvoy, J.W.; Chen, Y.; Ndumele, C.E.; Solomon, S.D.; Nambi, V.; Ballantyne, C.M.; Blumenthal, R.S.; Coresh, J.; Selvin, E. Six-Year Change in High-Sensitivity Cardiac Troponin T and Risk of Subsequent Coronary Heart Disease, Heart Failure, and Death. JAMA Cardiol. 2016, 1, 519–528. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of Serum Biomarkers in Cancer Patients Receiving Cardiotoxic Cancer Therapies: A Position Statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining Cardiovascular Toxicities of Cancer Therapies: An International Cardio-Oncology Society (Ic-Os) Consensus Statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef]
- Clerico, A.; Aimo, A.; Cantinotti, M. High-Sensitivity Cardiac Troponins in Pediatric Population. Clin. Chem. Lab. Med. 2022, 60, 18–32. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Aimo, A.; Cardinale, D.M.; Dittadi, R.; Sandri, M.T.; Perrone, M.A.; Belloni, L.; Fortunato, A.; Trenti, T.; et al. Variability of Cardiac Troponin Levels in Normal Subjects and in Patients with Cardiovascular Diseases: Analytical Considerations and Clinical Relevance. Clin. Chem. Lab. Med. 2023, 61, 1209–1229. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Gelehrter, S.K.; Vase, T.; Venkatramani, R.; Landier, W.; Wilson, K.D.; Herrera, C.; Reichman, L.; Menteer, J.D.; Mascarenhas, L.; et al. Screening for Cardiac Dysfunction in Anthracycline-Exposed Childhood Cancer Survivors. Clin. Cancer Res. 2014, 20, 6314–6323. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.B.; Howell, C.R.; Lu, L.; Plana, J.C.; Joshi, V.M.; Luepker, R.V.; Durand, J.B.; Ky, B.; Lenihan, D.J.; Jefferies, J.L.; et al. Cardiac Biomarkers and Association with Subsequent Cardiomyopathy and Mortality among Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort. Cancer 2021, 127, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Fish-Trotter, H.; Ferguson, J.F.; Patel, N.; Arora, P.; Allen, N.B.; Bachmann, K.N.; Daniels, L.B.; Reilly, M.P.; Lima, J.A.C.; Wang, T.J.; et al. Inflammation and Circulating Natriuretic Peptide Levels. Circ. Heart Fail 2020, 13, e006570. [Google Scholar] [CrossRef] [PubMed]
- Pourier, M.S.; Kapusta, L.; van Gennip, A.; Bökkerink, J.P.; Loonen, J.; Bellersen, L.; Mavinkurve-Groothuis, A.M. Values of High Sensitive Troponin T in Long-Term Survivors of Childhood Cancer Treated with Anthracyclines. Clin. Chim. Acta 2015, 441, 29–32. [Google Scholar] [CrossRef]
- Leerink, J.M.; Verkleij, S.J.; Feijen, E.A.M.; Mavinkurve-Groothuis, A.M.C.; Pourier, M.S.; Ylänen, K.; Tissing, W.J.E.; Louwerens, M.; van den Heuvel, M.M.; van Dulmen-den Broeder, E.; et al. Biomarkers to Diagnose Ventricular Dysfunction in Childhood Cancer Survivors: A Systematic Review. Heart 2019, 105, 210–216. [Google Scholar] [CrossRef] [PubMed]
- de Baat, E.C.; Feijen, E.A.M.; van Niekerk, J.B.; Mavinkurve-Groothuis, A.M.C.; Kapusta, L.; Loonen, J.; Kok, W.E.M.; Kremer, L.C.M.; van Dalen, E.C.; van der Pal, H.J.H. Electrocardiographic Abnormalities in Childhood Cancer Survivors Treated with Cardiotoxic Therapy: A Systematic Review. Pediatr. Blood Cancer 2022, 69, e29720. [Google Scholar] [CrossRef] [PubMed]
- Markman, T.M.; Ruble, K.; Loeb, D.; Chen, A.; Zhang, Y.; Beasley, G.S.; Thompson, W.R.; Nazarian, S. Electrophysiological Effects of Anthracyclines in Adult Survivors of Pediatric Malignancy. Pediatr. Blood Cancer 2017, 64, e26556. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, Z.; Liang, C.; Zhang, F.; Li, J.; Liu, R.; Chen, T.; Ji, B.; Chen, Y.; Wang, C. A Dynamic Learning-Based ECG Feature Extraction Method for Myocardial Infarction Detection. Physiol. Meas. 2023, 43, 124005. [Google Scholar] [CrossRef]
- Ehrhardt, M.J.; Leerink, J.M.; Mulder, R.L.; Mavinkurve-Groothuis, A.; Kok, W.; Nohria, A.; Nathan, P.C.; Merkx, R.; de Baat, E.; Asogwa, O.A.; et al. Systematic Review and Updated Recommendations for Cardiomyopathy Surveillance for Survivors of Childhood, Adolescent, and Young Adult Cancer from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2023, 24, e108–e120. [Google Scholar] [CrossRef]
- Gonzalez-Manzanares, R.; Castillo, J.C.; Molina, J.R.; Ruiz-Ortiz, M.; Mesa, D.; Ojeda, S.; Anguita, M.; Pan, M. Automated Global Longitudinal Strain Assessment in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancers 2022, 14, 1513. [Google Scholar] [CrossRef] [PubMed]
- Merkx, R.; Leerink, J.M.; Feijen, E.; de Baat, E.C.; Bellersen, L.; Bresters, D.; van Dalen, E.C.; van Dulmen-den Broeder, E.; van der Heiden-van der Loo, M.; van den Heuvel-Eibrink, M.M.; et al. Extensive Cardiac Function Analyses Using Contemporary Echocardiography in Childhood Cancer Survivors: A DCCSS LATER Study. Cardio Oncol. 2023, 5, 472–485. [Google Scholar] [CrossRef]
- Dorup, I.; Levitt, G.; Sullivan, I.; Sorensen, K. Prospective Longitudinal Assessment of Late Anthracycline Cardiotoxicity after Childhood Cancer: The Role of Diastolic Function. Heart 2004, 90, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Mertens, L.; Singh, G.; Armenian, S.; Chen, M.H.; Dorfman, A.L.; Garg, R.; Husain, N.; Joshi, V.; Leger, K.J.; Lipshultz, S.E.; et al. Multimodality Imaging for Cardiac Surveillance of Cancer Treatment in Children: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2023, 36, 1227–1253. [Google Scholar] [CrossRef] [PubMed]
- Long, T.M.; Lee, F.; Lam, K.; Wallman, K.E.; Walwyn, T.S.; Choong, C.S.; Naylor, L.H. Cardiovascular Testing Detects Underlying Dysfunction in Childhood Leukemia Survivors. Med. Sci. Sports Exerc. 2020, 52, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.G.; Jain, D.; Storozynsky, E. Traditional and Novel Methods to Assess and Prevent Chemotherapy-Related Cardiac Dysfunction Noninvasively. J. Nucl. Cardiol. 2013, 20, 443–464. [Google Scholar] [CrossRef] [PubMed]
- Tham, E.B.; Haykowsky, M.J.; Chow, K.; Spavor, M.; Kaneko, S.; Khoo, N.S.; Pagano, J.J.; Mackie, A.S.; Thompson, R.B. Diffuse Myocardial Fibrosis by T1-Mapping in Children with Subclinical Anthracycline Cardiotoxicity: Relationship to Exercise Capacity, Cumulative Dose and Remodeling. J. Cardiovasc. Magn. Reason 2013, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Bose, R.; Margossian, R.; Ames, B.L.; Moss, K.; Ehrhardt, M.J.; Armenian, S.H.; Yock, T.I.; Nekhlyudov, L.; Williams, D.; Hudson, M.; et al. Delphi Panel Consensus Recommendations for Screening and Managing Childhood Cancer Survivors at Risk for Cardiomyopathy. Cardio Oncol. 2022, 4, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, S.E.; Miller, T.L.; Scully, R.E.; Lipsitz, S.R.; Rifai, N.; Silverman, L.B.; Colan, S.D.; Neuberg, D.S.; Dahlberg, S.E.; Henkel, J.M.; et al. Changes in Cardiac Biomarkers During Doxorubicin Treatment of Pediatric Patients with High-Risk Acute Lymphoblastic Leukemia: Associations with Long-Term Echocardiographic Outcomes. J. Clin. Oncol. 2012, 30, 1042–1049. [Google Scholar] [CrossRef]
- Jirkovský, E.; Jirkovská, A.; Bavlovič-Piskáčková, H.; Skalická, V.; Pokorná, Z.; Karabanovich, G.; Kollárová-Brázdová, P.; Kubeš, J.; Lenčová-Popelová, O.; Mazurová, Y.; et al. Clinically Translatable Prevention of Anthracycline Cardiotoxicity by Dexrazoxane Is Mediated by Topoisomerase Ii Beta and Not Metal Chelation. Circ. Heart Fail 2021, 14, e008209. [Google Scholar] [CrossRef]
- Asselin, B.L.; Devidas, M.; Chen, L.; Franco, V.I.; Pullen, J.; Borowitz, M.J.; Hutchison, R.E.; Ravindranath, Y.; Armenian, S.H.; Camitta, B.M.; et al. Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J. Clin. Oncol. 2016, 34, 854–862. [Google Scholar] [CrossRef]
- de Baat, E.C.; Mulder, R.L.; Armenian, S.; Feijen, E.A.; Grotenhuis, H.; Hudson, M.M.; Mavinkurve-Groothuis, A.M.; Kremer, L.C.; van Dalen, E.C. Dexrazoxane for Preventing or Reducing Cardiotoxicity in Adults and Children with Cancer Receiving Anthracyclines. Cochrane Database Syst. Rev. 2022, 9, Cd014638. [Google Scholar] [CrossRef]
- Tebbi, C.K.; London, W.B.; Friedman, D.; Villaluna, D.; De Alarcon, P.A.; Constine, L.S.; Mendenhall, N.P.; Sposto, R.; Chauvenet, A.; Schwartz, C.L. Dexrazoxane-Associated Risk for Acute Myeloid Leukemia/Myelodysplastic Syndrome and Other Secondary Malignancies in Pediatric Hodgkin’s Disease. J. Clin. Oncol. 2007, 25, 493–500. [Google Scholar] [CrossRef]
- Chow, E.J.; Aplenc, R.; Vrooman, L.M.; Doody, D.R.; Huang, Y.V.; Aggarwal, S.; Armenian, S.H.; Baker, K.S.; Bhatia, S.; Constine, L.S.; et al. Late Health Outcomes after Dexrazoxane Treatment: A Report from the Children’s Oncology Group. Cancer 2022, 128, 788–796. [Google Scholar] [CrossRef]
- Kim, H.; Kang, H.J.; Park, K.D.; Koh, K.N.; Im, H.J.; Seo, J.J.; Lee, J.W.; Chung, N.G.; Cho, B.; Kim, H.K.; et al. Risk Factor Analysis for Secondary Malignancy in Dexrazoxane-Treated Pediatric Cancer Patients. Cancer Res. Treat. 2019, 51, 357–367. [Google Scholar] [CrossRef]
- de Baat, E.C.; van Dalen, E.C.; Mulder, R.L.; Hudson, M.M.; Ehrhardt, M.J.; Engels, F.K.; Feijen, E.A.M.; Grotenhuis, H.B.; Leerink, J.M.; Kapusta, L.; et al. Primary Cardioprotection with Dexrazoxane in Patients with Childhood Cancer Who Are Expected to Receive Anthracyclines: Recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Child Adolesc. Health 2022, 6, 885–894. [Google Scholar] [CrossRef]
- Chow, E.J.; Aggarwal, S.; Doody, D.R.; Aplenc, R.; Armenian, S.H.; Baker, K.S.; Bhatia, S.; Blythe, N.; Colan, S.D.; Constine, L.S.; et al. Dexrazoxane and Long-Term Heart Function in Survivors of Childhood Cancer. J. Clin. Oncol. 2023, 41, Jco2202423. [Google Scholar] [CrossRef]
- Walker, D.M.; Fisher, B.T.; Seif, A.E.; Huang, Y.S.; Torp, K.; Li, Y.; Aplenc, R. Dexrazoxane Use in Pediatric Patients with Acute Lymphoblastic or Myeloid Leukemia from 1999 and 2009: Analysis of a National Cohort of Patients in the Pediatric Health Information Systems Database. Pediatr. Blood Cancer 2013, 60, 616–620. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Li, X.R.; Cheng, X.H.; Zhang, G.N.; Wang, X.X.; Huang, J.M. Cardiac Safety Analysis of First-Line Chemotherapy Drug PEGylated Liposomal Doxorubicin in Ovarian Cancer. J. Ovarian. Res. 2022, 15, 96. [Google Scholar] [CrossRef]
- Creutzig, U.; Zimmermann, M.; Bourquin, J.P.; Dworzak, M.N.; Fleischhack, G.; Graf, N.; Klingebiel, T.; Kremens, B.; Lehrnbecher, T.; von Neuhoff, C.; et al. Randomized Trial Comparing Liposomal Daunorubicin with Idarubicin as Induction for Pediatric Acute Myeloid Leukemia: Results from Study Aml-Bfm 2004. Blood 2013, 122, 37–43. [Google Scholar] [CrossRef]
- Sieswerda, E.; Kremer, L.C.; Caron, H.N.; van Dalen, E.C. The Use of Liposomal Anthracycline Analogues for Childhood Malignancies: A Systematic Review. Eur. J. Cancer 2011, 47, 2000–2008. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Li, Y.; Tan, N.; Du, K.; Zhao, H.; Wang, J.; Zhang, J.; Wang, W.; Wang, Y. Protective Role of Enalapril in Anthracycline-Induced Cardiotoxicity: A Systematic Review. Front. Pharmacol. 2020, 11, 788. [Google Scholar] [CrossRef]
- Abdel-Qadir, H.; Ong, G.; Fazelzad, R.; Amir, E.; Lee, D.S.; Thavendiranathan, P.; Tomlinson, G. Interventions for Preventing Cardiomyopathy Due to Anthracyclines: A Bayesian Network Meta-Analysis. Ann. Oncol. 2017, 28, 628–633. [Google Scholar] [CrossRef]
- Silber, J.H.; Cnaan, A.; Clark, B.J.; Paridon, S.M.; Chin, A.J.; Rychik, J.; Hogarty, A.N.; Cohen, M.I.; Barber, G.; Rutkowski, M.; et al. Enalapril to Prevent Cardiac Function Decline in Long-Term Survivors of Pediatric Cancer Exposed to Anthracyclines. J. Clin. Oncol. 2004, 22, 820–828. [Google Scholar] [CrossRef]
- Cheuk, D.K.; Sieswerda, E.; van Dalen, E.C.; Postma, A.; Kremer, L.C. Medical Interventions for Treating Anthracycline-Induced Symptomatic and Asymptomatic Cardiotoxicity During and after Treatment for Childhood Cancer. Cochrane Database Syst. Rev. 2016, 2016, Cd008011. [Google Scholar] [CrossRef]
- He, D.; Hu, J.; Li, Y.; Zeng, X. Preventive Use of Beta-Blockers for Anthracycline-Induced Cardiotoxicity: A Network Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 968534. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Bjork, J.A.; Santos, M.S.; Leino, R.L.; Froberg, M.K.; Moreno, A.J.; Wallace, K.B. Carvedilol-Mediated Antioxidant Protection against Doxorubicin-Induced Cardiac Mitochondrial Toxicity. Toxicol. Appl. Pharmacol. 2004, 200, 159–168. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Tolba, O.A.; El-Shanshory, M.R.; El-Hawary, E.E. Protective Effect of Carvedilol on Adriamycin-Induced Left Ventricular Dysfunction in Children with Acute Lymphoblastic Leukemia. J. Card. Fail. 2012, 18, 607–613. [Google Scholar] [CrossRef]
- Henninger, C.; Fritz, G. Statins in Anthracycline-Induced Cardiotoxicity: Rac and Rho, and the Heartbreakers. Cell Death Dis. 2017, 8, e2564. [Google Scholar] [CrossRef]
- Pluimakers, V.G.; van Waas, M.; Neggers, S.; van den Heuvel-Eibrink, M.M. Metabolic Syndrome as Cardiovascular Risk Factor in Childhood Cancer Survivors. Crit. Rev. Oncol. Hematol. 2019, 133, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Feijen, E.A.M.; Leisenring, W.M.; Stratton, K.L.; Ness, K.K.; van der Pal, H.J.H.; van Dalen, E.C.; Armstrong, G.T.; Aune, G.J.; Green, D.M.; Hudson, M.M.; et al. Derivation of Anthracycline and Anthraquinone Equivalence Ratios to Doxorubicin for Late-Onset Cardiotoxicity. JAMA Oncol. 2019, 5, 864–871. [Google Scholar] [CrossRef]
- Moustafa, I.; Saka, S.; Viljoen, M.; Oosthuizen, F. Vitamin E and Levocarnitine as Prophylaxis against Doxorubicin-Induced Cardio Toxicity in the Adult Cancer Patient: A Review. J. Oncol. Pharm. Pract. 2022, 28, 1388–1399. [Google Scholar] [CrossRef]
- Lee, K.J.; Wright, G.; Bryant, H.; Wiggins, L.A.; Dal Zotto, V.L.; Schuler, M.; Malozzi, C.; Cohen, M.V.; Gassman, N.R. Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7439. [Google Scholar] [CrossRef]
- Rankovic, M.; Draginic, N.; Jeremic, J.; Samanovic, A.M.; Stojkov, S.; Mitrovic, S.; Jeremic, N.; Radonjic, T.; Srejovic, I.; Bolevich, S.; et al. Protective Role of Vitamin B(1) in Doxorubicin-Induced Cardiotoxicity in Rats: Focus on Hemodynamic, Redox, and Apoptotic Markers in Heart. Front. Physiol. 2021, 12, 690619. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, E.C.; Caron, H.N.; Dickinson, H.O.; Kremer, L.C. Cardioprotective Interventions for Cancer Patients Receiving Anthracyclines. Cochrane Database Syst. Rev. 2011, 2011, Cd003917. [Google Scholar] [CrossRef]
- Han, Z.; Guo, L.; Yu, X.; Guo, H.; Deng, X.; Yu, J.; Deng, X.; Xu, F.; Zhang, Z.; Huang, Y. Network-Driven Targeted Analysis Reveals That Astragali Radix Alleviates Doxorubicin-Induced Cardiotoxicity by Maintaining Fatty Acid Homeostasis. J. Ethnopharmacol. 2022, 287, 114967. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Guo, J.; Zhang, Q.; Yin, J.; Li, J.; Zhou, W.; Zhang, T.; Yuan, H.; Zhao, J.; Zhang, L.; et al. Erythropoietin Activates Sirt1 to Protect Human Cardiomyocytes against Doxorubicin-Induced Mitochondrial Dysfunction and Toxicity. Toxicol. Lett. 2017, 275, 28–38. [Google Scholar] [CrossRef]
- Li, L.; Takemura, G.; Li, Y.; Miyata, S.; Esaki, M.; Okada, H.; Kanamori, H.; Khai, N.C.; Maruyama, R.; Ogino, A.; et al. Preventive Effect of Erythropoietin on Cardiac Dysfunction in Doxorubicin-Induced Cardiomyopathy. Circulation 2006, 113, 535–543. [Google Scholar] [CrossRef]
- Li, K.; Sung, R.Y.; Huang, W.Z.; Yang, M.; Pong, N.H.; Lee, S.M.; Chan, W.Y.; Zhao, H.; To, M.Y.; Fok, T.F.; et al. Thrombopoietin Protects against in Vitro and in Vivo Cardiotoxicity Induced by Doxorubicin. Circulation 2006, 113, 2211–2220. [Google Scholar] [CrossRef]
- Scott, J.M.; Li, N.; Liu, Q.; Yasui, Y.; Leisenring, W.; Nathan, P.C.; Gibson, T.; Armenian, S.H.; Nilsen, T.S.; Oeffinger, K.C.; et al. Association of Exercise with Mortality in Adult Survivors of Childhood Cancer. JAMA Oncol. 2018, 4, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Bourdon, A.; Grandy, S.A.; Keats, M.R. Aerobic Exercise and Cardiopulmonary Fitness in Childhood Cancer Survivors Treated with a Cardiotoxic Agent: A Meta-Analysis. Support. Care Cancer 2018, 26, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Meeske, K.A.; Menteer, J.; Freyer, D.R. Exercise Recommendations for Childhood Cancer Survivors Exposed to Cardiotoxic Therapies: An Institutional Clinical Practice Initiative. J. Pediatr. Oncol. Nurs. 2012, 29, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Loucks, C.M.; Yan, K.; Tanoshima, R.; Ross, C.J.D.; Rassekh, S.R.; Carleton, B.C. Pharmacogenetic testing to guide therapeutic decision-making and improve outcomes for children undergoing anthracycline-based chemotherapy. Basic Clin. Pharmacol. Toxicol. 2022, 130 (Suppl. S1), 95–99. [Google Scholar] [CrossRef]
- Dionne, F.; Aminkeng, F.; Bhavsar, A.P.; Groeneweg, G.; Smith, A.; Visscher, H.; Rassekh, S.R.; Ross, C.; Carleton, B. An initial health economic evaluation of pharmacogenomic testing in patients treated for childhood cancer with anthracyclines. Pediatr. Blood Cancer 2018, 65, e26887. [Google Scholar] [CrossRef]
| Type | Drug | Mechanism | Study Subject | Challenges |
|---|---|---|---|---|
| Cardioprotective agent | Dexrazoxane | Iron-chelating agent | Human | Dispute of long-term efficacy and untoward effects |
| Liposome | Liposomal anthracycline, PEGylated liposomal doxorubicin | Different drug distribution | Human | Minimal research on pediatric data |
| Neurohormonal antagonists | ACEI, ARB, beta-blocker, | Neurohormonal antagonists | Human | Limited study quality Lack of clinical trials |
| aldosterone antagonist, renin-inhibitor | Animal | |||
| Statins | Atorvastatin | Antioxidative and anti-inflammatory effects | Human | Minimal research on pediatric data |
| Anthracycline derivatives | Epirubicin, daunorubicin, mitoxantrone | Equivalent replacement | Human | Conflicting information |
| Dietary supplements | Vitamins A, B, C, E, glutathione, selenium, l-carnitine, | Antioxidant | Human | Limited efficacy |
| coenzyme Q10, N-acetylcysteine | Mucolytic agent | Human | Limited efficacy | |
| Plant-based treatment | Flavonoids, apigenin, quercetin, pinocembrin | Ongoing | Animal | Lack of clinical trials |
| Hematopoietic stimulator | TPO EPO G-CSF | Ongoing | Animal | Lack of clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ge, S.; Zhang, A. Pediatric Cardio-Oncology: Screening, Risk Stratification, and Prevention of Cardiotoxicity Associated with Anthracyclines. Children 2024, 11, 884. https://doi.org/10.3390/children11070884
Liu X, Ge S, Zhang A. Pediatric Cardio-Oncology: Screening, Risk Stratification, and Prevention of Cardiotoxicity Associated with Anthracyclines. Children. 2024; 11(7):884. https://doi.org/10.3390/children11070884
Chicago/Turabian StyleLiu, Xiaomeng, Shuping Ge, and Aijun Zhang. 2024. "Pediatric Cardio-Oncology: Screening, Risk Stratification, and Prevention of Cardiotoxicity Associated with Anthracyclines" Children 11, no. 7: 884. https://doi.org/10.3390/children11070884






