Relations between Neurocognitive Function and Visual Acuity: A Cross-Sessional Study in a Cohort of Premature Children
Abstract
1. Introduction
2. Materials and Methods
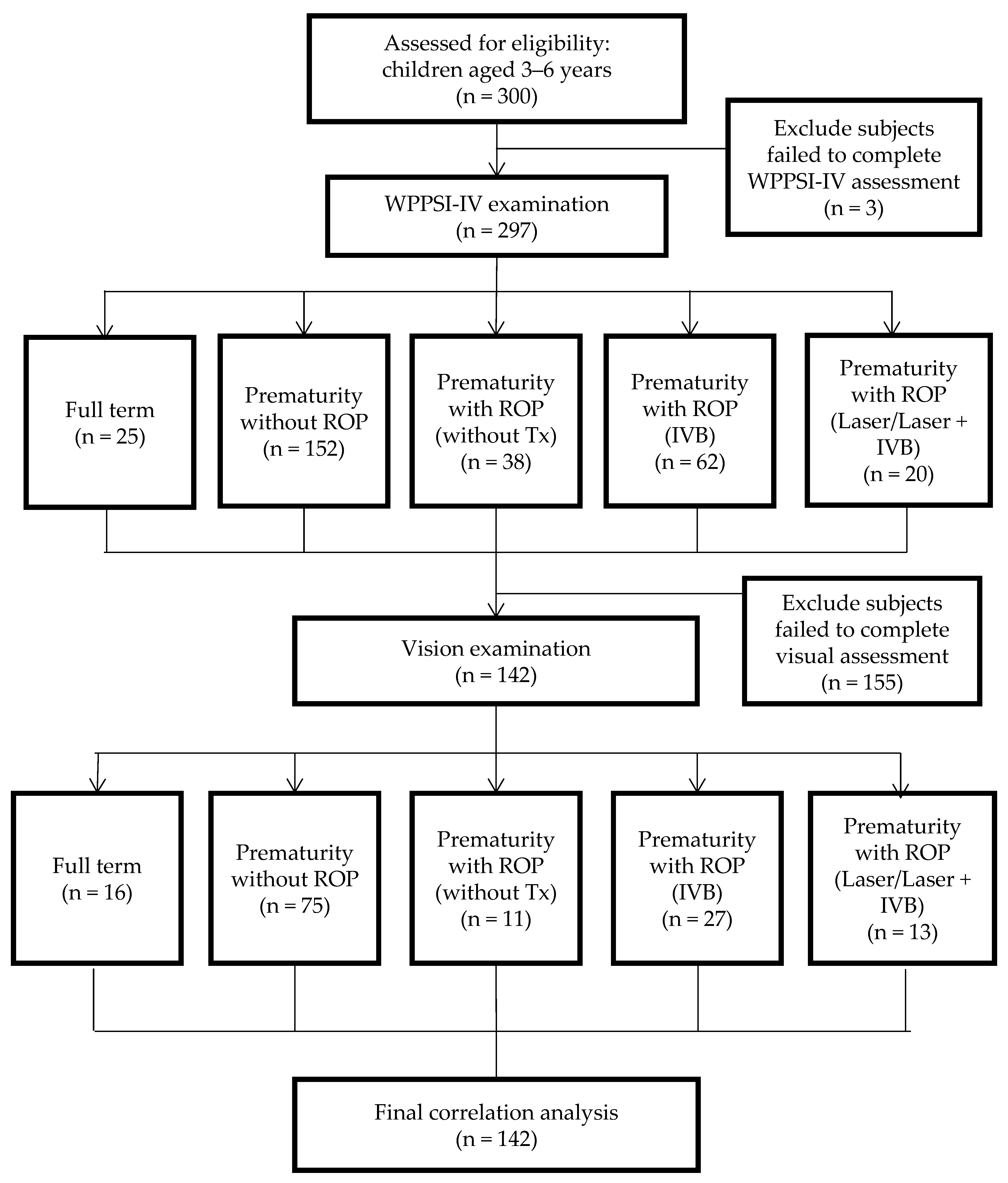
2.1. Procedures and Participants
2.2. Inclusion and Exclusion Criteria
2.3. Grouping
2.4. Data Collection
2.5. Ophthalmic Evaluation and Instruments
2.6. Cognitive Function Evaluation
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Aarnoudse-Moens, C.S.; Weisglas-Kuperus, N.; van Goudoever, J.B.; Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009, 124, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Stalnacke, S.R.; Tessma, M.; Bohm, B.; Herlenius, E. Cognitive Development Trajectories in Preterm Children with Very Low Birth Weight Longitudinally Followed Until 11 Years of Age. Front. Physiol. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 31 March 2023).
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.F.; Tsai, M.H.; Chu, S.M.; Fu, R.H.; Chiang, M.C.; Hwang, F.M.; Kuan, M.J.; Huang, Y.S. Early detection of minor neurodevelopmental dysfunctions at age 6 months in prematurely born neonates. Early Hum. Dev. 2013, 89, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Petrini, J.R.; Dias, T.; McCormick, M.C.; Massolo, M.L.; Green, N.S.; Escobar, G.J. Increased risk of adverse neurological development for late preterm infants. J. Pediatr. 2009, 154, 169–176. [Google Scholar] [CrossRef]
- Linsell, L.; Malouf, R.; Morris, J.; Kurinczuk, J.J.; Marlow, N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or with Very Low Birth Weight: A Systematic Review. JAMA Pediatr. 2015, 169, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, A.T.; Cleves, M.A.; Casey, P.H.; Cradock, M.M.; Anand, K.J. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA 2002, 288, 728–737. [Google Scholar] [CrossRef]
- O’Reilly, H.; Johnson, S.; Ni, Y.; Wolke, D.; Marlow, N. Neuropsychological Outcomes at 19 Years of Age Following Extremely Preterm Birth. Pediatrics 2020, 145, e20192087. [Google Scholar] [CrossRef]
- Twilhaar, E.S.; van Elburg, R.M.; Oosterlaan, J. Need for Further Analysis in Cognitive Outcomes of Children Born Preterm. JAMA Pediatr. 2018, 172, 889–890. [Google Scholar] [CrossRef]
- Stalnacke, J.; Lundequist, A.; Bohm, B.; Forssberg, H.; Smedler, A.C. Individual cognitive patterns and developmental trajectories after preterm birth. Child. Neuropsychol. 2015, 21, 648–667. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.S.; Santhakumaran, S.; Cowan, F.M.; Modi, N.; Medicines for Neonates Investigator Group. Developmental Assessments in Preterm Children: A Meta-analysis. Pediatrics 2016, 138, e20160251. [Google Scholar] [CrossRef] [PubMed]
- Mangin, K.S.; Horwood, L.J.; Woodward, L.J. Cognitive Development Trajectories of Very Preterm and Typically Developing Children. Child. Dev. 2017, 88, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.P.; Thompson, B.; Black, J.; Dai, S.; Alsweiler, J.M. The effects of preterm birth on visual development. Clin. Exp. Optom. 2018, 101, 4–12. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, C.G.; Salthouse, T.A. Out of sight, out of mind? Relations between visual acuity and cognition. Psychon. Bull. Rev. 2014, 21, 1202–1208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wheatley, C.M.; Dickinson, J.L.; Mackey, D.A.; Craig, J.E.; Sale, M.M. Retinopathy of prematurity: Recent advances in our understanding. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F78–F82. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C.; Fielder, A.; Gordillo, L.; Quinn, G.; Semiglia, R.; Visintin, P.; Zin, A.; on behalf of the International NO-ROP Group. Characteristics of infants with severe retinopathy of prematurity in countries with low, moderate, and high levels of development: Implications for screening programs. Pediatrics 2005, 115, e518–e525. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum. Dev. 2008, 84, 77–82. [Google Scholar] [CrossRef]
- O’Connor, A.R.; Wilson, C.M.; Fielder, A.R. Ophthalmological problems associated with preterm birth. Eye 2007, 21, 1254–1260. [Google Scholar] [CrossRef]
- Casey, P.H.; Whiteside-Mansell, L.; Barrett, K.; Bradley, R.H.; Gargus, R. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: An 8-year longitudinal evaluation. Pediatrics 2006, 118, 1078–1086. [Google Scholar] [CrossRef]
- Hack, M.; Flannery, D.J.; Schluchter, M.; Cartar, L.; Borawski, E.; Klein, N. Outcomes in young adulthood for very-low-birth-weight infants. N. Engl. J. Med. 2002, 346, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Wada, K.; Arahori, H.; Kuno, N.; Imoto, K.; Iwahashi-Shima, C.; Kusaka, S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am. J. Ophthalmol. 2012, 153, 327–333.e321. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Lien, R.; Liao, P.J.; Wang, N.K.; Chen, Y.P.; Chao, A.N.; Chen, K.J.; Chen, T.L.; Hwang, Y.S.; Lai, C.C. Serum levels of vascular endothelial growth factor and related factors after intravitreous bevacizumab injection for retinopathy of prematurity. JAMA Ophthalmol. 2015, 133, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Morin, J.; Luu, T.M.; Superstein, R.; Ospina, L.H.; Lefebvre, F.; Simard, M.N.; Shah, V.; Shah, P.S.; Kelly, E.N.; Canadian Neonatal, N.; et al. Neurodevelopmental Outcomes Following Bevacizumab Injections for Retinopathy of Prematurity. Pediatrics 2016, 137, e20153218. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Akiyama, M.; Fujiwara, K.; Mori, Y.; Inoue, H.; Seki, E.; Nakama, T.; Tsukamoto, S.; Ochiai, M.; Ohga, S.; et al. Neurodevelopmental outcomes following intravitreal bevacizumab injection in Japanese preterm infants with type 1 retinopathy of prematurity. PLoS ONE 2020, 15, e0230678. [Google Scholar] [CrossRef] [PubMed]
- Hard, A.L.; Hellstrom, A. On safety, pharmacokinetics and dosage of bevacizumab in ROP treatment—A review. Acta Paediatr. 2011, 100, 1523–1527. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Yeh, P.T.; Tsao, P.N.; Chung, Y.E.; Chang, Y.S.; Lai, T.T. Neurodevelopmental Outcomes after Bevacizumab Treatment for Retinopathy of Prematurity: A Meta-analysis. Ophthalmology 2021, 128, 877–888. [Google Scholar] [CrossRef]
- Vohr, B.R.; Stephens, B.E.; Higgins, R.D.; Bann, C.M.; Hintz, S.R.; Das, A.; Newman, J.E.; Peralta-Carcelen, M.; Yolton, K.; Dusick, A.M.; et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J. Pediatr. 2012, 161, 222–228.e223. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Burnett, A. Assessing developmental delay in early childhood—Concerns with the Bayley-III scales. Clin. Neuropsychol. 2017, 31, 371–381. [Google Scholar] [CrossRef]
- Martinez-Castellanos, M.A.; Schwartz, S.; Hernandez-Rojas, M.L.; Kon-Jara, V.A.; Garcia-Aguirre, G.; Guerrero-Naranjo, J.L.; Chan, R.V.; Quiroz-Mercado, H. Long-term effect of antiangiogenic therapy for retinopathy of prematurity up to 5 years of follow-up. Retina 2013, 33, 329–338. [Google Scholar] [CrossRef]
- Lien, R.; Yu, M.H.; Hsu, K.H.; Liao, P.J.; Chen, Y.P.; Lai, C.C.; Wu, W.C. Neurodevelopmental Outcomes in Infants with Retinopathy of Prematurity and Bevacizumab Treatment. PLoS ONE 2016, 11, e0148019. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Huang, Y.S.; Huang, C.Y.; Hsu, J.F.; Shih, C.P.; Hwang, Y.S.; Yao, T.C.; Lai, C.C.; Wu, W.C. Neurodevelopmental Outcomes after Intravitreal Bevacizumab Therapy for Retinopathy of Prematurity: A Prospective Case-Control Study. Ophthalmology 2019, 126, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.D.; Shih, C.P.; Huang, Y.S.; Liu, L.; Lai, C.C.; Chen, K.J.; Hwang, Y.S.; Wu, W.C. Cognitive Outcomes Following Intravitreal Bevacizumab for Retinopathy of Prematurity: 4- to 6-Year Outcomes in a Prospective Cohort. Am. J. Ophthalmol. 2022, 234, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ohnell, H.M.; Andreasson, S.; Granse, L. Dexamethasone Eye Drops for the Treatment of Retinopathy of Prematurity. Ophthalmol. Retina 2022, 6, 181–182. [Google Scholar] [CrossRef]
- Fielder, A.; Blencowe, H.; O’Connor, A.; Gilbert, C. Impact of retinopathy of prematurity on ocular structures and visual functions. Arch. Dis. Child. Fetal Neonatal Ed. 2015, 100, F179–F184. [Google Scholar] [CrossRef] [PubMed]
- Ingvaldsen, S.H.; Hansen, T.I.; Haberg, A.K.; Moholdt, V.; Evensen, K.A.I.; Dammann, O.; Austeng, D.; Morken, T.S. Visual function correlates with neurodevelopment in a population cohort of school-aged children born extremely preterm. Acta Paediatr. 2023, 112, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Fazzi, E.; Bova, S.; Giovenzana, A.; Signorini, S.; Uggetti, C.; Bianchi, P. Cognitive visual dysfunctions in preterm children with periventricular leukomalacia. Dev. Med. Child. Neurol. 2009, 51, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Good, W.V.; Jan, J.E.; Burden, S.K.; Skoczenski, A.; Candy, R. Recent advances in cortical visual impairment. Dev. Med. Child. Neurol. 2001, 43, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Norcia, A.M.; Madan, A.; Tith, S.; Agarwal, R.; Good, W.V. Visual cortical function in very low birth weight infants without retinal or cerebral pathology. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9091–9098. [Google Scholar] [CrossRef]
- Ingvaldsen, S.H.; Morken, T.S.; Austeng, D.; Dammann, O. Visuopathy of prematurity: Is retinopathy just the tip of the iceberg? Pediatr. Res. 2022, 91, 1043–1048. [Google Scholar] [CrossRef]
- Thebaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary dysplasia. Nat. Rev. Dis. Primers 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.A.; Markel, T.A. Preface: Necrotizing enterocolitis. Semin. Pediatr. Surg. 2023, 32, 151303. [Google Scholar] [CrossRef] [PubMed]
- Tsao, P.C. Pathogenesis and Prevention of Intraventricular Hemorrhage in Preterm Infants. J. Korean Neurosurg. Soc. 2023, 66, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.; Shyamsundar, K.; Taank, P.; Singh, A. Periventricular leukomalacia: An ophthalmic perspective. Med. J. Armed Forces India 2021, 77, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Abiramalatha, T.; Bandyopadhyay, T.; Ramaswamy, V.V.; Shaik, N.B.; Thanigainathan, S.; Pullattayil, A.K.; Amboiram, P. Risk Factors for Periventricular Leukomalacia in Preterm Infants: A Systematic Review, Meta-analysis, and GRADE-Based Assessment of Certainty of Evidence. Pediatr. Neurol. 2021, 124, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised Indications for the Treatment of Retinopathy of Prematurity: Results of the Early Treatment for Retinopathy of Prematurity Randomized Trial. Arch. Ophthalmol. 2003, 121, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; , Chen, Y.H. WPPSI-IV (Chinese Version). Available online: www.mytest.com.tw/Infant_page.aspx?title=I_WPPSI_IV (accessed on 24 January 2024).
- Wechsler, D. Wechsler Preschool and Primary Scale of Intelligence, 4th ed.; The Psychological Corporation: San Antonio, TX, USA, 2012. [Google Scholar]
- Syeda, M.M.; Climie, E.A. Test Review: Wechsler Preschool and Primary Scale of Intelligence–Fourth Edition. J. Psychoeduc. Assess. 2014, 32, 265–272. [Google Scholar] [CrossRef]
- Raiford, S.E.; Coalson, D.L.; Gallemore, E. Essentials of WPPSI™-IV Assessment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Wu, W.C.; Yeh, P.T.; Chen, S.N.; Yang, C.M.; Lai, C.C.; Kuo, H.K. Effects and complications of bevacizumab use in patients with retinopathy of prematurity: A multicenter study in taiwan. Ophthalmology 2011, 118, 176–183. [Google Scholar] [CrossRef]
- Stephens, B.E.; Vohr, B.R. Neurodevelopmental outcome of the premature infant. Pediatr. Clin. North Am. 2009, 56, 631–646. [Google Scholar] [CrossRef]
- Murray, A.L.; Scratch, S.E.; Thompson, D.K.; Inder, T.E.; Doyle, L.W.; Anderson, J.F.; Anderson, P.J. Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology 2014, 28, 552–562. [Google Scholar] [CrossRef]
- Johnson, S.; Fawke, J.; Hennessy, E.; Rowell, V.; Thomas, S.; Wolke, D.; Marlow, N. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics 2009, 124, e249–e257. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Min, A.; Kim, K.; Kim, K.A.; Oh, M.Y.; Lee, H.J.; Park, H.K.; Park, H. Cognitive Function, Emotional and Behavioral Problems, and Temperament of Premature Children. Soa Chongsonyon Chongsin Uihak 2019, 30, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kaul, Y.F.; Johansson, M.; Mansson, J.; Stjernqvist, K.; Farooqi, A.; Serenius, F.; Thorell, L.B. Cognitive profiles of extremely preterm children: Full-Scale IQ hides strengths and weaknesses. Acta Paediatr. 2021, 110, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Lohaugen, G.C.; Gramstad, A.; Evensen, K.A.; Martinussen, M.; Lindqvist, S.; Indredavik, M.; Vik, T.; Brubakk, A.M.; Skranes, J. Cognitive profile in young adults born preterm at very low birthweight. Dev. Med. Child. Neurol. 2010, 52, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.W.; Foulder-Hughes, L.; Newsham, D.; Clarke, D. Ophthalmic impairment at 7 years of age in children born very preterm. Arch. Dis. Child. Fetal Neonatal Ed. 2004, 89, F249–F253. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Braddick, O. Visual and visuocognitive development in children born very prematurely. Prog. Brain Res. 2007, 164, 123–149. [Google Scholar] [CrossRef] [PubMed]
- Tomás, C.C.; Oliveira, E.; Sousa, D.; Uba-Chupel, M.; Furtado, G.; Rocha, C.; Teixeira, A.; Ferreira, P.; Alves, C.; Gisin, S.; et al. Proceedings of the 3rd IPLeiria’s International Health Congress: Leiria, Portugal. 6–7 May 2016. BMC Health Serv. Res. 2016, 16 (Suppl. S3), 200. [Google Scholar] [CrossRef]
- Brambring, M.; Tröster, H. The Assessment of Cognitive Development in Blind Infants and Preschoolers. J. Vis. Impair. Blind. 1994, 88, 9–18. [Google Scholar] [CrossRef]
- Roizen, N.; Kasza, K.; Karrison, T.; Mets, M.; Noble, A.G.; Boyer, K.; Swisher, C.; Meier, P.; Remington, J.; Jalbrzikowski, J.; et al. Impact of Visual Impairment on Measures of Cognitive Function for Children with Congenital Toxoplasmosis: Implications for Compensatory Intervention Strategies. Pediatrics 2006, 118, e379–e390. [Google Scholar] [CrossRef]
- Good, W.V.; Hardy, R.J.; Dobson, V.; Palmer, E.A.; Phelps, D.L.; Tung, B.; Redford, M.; Early Treatment for Retinopathy of Prematurity Cooperative Group. Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch. Ophthalmol. 2010, 128, 663–671. [Google Scholar] [CrossRef]
- Chin, W.C.; Wu, W.C.; Hsu, J.F.; Tang, I.; Yao, T.C.; Huang, Y.S. Correlation Analysis of Attention and Intelligence of Preterm Infants at Preschool Age: A Premature Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3357. [Google Scholar] [CrossRef] [PubMed]
- Pouw, W.; Rop, G.; de Koning, B.; Paas, F. The cognitive basis for the split-attention effect. J. Exp. Psychol. Gen. 2019, 148, 2058–2075. [Google Scholar] [CrossRef] [PubMed]
- Böhm, B.; Smedler, A.C.; Forssberg, H. Impulse control, working memory and other executive functions in preterm children when starting school. Acta Paediatr. 2004, 93, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Msall, M.E.; Buck, G.M.; Rogers, B.T.; Catanzaro, N.L. Kindergarten readiness after extreme prematurity. Am. J. Dis. Child. 1992, 146, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
| (Mean ± SD) | 1. Full-Term (n = 25) | 2. Prematurity without ROP (n = 154) | 3. Prematurity with ROP (without Tx) (n = 39) | 4. Prematurity with ROP (IVB) (n = 62) | 5. Prematurity with ROP (Laser/Laser + IVB) (n = 20) | p Value | Post Hoc (Scheffe; Bonferroni) | Effect Size a |
|---|---|---|---|---|---|---|---|---|
| Age (yrs) | 4.06 ± 1.03 | 3.97 ± 0.98 | 3.79 ± 0.75 | 3.98 ± 0.95 | 4.93 ± 1.30 | <0.001 *** | 5 > 2,3,4 | 0.067 |
| Male, n (%) | 17 (68.0%) | 82 (53.2%) | 21 (53.8%) | 35 (56.5%) | 14 (70.0%) | 0.463 | n.s. | |
| GA (wks) | 38.33 ± 2.38 | 31.82 ± 2.98 | 28.52 ± 2.35 | 26.83 ± 2.53 | 25.72 ± 1.49 | <0.001 *** | 1 > 2 > 3,4,5 | 0.615 |
| BW (gm) | 3162.6 ± 494.11 | 1635.68 ± 601.44 | 1074.29 ± 366.28 | 914.66 ± 302.87 | 778.67 ± 147.43 | <0.001 *** | 1 > 2 > 3 > 5 1 > 2 > 4 | 0.628 |
| Apgar score, 1 min | 8.6 ± 0.58 | 7.3 ± 1.61 | 4.95 ± 2.04 | 5.35 ± 2.07 | 4.45 ± 1.88 | <0.001 *** | 1 > 2 > 3,4,5 | 0.345 |
| Apgar score, 5 min | 9.6 ± 0.58 | 8.87 ± 1.25 | 7.34 ± 1.62 | 7.44 ± 1.67 | 6.85 ± 1.46 | <0.001 *** | 2,1 > 3,4,5 | 0.281 |
| ROP stage | <0.001 *** | 0.626 | ||||||
| Stage 1 | 0 (0%) | 0 (0%) | 19 (50%) | 3 (4.9%) | 1 (5.0%) | 3 > 1,2,4,5 | ||
| Stage 2 | 0 (0%) | 0 (0%) | 17 (44.7%) | 6 (9.8%) | 4 (20.0%) | 3,5 > 4 > 2 3 > 1 | ||
| Stage 3 | 0 (0%) | 0 (0%) | 3 (5.3%) | 51 (83.6%) | 15 (75.0%) | 4,5, > 1,2,3 3 > 2 | ||
| Stage 4 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Stage 5 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.6%) | 0 (0%) | |||
| ASD | 1 (4.0%) | 28 (18.2%) | 6 (15.4%) | 19 (30.6%) | 8 (40.0%) | 0.008 ** | 5 > 1 | 0.215 |
| VSD | 0 (0.0%) | 2 (1.3%) | 1 (2.6%) | 0 (0.0%) | 0 (0.0%) | 0.702 | n.s. | |
| BPD | 0 (0.0%) | 34 (22.1%) | 29 (74.4%) | 49 (79.0%) | 20 (100.0%) | <0.001 *** | 3,4,5 > 1,2 | 0.634 |
| Pneumonia | 0 (0.0%) | 16 (10.4%) | 16 (41.0%) | 33 (53.2%) | 12 (60.0%) | <0.001 *** | 3,4,5 > 1,2 | 0.481 |
| Pulmonary hypertension | 0 (0.0%) | 4 (2.6%) | 7 (17.9%) | 7 (11.3%) | 4 (20.0%) | 0.001 ** | 4,5 > 2 | 0.256 |
| PDA | 0 (0.0%) | 28 (18.2%) | 22 (56.4%) | 44 (71.0%) | 12 (60.0%) | <0.001 *** | 3,4,5 > 1,2 | 0.519 |
| RDS | 0 (0.0%) | 88 (57.1%) | 37 (94.9%) | 62 (100.0%) | 20 (100.0%) | <0.001 *** | 3,4,5 > 2 > 1 | 0.619 |
| RDS grade | <0.001 *** | 0.370 | ||||||
| Grade 1 | 0 (0.0%) | 46 (29.9%) | 5 (13.9%) | 6 (10.3%) | 0 (0.0%) | 2 > 1,4 | ||
| Grade 2 | 0 (0.0%) | 18 (11.7%) | 9 (25.0%) | 24 (41.4%) | 5 (29.4%) | 4 > 1,2; 5 > 1 | ||
| Grade 3 | 0 (0.0%) | 18 (11.7%) | 8 (22.2%) | 14 (24.1%) | 8 (47.1%) | 5 > 1,2 | ||
| Grade 4 | 0 (0.0%) | 7 (4.5%) | 11 (30.6%) | 13 (22.4%) | 4 (23.5%) | 3,4,5 > 2; 3 > 1 | ||
| NEC | 0 (0.0%) | 8 (5.2%) | 7 (17.9%) | 8 (12.9%) | 2 (10.0%) | 0.030 * | 3 > 2 | 0.200 |
| NEC Stage | 0.014 * | 0.161 | ||||||
| 1A | 0 (0.0%) | 1 (0.6%) | 4 (10.3%) | 2 (3.2%) | 0 (0.0%) | 5 > 2 | ||
| 1B | 0 (0.0%) | 5 (3.2%) | 3 (7.7%) | 2 (3.2%) | 0 (0.0%) | |||
| 2B | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | 2 (3.2%) | 0 (0.0%) | |||
| 3B | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (3.2%) | 1 (5.3%) | |||
| IVH | 0 (0.0%) | 26 (16.9%) | 14 (35.9%) | 19 (30.6%) | 8 (40.0%) | <0.001 *** | 3,4,5 > 1 | 0.258 |
| IVH Stage (stage 3 &4) | 0 (0.0%) | 5 (3.2%) | 5 (13.2%) | 5 (8.2%) | 1 (5.0%) | 0.082 | n.s. | |
| PVL | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | 1 (1.6%) | 0 (0.0%) | 0.844 | n.s. | |
| Anemia | 0 (0.0%) | 92 (59.7%) | 39 (100.0%) | 62 (100.0%) | 17 (85.0%) | <0.001 *** | 3,4 > 2 > 1 4 > 5 > 1 | 0.610 |
| Blood transfusion | 0 (0.0%) | 88 (57.1%) | 32 (82.1%) | 62 (100.0%) | 20 (100.0%) | <0.001 *** | 4 > 3 > 2 > 1 5 > 2 > 1 | 0.584 |
| Sepsis | 1 (4.0%) | 36 (23.4%) | 17 (43.6%) | 30 (48.4%) | 11 (55.0%) | <0.001 *** | 4,5 > 1,2; 3 > 1 | 0.313 |
| Surfactant use | 0 (0.0%) | 32 (20.9%) | 23 (59.0%) | 47 (77.0%) | 17 (85.0%) | <0.001 *** | 3,4,5 > 1,2 | 0.572 |
| 1. Full-Term (n = 25) | 2. Prematurity without ROP (n = 152) | 3. Prematurity with ROP (without Tx) (n = 38) | 4. Prematurity with ROP (IVB) (n = 62) | 5. Prematurity with ROP (Laser/Laser + IVB) (n = 20) | p Value | Post Hoc (Bonferroni) | Effect Size a | |
|---|---|---|---|---|---|---|---|---|
| FSIQ-I | 100.7 ± 3.98 | 95.03 ± 1.27 | 89.2 ± 2.43 | 88.71 ± 2.19 | 85.07 ± 3.7 | 0.047 * | n.s. | |
| FSIQ-PR | 52.77 ± 7.26 | 39.72 ± 2.32 | 31.60 ± 4.43 | 31.45 ± 3.99 | 23.93 ± 6.75 | 0.100 | n.s. | |
| Index scores | ||||||||
| VCI | 106.77 ± 4.76 | 99.19 ± 1.48 | 94.49 ± 2.92 | 91.41 ± 2.91 | 88.1 ± 5.16 | 0.108 | n.s. | |
| VC-PR | 63.21 ± 7.95 | 48.17 ± 2.49 | 40.07 ± 4.88 | 39.46 ± 4.92 | 27.10 ± 8.63 | 0.086 | n.s. | |
| VSI | 97.76 ± 4.37 | 95.70 ± 1.37 | 93.36 ± 2.68 | 88.71 ± 2.71 | 86.90 ± 4.75 | 0.245 | n.s. | |
| VS-PR | 46.90 ± 7.68 | 43.24 ± 2.40 | 40.22 ± 4.70 | 36.85 ± 4.69 | 30.54 ± 8.32 | 0.666 | n.s. | |
| FRI | 109.58 ± 6.48 | 99.20 ± 2.15 | 88.55 ± 4.60 | 82.56 ± 3.78 | 78.05 ± 5.58 | 0.004 ** | 1,2 > 4,5 | 0.121 |
| FR-PR | 65.15 ± 10.93 | 47.82 ± 3.66 | 27.57 ± 7.79 | 23.09 ± 6.49 | 13.16 ± 9.49 | 0.008 ** | n.s. | |
| WMI | 92.18 ± 3.88 | 94.42 ± 1.23 | 87.22 ± 2.36 | 88.83 ± 2.41 | 88.87 ± 4.19 | 0.080 | n.s. | |
| WM-PR | 35.18 ± 7.07 | 40.21 ± 2.23 | 26.60 ± 4.30 | 31.37 ± 4.38 | 29.90 ± 7.63 | 0.072 | n.s. | |
| PSI | 101.24 ± 7.40 | 89.72 ± 2.37 | 87.88 ± 5.53 | 81.14 ± 4.30 | 79.02 ± 6.68 | 0.350 | n.s. | |
| PS-PR | 49.65 ± 10.81 | 34.89 ± 3.47 | 27.26 ± 8.04 | 21.64 ± 6.46 | 21.51 ± 9.93 | 0.405 | n.s. | |
| Subtest scores | ||||||||
| SI | 11.25 ± 1.07 | 9.74 ± 0.39 | 9.41 ± 0.75 | 9.32 ± 0.54 | 9.40 ± 0.79 | 0.683 | n.s. | |
| IN | 10.55 ± 0.78 | 9.40 ± 0.25 | 9.04 ± 0.49 | 9.17 ± 0.44 | 7.86 ± 0.75 | 0.249 | n.s. | |
| OA | 10.24 ± 0.85 | 9.23 ± 0.27 | 8.81 ± 0.53 | 7.79 ± 0.48 | 6.46 ± 0.90 | 0.034 * | 1,2 > 5 | 0.037 |
| BD | 8.81 ± 0.90 | 9.19 ± 0.29 | 8.50 ± 0.54 | 8.02 ± 0.49 | 7.59 ± 0.83 | 0.275 | n.s. | |
| PC | 10.75 ± 1.36 | 9.95 ± 0.45 | 8.40 ± 0.94 | 7.35 ± 0.80 | 5.71 ± 1.22 | 0.034 * | 1,2 > 5 | 0.087 |
| MR | 10.26 ± 0.82 | 9.35 ± 0.26 | 8.15 ± 0.50 | 8.34 ± 0.45 | 8.43 ± 0.79 | 0.190 | n.s. | |
| ZL | 7.76 ± 0.71 | 9.26 ± 0.22 | 8.29 ± 0.44 | 8.12 ± 0.43 | 8.28 ± 0.73 | 0.014 * | n.s. | |
| PM | 9.33 ± 0.83 | 8.75 ± 0.26 | 7.43 ± 0.51 | 8.11 ± 0.52 | 8.30 ± 0.90 | 0.255 | n.s. | |
| CA | 9.77 ± 1.21 | 9.04 ± 0.40 | 7.57 ± 0.84 | 6.96 ± 0.71 | 7.19 ± 1.03 | 0.230 | n.s. | |
| BS | 11.76 ± 1.39 | 8.44 ± 0.46 | 7.12 ± 0.97 | 5.93 ± 0.82 | 4.47 ± 1.22 | 0.020 * | 1 > 4,5 | 0.095 |
| 1. Full-Term (n = 16) | 2. Prematurity without ROP (n = 75) | 3. Prematurity with ROP (without Tx) (n = 11) | 4. Prematurity with ROP (IVB) (n = 27) | 5. Prematurity with ROP (Laser/Laser + IVB) (n = 13) | p Value | Post Hoc (Bonferroni) | Effect Size a | |
|---|---|---|---|---|---|---|---|---|
| Uncorrected VA | 0.17 ± 0.16 | 0.23 ± 0.25 | 0.23 ± 0.14 | 0.3 ± 0.27 | 0.64 ± 0.42 | <0.001 *** | 5 > 1,2,3,4 | 0.182 |
| BCVA | 0.05 ± 0.09 | 0.1 ± 0.14 | 0.08 ± 0.06 | 0.09 ± 0.1 | 0.14 ± 0.15 | 0.384 | n.s. | |
| SPH | 1.02 ± 1.43 | 0.85 ± 1.25 | 0.5 ± 0.68 | 1.07 ± 2.29 | −1.62 ± 3.16 | <0.001 *** | 1,2,3,4 > 5 | 0.167 |
| CYL | −1.13 ± 0.63 | −4.17 ± 16.47 | −1.21 ± 1.1 | −1.13 ± 0.99 | −1.9 ± 0.65 | 0.865 | n.s. | |
| SE | 1 ± 1.89 | −1.09 ± 8.6 | −0.36 ± 1.18 | 1.1 ± 1.35 | −3.18 ± 3.4 | 0.416 | n.s. |
| (a) | ||||
|---|---|---|---|---|
| BCVA | SPH | CYL | SE | |
| Age | −0.207 * | −0.230 ** | −0.037 | −0.141 |
| Gender | 0.054 | 0.084 | −0.147 | −0.079 |
| GA | −0.230 ** | 0.139 | −0.079 | −0.012 ** |
| BW | −0.200 * | 0.148 | −0.095 | −0.010 |
| FSIQ-I | −0.173 * | −0.034 | 0.095 | 0.086 |
| FSIQ-PR | −0.183 * | −0.029 | 0.108 | 0.096 |
| Index scores | ||||
| VCI | −0.176 | −0.081 | 0.062 | 0.040 |
| VC-PR | −0.291 ** | −0.060 | 0.106 | 0.090 |
| VSI | −0.109 | 0.043 | −0.105 | −0.061 |
| VS-PR | −0.107 | 0.050 | −0.138 | −0.088 |
| FRI | −0.098 | 0.039 | 0.057 | 0.063 |
| FR-PR | −0.109 | 0.055 | 0.081 | 0.088 |
| WMI | −0.011 | 0.058 | 0.158 | 0.171 |
| WM-PR | −0.019 | 0.103 | 0.151 | 0.177 |
| PSI | −0.158 | −0.033 | −0.031 | −0.029 |
| PS-PR | −0.200 | −0.013 | 0.010 | 0.020 |
| Subtest scores | ||||
| SI | −0.231 * | −0.194 | 0.000 | −0.043 |
| IN | −0.278 ** | −0.026 | 0.136 | 0.137 |
| OA | −0.055 | 0.041 | −0.181 | −0.130 |
| BD | −0.142 | 0.034 | −0.001 | 0.031 |
| PC | −0.080 | 0.048 | −0.032 | −0.012 |
| MR | −0.059 | −0.067 | 0.083 | 0.041 |
| ZL | −0.141 | 0.007 | 0.237 * | 0.222 |
| PM | 0.133 | 0.071 | 0.030 | 0.058 |
| CA | −0.100 | 0.033 | −0.170 | −0.136 |
| BS | −0.317 ** | −0.004 | 0.117 | 0.120 |
| (b) | ||||
| Variable | BCVA | SPH | CYL | SE |
| FSIQ_I | −0.12 | −0.164 | 0.144 | 0.094 |
| FSIQ_PR | −0.122 | −0.15 | 0.16 | 0.11 |
| VCI | −0.192 * | −0.176 | 0.092 | 0.05 |
| VCI_PR | −0.286 ** | −0.154 | 0.151 | 0.115 |
| VSI | −0.056 | −0.073 | −0.082 | −0.087 |
| VSI_PR | −0.045 | −0.073 | −0.121 | −0.12 |
| FRI | 0.007 | −0.021 | 0.096 | 0.087 |
| FRI_PR | 0.017 | −0.005 | 0.128 | 0.12 |
| WMI | 0.086 | −0.047 | 0.211 | 0.168 |
| WMI_PR | 0.088 | 0.017 | 0.208 | 0.183 |
| PSI | −0.071 | −0.085 | −0.006 | −0.016 |
| PSI_PR | −0.119 | −0.065 | 0.055 | 0.05 |
| SI | −0.108 | −0.255 * | 0.038 | −0.009 |
| IN | −0.299 *** | −0.119 | 0.157 | 0.127 |
| OA | 0.04 | −0.032 | −0.164 | −0.143 |
| BD | −0.083 | −0.061 | 0.027 | 0.017 |
| PC | 0.038 | −0.009 | 0.000 | 0.004 |
| MR | 0.035 | −0.121 | 0.121 | 0.063 |
| ZL | −0.038 | −0.082 | 0.301 * | 0.232 |
| PM | 0.21 * | −0.034 | 0.045 | 0.028 |
| CA | −0.042 | −0.013 | −0.15 | −0.127 |
| BS | −0.228 * | −0.073 | 0.161 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, C.-H.; Wu, W.-C.; Chin, W.-C.; Hsu, S.-C.; Tang, I.; Hsu, J.-F.; Chou, H.-D.; Kang, E.Y.-C.; Huang, Y.-S. Relations between Neurocognitive Function and Visual Acuity: A Cross-Sessional Study in a Cohort of Premature Children. Children 2024, 11, 894. https://doi.org/10.3390/children11080894
Tu C-H, Wu W-C, Chin W-C, Hsu S-C, Tang I, Hsu J-F, Chou H-D, Kang EY-C, Huang Y-S. Relations between Neurocognitive Function and Visual Acuity: A Cross-Sessional Study in a Cohort of Premature Children. Children. 2024; 11(8):894. https://doi.org/10.3390/children11080894
Chicago/Turabian StyleTu, Chun-Hsien, Wei-Chi Wu, Wei-Chih Chin, Shih-Chieh Hsu, I Tang, Jen-Fu Hsu, Hung-Da Chou, Eugene Yu-Chuan Kang, and Yu-Shu Huang. 2024. "Relations between Neurocognitive Function and Visual Acuity: A Cross-Sessional Study in a Cohort of Premature Children" Children 11, no. 8: 894. https://doi.org/10.3390/children11080894
APA StyleTu, C.-H., Wu, W.-C., Chin, W.-C., Hsu, S.-C., Tang, I., Hsu, J.-F., Chou, H.-D., Kang, E. Y.-C., & Huang, Y.-S. (2024). Relations between Neurocognitive Function and Visual Acuity: A Cross-Sessional Study in a Cohort of Premature Children. Children, 11(8), 894. https://doi.org/10.3390/children11080894








