Feasibility of Brain Ultrasound Performed by Nurses in the Evaluation of Newborns Who Are HIV Exposed in Utero and Uninfected: A Pilot Study in Botswana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Maternal and Infant Clinical and Laboratory Data
2.3. Brain US Protocol
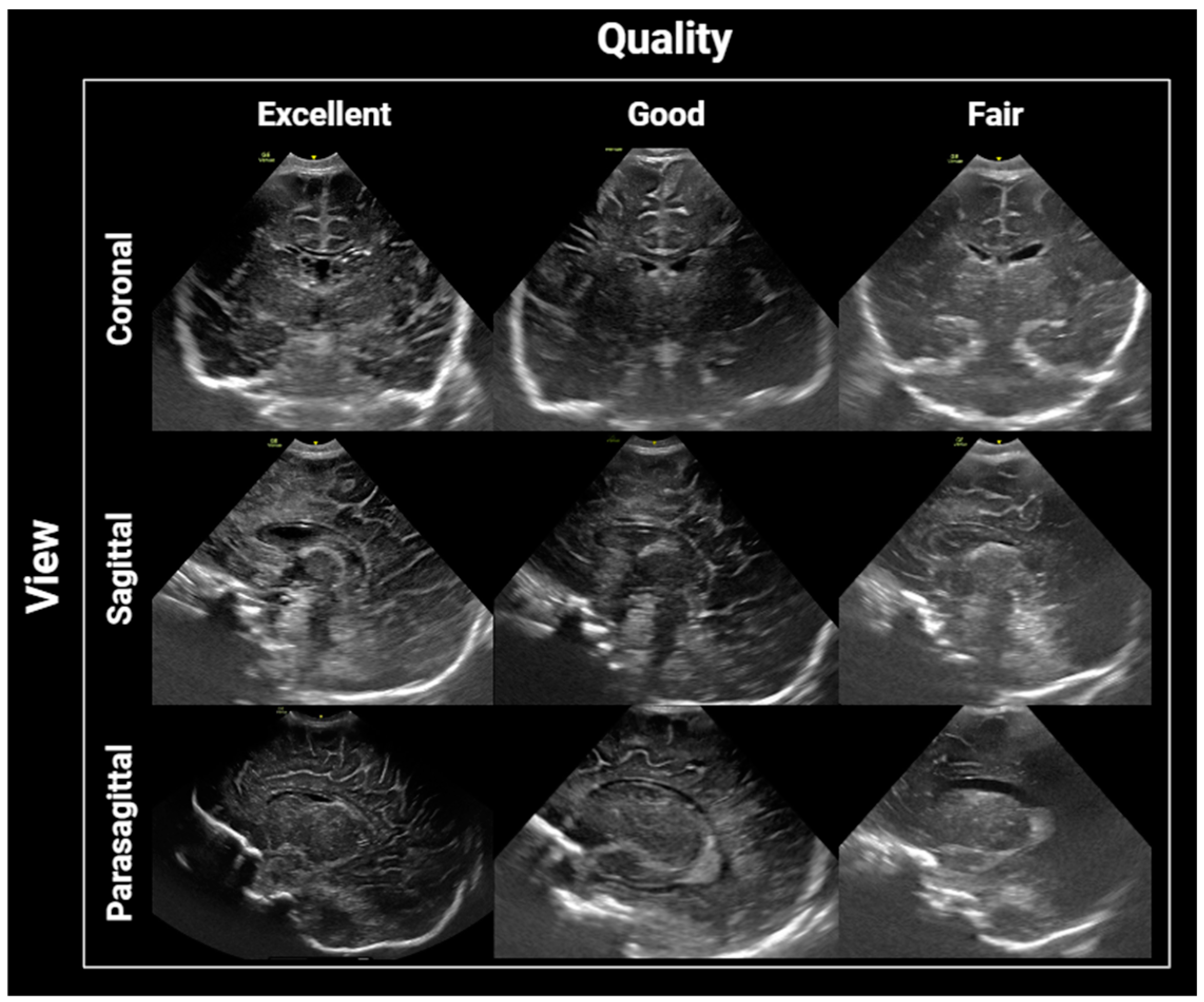
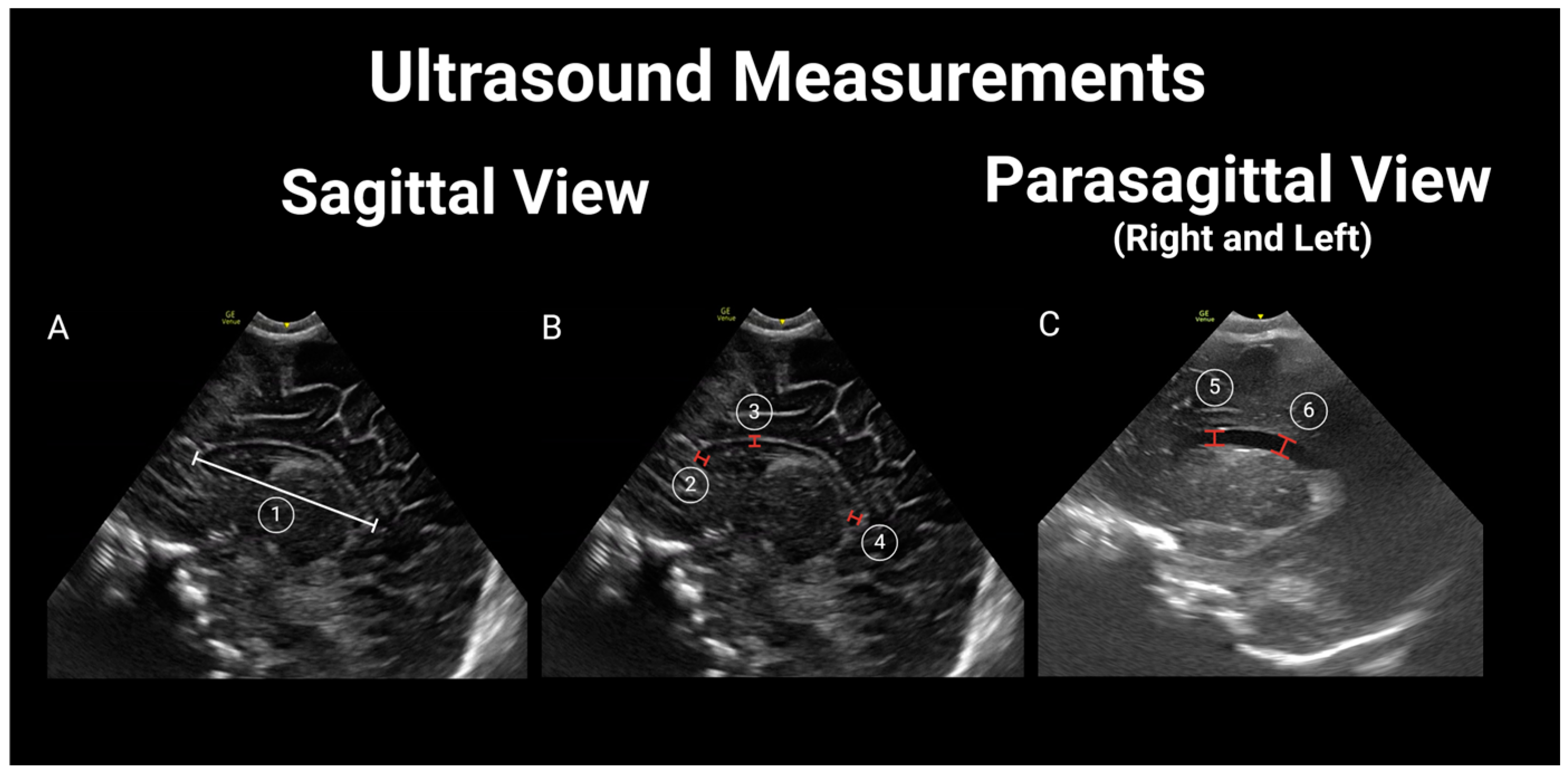
2.4. Image Interpretation and Analysis
2.5. Statistical Analysis
3. Results
3.1. Final Sample
3.2. Image Interpretation and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- George, R.; Andronikou, S.; du Plessis, J.; du Plessis, A.-M.; Van Toorn, R.; Maydell, A. Central nervous system manifestations of HIV infection in children. Pediatr. Radiol. 2009, 39, 575–585. [Google Scholar] [CrossRef]
- Cordeiro, C.N.; Tsimis, M.; Burd, I. Infections and brain development. Obstet. Gynecol. Surv. 2015, 70, 644–655. [Google Scholar] [CrossRef]
- Donald, K.A.; Walker, K.G.; Kilborn, T.; Carrara, H.; Langerak, N.G.; Eley, B.; Wilmshurst, J.M. HIV Encephalopathy: Pediatric case series description and insights from the clinic coalface. AIDS Res. Ther. 2015, 12, 2. [Google Scholar] [CrossRef]
- Slogrove, A.L.; Powis, K.M.; Johnson, L.F.; Stover, J.; Mahy, M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–2018: A modelling study. Lancet Glob. Health 2020, 8, e67–e75. [Google Scholar] [CrossRef]
- Piske, M.; Budd, M.A.; Qiu, A.Q.; Maan, E.J.; Sauvé, L.J.; Forbes, J.C.; Alimenti, A.; Janssen, P.; Côté, H.C.F.; CIHR Team Grant on Cellular Aging and HIV Comorbidities in Women and Children (CARMA). Neurodevelopmental outcomes and in-utero antiretroviral exposure in HIV-exposed uninfected children. AIDS 2018, 32, 2583–2592. [Google Scholar] [CrossRef]
- McHenry, M.S.; Balogun, K.A.; McDonald, B.C.; Vreeman, R.C.; Whipple, E.C.; Serghides, L. In utero exposure to HIV and/or antiretroviral therapy: A systematic review of preclinical and clinical evidence of cognitive outcomes. J. Int. AIDS Soc. 2019, 22, e25275. [Google Scholar] [CrossRef]
- Young, J.M.; Bitnun, A.; Read, S.E.; Smith, M.L. Early academic achievement of HIV-exposed uninfected children compared to HIV-unexposed uninfected children at 5 years of age. Child Neuropsychol. 2021, 27, 532–547. [Google Scholar] [CrossRef]
- Powis, K.M.; Lebanna, L.; Schenkel, S.; Masasa, G.; Kgole, S.W.; Ngwaca, M.; Kgathi, C.; Williams, P.L.; Slogrove, A.L.; Shapiro, R.L.; et al. Lower academic performance among children with perinatal HIV exposure in Botswana. J. Int. AIDS Soc. 2023, 26 (Suppl. S4), e26165. [Google Scholar] [CrossRef]
- Kandawasvika, G.Q.; Ogundipe, E.; Gumbo, F.Z.; Kurewa, E.N.; Mapingure, M.P.; Stray-Pedersen, B. Neurodevelopmental impairment among infants born to mothers infected with human immunodeficiency virus and uninfected mothers from three peri-urban primary care clinics in Harare, Zimbabwe. Dev. Med. Child Neurol. 2011, 53, 1046–1052. [Google Scholar] [CrossRef]
- Benki-Nugent, S.F.; Yunusa, R.; Mueni, A.; Laboso, T.; Tamasha, N.; Njuguna, I.; Gómez, L.; Wamalwa, D.C.; Tapia, K.; Maleche-Obimbo, E.; et al. Lower Neurocognitive Functioning in HIV-Exposed Uninfected Children Compared with That in HIV-Unexposed Children. J. Acquir. Immune Defic. Syndr. 2022, 89, 441–447. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Evans, C. Children who are HIV-exposed and uninfected: Evidence for action. AIDS 2023, 37, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Williams, P.L.; Mayondi, G.K.; Leidner, J.; Holding, P.; Tepper, V.; Nichols, S.; Magetse, J.; Sakoi, M.; Moabi, K.; et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Pediatrics 2017, 140, e20170988. [Google Scholar] [CrossRef]
- Debeaudrap, P.; Bodeau-Livinec, F.; Pasquier, E.; Germanaud, D.; Ndiang, S.T.; Nlend, A.N.; Ndongo, F.A.; Guemkam, G.; Penda, C.I.; Warszawski, J.; et al. Neurodevelopmental outcomes in HIV-infected and uninfected African children. AIDS 2018, 32, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, E.E.; Zeffiro, T.A.; Zeffiro, T.A. Brain Structural Changes following HIV Infection: Meta-Analysis. AJNR Am. J. Neuroradiol. 2018, 39, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Musielak, K.A.; Fine, J.G. An Updated Systematic Review of Neuroimaging Studies of Children and Adolescents with Perinatally Acquired HIV. J. Pediatr. Neuropsychol. 2016, 2, 34–49. [Google Scholar] [CrossRef]
- Jahanshad, N.; Couture, M.-C.; Prasitsuebsai, W.; Nir, T.M.; Aurpibul, L.; Thompson, P.M.; Pruksakaew, K.; Lerdlum, S.; Visrutaratna, P.; Catella, S.; et al. Brain Imaging and Neurodevelopment in HIV-uninfected Thai Children Born to HIV-infected Mothers. Pediatr. Infect. Dis. J. 2015, 34, e211–e216. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Weldon, E.; Bertran-Cobo, C.; Rehman, A.M.; Stein, D.J.; Gibb, D.M.; Yeung, S.; Prendergast, A.J.; Donald, K.A. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2022, 6, 393–408. [Google Scholar] [CrossRef]
- Wedderburn, C.J.; Yeung, S.; Subramoney, S.; Fouche, J.-P.; Joshi, S.H.; Narr, K.L.; Rehman, A.M.; Roos, A.; Gibb, D.M.; Zar, H.J.; et al. Association of in utero HIV exposure with child brain structure and language development: A South African birth cohort study. BMC Med. 2024, 22, 129. [Google Scholar] [CrossRef]
- Magondo, N.; Meintjes, E.M.; Warton, F.L.; Little, F.; van der Kouwe, A.J.W.; Laughton, B.; Jankiewicz, M.; Holmes, M.J. Distinct alterations in white matter properties and organization related to maternal treatment initiation in neonates exposed to HIV but uninfected. Sci. Rep. 2024, 14, 8822. [Google Scholar] [CrossRef]
- Tran, L.T.; Roos, A.; Fouche, J.-P.; Koen, N.; Woods, R.P.; Zar, H.J.; Narr, K.L.; Stein, D.J.; Donald, K.A. White Matter Microstructural Integrity and Neurobehavioral Outcome of HIV-Exposed Uninfected Neonates. Medicine 2016, 95, e2577. [Google Scholar] [CrossRef]
- Jalloul, M.; Miranda-Schaeubinger, M.; Noor, A.M.; Stein, J.M.; Amiruddin, R.; Derbew, H.M.; Mango, V.L.; Akinola, A.; Hart, K.; Weygand, J.; et al. MRI scarcity in low- and middle-income countries. NMR Biomed. 2023, 36, e5022. [Google Scholar] [CrossRef] [PubMed]
- Daneman, A.; Epelman, M.; Blaser, S.; Jarrin, J.R. Imaging of the brain in full-term neonates: Does sonography still play a role? Pediatr. Radiol. 2006, 36, 636–646. [Google Scholar] [CrossRef]
- Dudink, J.; Jeanne Steggerda, S.; Horsch, S.; eurUS.Brain Group. State-of-the-art neonatal cerebral ultrasound: Technique and reporting. Pediatr. Res. 2020, 87, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Abrokwa, S.K.; Ruby, L.C.; Heuvelings, C.C.; Bélard, S. Task shifting for point of care ultrasound in primary healthcare in low- and middle-income countries-a systematic review. EClinicalMedicine 2022, 45, 101333. [Google Scholar] [CrossRef]
- Fleming, K.A.; Horton, S.; Wilson, M.L.; Atun, R.; DeStigter, K.; Flanigan, J.; Sayed, S.; Adam, P.; Aguilar, B.; Andronikou, S.; et al. The Lancet Commission on diagnostics: Transforming access to diagnostics. Lancet 2021, 398, 1997–2050. [Google Scholar] [CrossRef] [PubMed]
- Hand, I.L.; Shellhaas, R.A.; Milla, S.S.; Committee on Fetus and Newborn, Section on Neurology, Section on Radiology. Routine neuroimaging of the preterm brain. Pediatrics 2020, 146, e2020029082. [Google Scholar] [CrossRef]
- Committee on Obstetric Practice, American Institute of Ultrasound in Medicine; Society for Maternal-Fetal Medicine. Committee opinion no 700: Methods for estimating the due date. Obstet. Gynecol. 2017, 129, e150–e154. [Google Scholar] [CrossRef]
- Fox, L.M.; Choo, P.; Rogerson, S.R.; Spittle, A.J.; Anderson, P.J.; Doyle, L.; Cheong, J.L.Y. The relationship between ventricular size at 1 month and outcome at 2 years in infants less than 30 weeks’ gestation. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, F209–F214. [Google Scholar] [CrossRef]
- Cuzzilla, R.; Spittle, A.J.; Lee, K.J.; Rogerson, S.; Cowan, F.M.; Doyle, L.W.; Cheong, J.L.Y. Postnatal brain growth assessed by sequential cranial ultrasonography in infants born <30 weeks’ gestational age. AJNR Am. J. Neuroradiol. 2018, 39, 1170–1176. [Google Scholar] [CrossRef]
- Foss-Skiftesvik, J.; Andresen, M.; Juhler, M. Childhood hydrocephalus—Is radiological morphology associated with etiology. Springerplus 2013, 2, 11. [Google Scholar] [CrossRef]
- Benchoufi, M.; Matzner-Lober, E.; Molinari, N.; Jannot, A.S.; Soyer, P. Interobserver agreement issues in radiology. Diagn. Interv. Imaging 2020, 101, 639–641. [Google Scholar] [CrossRef]
- Mubuuke, A.G.; Erem, G.; Nassanga, R.; Kiguli-Malwadde, E. Point of care obstetric ultrasound training for midwives and nurses: Implementation and experiences of trainees at a rural based hospital in Sub-saharan Africa: A qualitative study. BMC Res. Notes 2023, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Santos, N.; Kisa, R.; Maxwell, O.M.; Mulowooza, J.; Walker, D.; Muruganandan, K.M. Efficacy of an ultrasound training program for nurse midwives to assess high-risk conditions at labor triage in rural Uganda. PLoS ONE 2020, 15, e0235269. [Google Scholar] [CrossRef]
- Vinci, F.; Tiseo, M.; Colosimo, D.; Calandrino, A.; Ramenghi, L.A.; Biasucci, D.G. Point-of-care brain ultrasound and transcranial doppler or color-coded doppler in critically ill neonates and children. Eur. J. Pediatr. 2024, 183, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Kolnik, S.E.; Sahota, A.; Wood, T.R.; German, K.; Puia-Dumitrescu, M.; Mietzsch, U.; Dighe, M.; Law, J.B. Cranial Point-of-Care Ultrasound for Neonatal Providers: A Feasibility Study. J. Ultrasound Med. 2024, 43, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Brunelle, F.; Raybaud, C.; Ball, W.; Barret, B.; Pautard, B.; Lachassine, E.; Mayaux, M.-J.; Blanche, S. Cerebral MR imaging in uninfected children born to HIV-seropositive mothers and perinatally exposed to zidovudine. AJNR Am. J. Neuroradiol. 2005, 26, 695–701. [Google Scholar] [PubMed]
- Wedderburn, C.J.; Groenewold, N.A.; Roos, A.; Yeung, S.; Fouche, J.-P.; Rehman, A.M.; Gibb, D.M.; Narr, K.L.; Zar, H.J.; Stein, D.J.; et al. Early structural brain development in infants exposed to HIV and antiretroviral therapy in utero in a South African birth cohort. J. Int. AIDS Soc. 2022, 25, e25863. [Google Scholar] [CrossRef]
- Barkovich, A.J.; Kjos, B.O. Normal postnatal development of the corpus callosum as demonstrated by MR imaging. AJNR Am. J. Neuroradiol. 1988, 9, 487–491. [Google Scholar]
- Harreld, J.H.; Bhore, R.; Chason, D.P.; Twickler, D.M. Corpus callosum length by gestational age as evaluated by fetal MR imaging. AJNR Am. J. Neuroradiol. 2011, 32, 490–494. [Google Scholar] [CrossRef]
- Kier, E.L.; Truwit, C.L. The normal and abnormal genu of the corpus callosum: An evolutionary, embryologic, anatomic, and MR analysis. AJNR Am. J. Neuroradiol. 1996, 17, 1631–1641. [Google Scholar]
- Andronikou, S.; Ackermann, C.; Laughton, B.; Cotton, M.; Tomazos, N.; Spottiswoode, B.; Mauff, K.; Pettifor, J.M. Corpus callosum thickness on mid-sagittal MRI as a marker of brain volume: A pilot study in children with HIV-related brain disease and controls. Pediatr. Radiol. 2015, 45, 1016–1025. [Google Scholar] [CrossRef]
- Andronikou, S.; Pillay, T.; Gabuza, L.; Mahomed, N.; Naidoo, J.; Hlabangana, L.T.; du Plessis, V.; Prabhu, S.P. Corpus callosum thickness in children: An MR pattern-recognition approach on the midsagittal image. Pediatr. Radiol. 2015, 45, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Jankiewicz, M.; Holmes, M.J.; Taylor, P.A.; Cotton, M.F.; Laughton, B.; van der Kouwe, A.J.W.; Meintjes, E.M. White Matter Abnormalities in Children with HIV Infection and Exposure. Front. Neuroanat. 2017, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Gupta, R.K.; Hashem, S.; Nisar, S.; Azeem, T.; Bhat, A.A.; Syed, N.; Garg, R.K.; Venkatesh, V.; Kamal, M.; et al. Brain microstructural changes support cognitive deficits in HIV uninfected children born to HIV infected mothers. Brain Behav. Immun. Health 2020, 2, 100039. [Google Scholar] [CrossRef]
- Li, J.; Gao, L.; Ye, Z. Study of brain structure in HIV vertically infected adolescents. AIDS Res. Hum. Retroviruses 2021, 37, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Westerhausen, R.; Friesen, C.-M.; Rohani, D.A.; Krogsrud, S.K.; Tamnes, C.K.; Skranes, J.S.; Håberg, A.K.; Fjell, A.M.; Walhovd, K.B. The corpus callosum as anatomical marker of intelligence? A critical examination in a large-scale developmental study. Brain Struct. Funct. 2018, 223, 285–296. [Google Scholar] [CrossRef]
- Dibble, M.; Ang, J.Z.; Mariga, L.; Molloy, E.J.; Bokde, A.L.W. Diffusion Tensor Imaging in Very Preterm, Moderate-Late Preterm and Term-Born Neonates: A Systematic Review. J. Pediatr. 2021, 232, 48–58.e3. [Google Scholar] [CrossRef]
- Nosarti, C.; Rushe, T.M.; Woodruff, P.W.R.; Stewart, A.L.; Rifkin, L.; Murray, R.M. Corpus callosum size and very preterm birth: Relationship to neuropsychological outcome. Brain 2004, 127, 2080–2089. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, Z.; Wang, K.; Moon, B.F.; Zhang, B.; Shen, Y.; Wang, Z.; Zhao, X.; Zhang, X. Assessment of brain structure and volume reveals neurodevelopmental abnormalities in preterm infants with low-grade intraventricular hemorrhage. Sci. Rep. 2024, 14, 5709. [Google Scholar] [CrossRef]
- Lubián-Gutiérrez, M.; Benavente-Fernández, I.; Marín-Almagro, Y.; Jiménez-Luque, N.; Zuazo-Ojeda, A.; Sánchez-Sandoval, Y.; Lubián-López, S.P. Corpus callosum long-term biometry in very preterm children related to cognitive and motor outcomes. Pediatr. Res. 2024, 96, 409–417. [Google Scholar] [CrossRef]
- Hagmann, C.F.; Robertson, N.J.; Acolet, D.; Chan, D.; Onda, S.; Nyombi, N.; Nakakeeto, M.; Cowan, F.M. Cranial ultrasound findings in well newborn Ugandan infants. Arch. Dis. Child. Fetal Neonatal Ed. 2010, 95, F338–F344. [Google Scholar] [CrossRef] [PubMed]
- Mulkey, S.B.; Bulas, D.I.; Vezina, G.; Fourzali, Y.; Morales, A.; Arroyave-Wessel, M.; Swisher, C.B.; Cristante, C.; Russo, S.M.; Encinales, L.; et al. Sequential neuroimaging of the fetus and newborn with in utero zika virus exposure. JAMA Pediatr. 2019, 173, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Richer, E.J.; Riedesel, E.L.; Linam, L.E. Review of neonatal and infant cranial US. Radiographics 2021, 41, E206–E207. [Google Scholar] [CrossRef]
- Richer, E.J.; Riedesel, E.L. Pediatric cranial ultrasound revisited: A comprehensive review. Ultrasound Q. 2024, 40, e00684. [Google Scholar] [CrossRef]
- Robba, C.; Goffi, A.; Geeraerts, T.; Cardim, D.; Via, G.; Czosnyka, M.; Park, S.; Sarwal, A.; Padayachy, L.; Rasulo, F.; et al. Brain ultrasonography: Methodology, basic and advanced principles and clinical applications. A narrative review. Intensive Care Med. 2019, 45, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Terry, B.; Polan, D.L.; Nambaziira, R.; Mugisha, J.; Bisanzo, M.; Gaspari, R. Rapid, remote education for point-of-care ultrasound among non-physician emergency care providers in a resource limited setting. Afr. J. Emerg. Med. 2019, 9, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, K.J.; Uiterwaal, C.S.P.M.; Beek, F.J.A.; van Haastert, I.C.; Lieftink, A.F.; Groenendaal, F.; Grobbee, D.E.; de Vries, L.S. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F489–F493. [Google Scholar] [CrossRef]
- Debillon, T.; N’Guyen, S.; Muet, A.; Quere, M.P.; Moussaly, F.; Roze, J.C. Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F275–F279. [Google Scholar] [CrossRef]
- Bosdriesz, J.R.; Stel, V.S.; van Diepen, M.; Meuleman, Y.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Evidence-based medicine-When observational studies are better than randomized controlled trials. Nephrology 2020, 25, 737–743. [Google Scholar] [CrossRef]
- Meijler, G. Limitations of cranial ultrasonography and recommendations for MRI. In Neonatal Cranial Ultrasonography; Springer: Berlin/Heidelberg, Germany, 2012; pp. 91–95. ISBN 978-3-642-21319-9. [Google Scholar]
- Benavente-Fernández, I.; Ruiz-González, E.; Lubian-Gutiérrez, M.; Lubián-Fernández, S.P.; Cabrales Fontela, Y.; Roca-Cornejo, C.; Olmo-Duran, P.; Lubián-López, S.P. Ultrasonographic estimation of total brain volume: 3D reliability and 2D estimation. enabling routine estimation during NICU admission in the preterm infant. Front. Pediatr. 2021, 9, 708396. [Google Scholar] [CrossRef]

| Variable | HEU (n = 35) | HU (n = 24) | p-Value |
|---|---|---|---|
| Mode of Delivery | 0.5 | ||
| Vaginal | 32 | 20 | |
| C-Section | 3 | 3 | |
| Assisted (vacuum) | 0 | 1 | |
| Gestational age (weeks) (median, IQR) | 39 (37.2, 39.8) | 38 (37.6, 39.1) | 0.33 |
| Boys | 22 (62.9%) | 13 (54.2%) | 0.59 |
| Girls | 13 (37.1%) | 11 (45.8%) | |
| Birth weight (kg) (mean, SD) | 2.96 (0.43) | 3.13 (0.67) | 0.24 |
| Birth length (cm) (mean, SD) | 50.8 (3.04) | 51.3 (4.05) | 0.51 |
| Head circumference (cm) (mean, SD) | 34.03 (1.67) | 34.98 (1.79) | 0.71 |
| Apgar 1-min (n, (%)) | 0.53 | ||
| 9 | 28 (80%) | 17 (70.8%) | |
| 8 | 7 (20%) | 3 (12.5%) | |
| ≤7 | 0 | 4 (16.7%) | |
| Apgar 10-min | 0.38 | ||
| 10 | 34 (97.1%) | 21 (87.5%) | |
| 9 | 1 (2.9%) | 2 (8.3%) | |
| 8 | 0 | 1 (4.2%) | |
| ≤7 | 0 | 0 |
| Variable | Yes | No | Unknown |
|---|---|---|---|
| ART initiated prior to conception | 35 (100%) | 0 | 0 |
| ART (Lamivudine, Dolutegravir, Tenofovir) | 35 | 0 | 0 |
| Treatment interruption (>1 day in a row) | 0 | 35 | 0 |
| Last known CD4 count: mean (SD) | 754.6 (394.9) | ||
| Viral load during pregnancy | |||
| Undetectable | 22 | ||
| 20–400 copies/mL | 9 | ||
| ≥400 copies/mL | 1 | ||
| Unknown | 3 |
| Exposed (n = 35) Median (IQR) | Unexposed (n = 24) Median (IQR) | p-Value ^ | ICC between 2-Readers ^ | |
|---|---|---|---|---|
| Structural findings | n/a | n/a | ||
| Lenticulostriate vasculopathy | 0 | 2 | ||
| Choroid plexus cyst | 3 | 0 | ||
| Subependymal cyst | 1 | 0 | ||
| Subarachnoid spaces | ||||
| Cranio-cortical width | 1.35 (0.91–1.88) | 1.36 (1.03–1.99) | 0.55 | 0.89 |
| Sino-cortical width | 1.75 (1.16–2.52) | 1.85 (1.61–2.36) | 0.57 | 0.90 |
| Interhemispheric fissure width | 1.7 (1.0–2.33) | 1.7 (1.2–2.14) | 0.98 | 0.93 |
| Coronal view: level of third ventricle | ||||
| Biparietal diameter | 84.73 (80.20–87.50) | 85 (83.0–88.56) | 0.31 | 0.96 |
| Evans Index (*) | 0.225 (0.19–0.26) | 0.027 (0.13–0.26) | 0.78 | n/a |
| Lateral ventricular transverse width | 18.96 (15–22.77) | 18.39 (15.29–23.45) | 0.88 | 0.86 |
| Lateral ventricle anterior horn width (right) | 1.13 (0.7–1.52) | 1.15 (0.9–1.76) | 0.45 | 0.49 |
| Lateral ventricle anterior horn width (left) | 1.00 (0.8–1.50) | 1.25 (0.7–1.63) | 0.46 | 0.20 |
| Lateral ventricular index (right) | 9.03 (6.98–10.57) | 9.1 (6.76–11.12) | 0.89 | 0.89 |
| Lateral ventricular index (left) | 8.35 (7–10.72) | 9.27 (6.71–11.11) | 0.98 | 0.83 |
| Parasagittal | ||||
| Ventricle height (right) | 1.3 (1–1.60) | 1.25 (0.8–1.81) | 0.98 | 0.77 |
| Ventricle height (left) | 1.2 (1–1.48) | 1.31 (0.97–1.59) | 0.59 | 0.85 |
| Ventricle midbody height (right) | 2.1 (1.33–2.55) | 1.99 (1.26–2.59) | 0.86 | 0.91 |
| Ventricle midbody height (left) | 2.07 (1.53–2.62) | 1.92 (1.23–2.33) | 0.51 | 0.91 |
| Mid-sagittal plane | ||||
| Corpus callosum length | 45.58 (43.9–47.2) | 47.37 (44.82–49.5) | 0.03 | 0.36 |
| Corpus callosum body height | 1.72 (1.51–1.90) | 1.80 (1.62–2.24) | 0.15 | 0.78 |
| Corpus callosum genu width | 3.95 (3.33–4.55) | 4.2 (3.25–4.95) | 0.5 | 0.47 |
| Corpus callosum splenium width | 3.65 (3.07–4.00) | 3.85 (3.26–4.96) | 0.11 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero, H.J.; Miranda-Schaeubinger, M.; Schenkel, S.R.; Ramirez-Suarez, K.I.; Cerron-Vela, C.R.; Wannasarnmetha, M.; Kgole, S.W.; Masasa, G.; Ngwaca, M.; Phale, B.; et al. Feasibility of Brain Ultrasound Performed by Nurses in the Evaluation of Newborns Who Are HIV Exposed in Utero and Uninfected: A Pilot Study in Botswana. Children 2024, 11, 1039. https://doi.org/10.3390/children11091039
Otero HJ, Miranda-Schaeubinger M, Schenkel SR, Ramirez-Suarez KI, Cerron-Vela CR, Wannasarnmetha M, Kgole SW, Masasa G, Ngwaca M, Phale B, et al. Feasibility of Brain Ultrasound Performed by Nurses in the Evaluation of Newborns Who Are HIV Exposed in Utero and Uninfected: A Pilot Study in Botswana. Children. 2024; 11(9):1039. https://doi.org/10.3390/children11091039
Chicago/Turabian StyleOtero, Hansel J., Monica Miranda-Schaeubinger, Sara Rae Schenkel, Karen I. Ramirez-Suarez, Carmen R. Cerron-Vela, Mix Wannasarnmetha, Samuel W. Kgole, Gosego Masasa, Martha Ngwaca, Boitshepo Phale, and et al. 2024. "Feasibility of Brain Ultrasound Performed by Nurses in the Evaluation of Newborns Who Are HIV Exposed in Utero and Uninfected: A Pilot Study in Botswana" Children 11, no. 9: 1039. https://doi.org/10.3390/children11091039
APA StyleOtero, H. J., Miranda-Schaeubinger, M., Schenkel, S. R., Ramirez-Suarez, K. I., Cerron-Vela, C. R., Wannasarnmetha, M., Kgole, S. W., Masasa, G., Ngwaca, M., Phale, B., Ralegoreng, T., Makhema, J. M., Mokane, T., Lowenthal, E. D., & Powis, K. M. (2024). Feasibility of Brain Ultrasound Performed by Nurses in the Evaluation of Newborns Who Are HIV Exposed in Utero and Uninfected: A Pilot Study in Botswana. Children, 11(9), 1039. https://doi.org/10.3390/children11091039






