Assessing Response Rates and Sleep Disorder Prevalence: Insights from a Propranolol Treatment Study for Infantile Haemangiomas
Abstract
1. Introduction
1.1. Background
1.2. Objectives
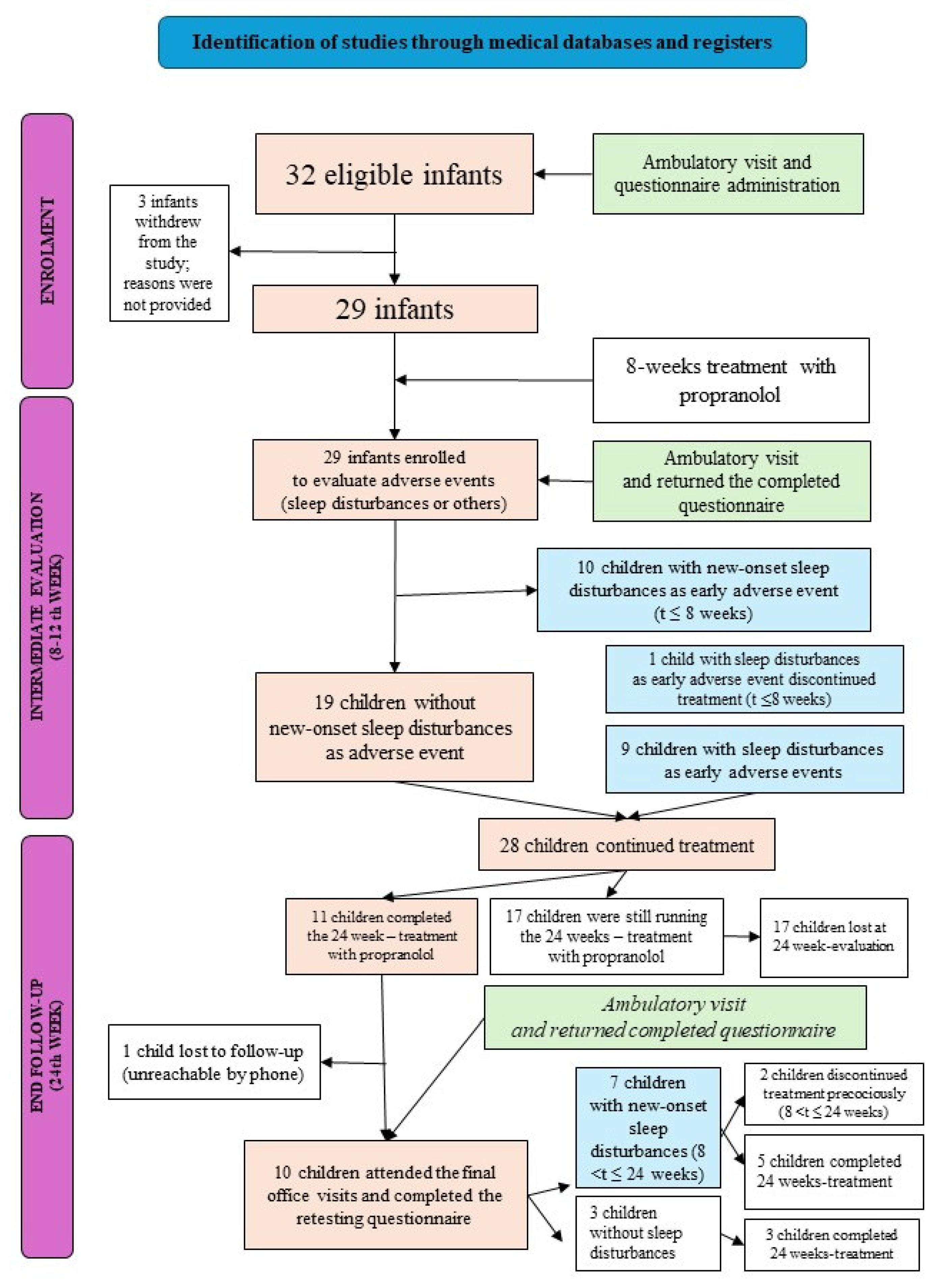
2. Materials and Methods
2.1. Design and Setting of the Study
2.2. Study Population
3. Data Collection
3.1. Office Visits
3.2. Structured Electronic Questionnaire
3.3. Dataset Management
3.4. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADR | Adverse drug reaction |
| AE | Adverse event |
| BISQ | Brief Infant Sleep Questionnaire |
| IHs | Infantile haemangiomas |
| IQR | Interquartile range |
| SD | Standard deviation |
References
- Holm, A.; Mulliken, J.B.; Bischoff, J. Infantile hemangioma: The common and enigmatic vascular tumor. J. Clin. Investig. 2024, 134, e172836. [Google Scholar] [CrossRef] [PubMed]
- Krowchuk, D.P.; Frieden, I.J.; Mancini, A.J.; Darrow, D.H.; Blei, F.; Greene, A.K.; Annam, A.; Baker, C.N.; Frommelt, P.C.; Hodak, A.; et al. Clinical Practice Guideline for the Management of Infantile Hemangiomas. Pediatrics 2019, 143, e20183475. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Guo, S.; Wang, C. Propranolol in the Treatment of Infantile Hemangiomas. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1155–1163. [Google Scholar] [CrossRef]
- Darrow, D.H.; Greene, A.K.; Mancini, A.J.; Nopper, A.J. Diagnosis and Management of Infantile Hemangioma. Pediatrics 2015, 136, e1060–e1104. [Google Scholar] [CrossRef]
- Solman, L.; Glover, M.; Beattie, P.E.; Buckley, H.; Clark, S.; Gach, J.E.; Giardini, A.; Helbling, I.; Hewitt, R.J.; Laguda, B.; et al. Oral propranolol in the treatment of proliferating infantile haemangiomas: British Society for Paediatric Dermatology consensus guidelines. Br. J. Dermatol. 2018, 179, 582–589. [Google Scholar] [CrossRef]
- Macca, L.; Altavilla, D.; Di Bartolomeo, L.; Irrera, N.; Borgia, F.; Li Pomi, F.; Vaccaro, F.; Squadrito, V.; Squadrito, F.; Vaccaro, M. Update on Treatment of Infantile Hemangiomas: What’s New in the Last Five Years? Front. Pharmacol. 2022, 13, 879602. [Google Scholar] [CrossRef] [PubMed]
- Drolet, B.A.; Frommelt, P.C.; Chamlin, S.L.; Haggstrom, A.; Bauman, N.M.; Chiu, Y.E.; Chun, R.H.; Garzon, M.C.; Holland, K.E.; Liberman, L.; et al. Initiation and use of propranolol for infantile hemangioma: Report of a consensus conference. Pediatrics 2013, 131, 128–140. [Google Scholar] [CrossRef]
- Schreiber, P.W.; Sax, H.; Wolfensberger, A.; Clack, L.; Kuster, S.P. The preventable proportion of healthcare-associated infections 2005-2016: Systematic review and meta-analysis. Infect Control Hosp. Epidemiol. 2018, 39, 1277–1295. [Google Scholar] [CrossRef]
- Behar, J.A.; Liu, C.; Kotzen, K.; Tsutsui, K.; Corino, V.D.A.; Singh, J.; Pimentel, M.A.F.; Warrick, P.; Zaunseder, S.; Andreotti, F.; et al. Remote health diagnosis and monitoring in the time of COVID-19. Physiol. Meas. 2020, 41, 10TR01. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.A.; Janecek, E.; Domecq, C.; Greenblatt, D.J. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Mindell, J.A.; Owens, J.A. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA; London, UK, 2009. [Google Scholar]
- Sadeh, A. A brief screening questionnaire for infant sleep problems: Validation and findings for an Internet sample. Pediatrics 2004, 113, e570–e577. [Google Scholar] [CrossRef]
- Mindell, J.A.; Leichman, E.S.; Composto, J.; Lee, C.; Bhullar, B.; Walters, R.M. Development of infant and toddler sleep patterns: Real-world data from a mobile application. J. Sleep Res. 2016, 25, 508–516. [Google Scholar] [CrossRef]
- Hill, J.; Ogle, K.; Santen, S.A.; Gottlieb, M.; Artino, A.R., Jr. Educator’s blueprint: A how-to guide for survey design. AEM Educ. Train. 2022, 6, e10796. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.J. Good surveys guide. BMJ 1991, 302, 302–303. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marqueling, A.L.; Oza, V.; Frieden, I.J.; Puttgen, K.B. Propranolol and infantile hemangiomas four years later: A systematic review. Pediatr. Dermatol. 2013, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Léaute-Labrèze, C.; Boccara, O.; Degrugillier-Chopinet, C.; Mazereeuw-Hautier, J.; Prey, S.; Lebbé, G.; Gautier, S.; Ortis, V.; Lafon, M.; Montagne, A.; et al. Safety of Oral Propranolol for the Treatment of Infantile Hemangioma: A Systematic Review. Pediatrics 2016, 138, e20160353. [Google Scholar] [CrossRef]
- Adair, R.; Bauchner, H.; Philipp, B.; Levenson, S.; Zuckerman, B. Night waking during infancy: Role of parental presence at bedtime. Pediatrics 1991, 87, 500–504. [Google Scholar] [CrossRef]
- Hayes, M.J.; Parker, K.G.; Sallinen, B.; Davare, A.A. Bedsharing, temperament, and sleep disturbance in early childhood. Sleep 2001, 24, 657–662. [Google Scholar] [CrossRef]
- Bruni, O.; Baumgartner, E.; Sette, S.; Ancona, M.; Caso, G.; Di Cosimo, M.E.; Mannini, A.; Ometto, M.; Pasquini, A.; Ulliana, A.; et al. Longitudinal study of sleep behavior in normal infants during the first year of life. J. Clin. Sleep Med. 2014, 10, 1119–1127. [Google Scholar] [CrossRef]
- Hysing, M.; Harvey, A.G.; Torgersen, L.; Ystrom, E.; Reichborn-Kjennerud, T.; Sivertsen, B. Trajectories and predictors of nocturnal awakenings and sleep duration in infants. J. Dev. Behav. Pediatr. 2014, 35, 309–316. [Google Scholar] [CrossRef]
- Sharkey, K.M.; Iko, I.N.; Machan, J.T.; Thompson-Westra, J.; Pearlstein, T.B. Infant sleep and feeding patterns are associated with maternal sleep, stress, and depressed mood in women with a history of major depressive disorder (MDD). Arch. Womens Ment. Health 2016, 19, 209–218. [Google Scholar] [CrossRef]
- Bruni, O.; Alonso-Alconada, D.; Besag, F.; Biran, V.; Braam, W.; Cortese, S.; Moavero, R.; Parisi, P.; Smits, M.; Van der Heijden, K.; et al. Current role of melatonin in pediatric neurology: Clinical recommendations. Eur. J. Paediatr. Neurol. 2015, 19, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Anderson de Moreno, L.C.; Matt, B.H.; Montgomery, G.; Kim, Y.J. Propranolol in the treatment of upper airway hemangiomas. Ear Nose Throat J. 2013, 92, 209–214. [Google Scholar] [CrossRef]
- Theiler, M.; Knöpfel, N.; von der Heydt, S.; Schwieger-Briel, A.; Luchsinger, I.; Smith, A.; Kernland-Lang, K.; Waelchli, R.; Neuhaus, K.; Kohler, M.; et al. Sleep behavior of infants with infantile hemangioma treated with propranolol-a cohort study. Eur. J. Pediatr. 2021, 180, 2655–2668. [Google Scholar] [CrossRef]
- Pandey, V.; Tiwari, P.; Imran, M.; Mishra, A.; Kumar, D.; Sharma, S.P. Adverse Drug Reactions Following Propranolol in Infantile Hemangioma. Indian Pediatr. 2021, 58, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.K.; Kim, S.S. Behavioral insomnia in infants and young children. Clin. Exp. Pediatr. 2021, 64, 111–116. [Google Scholar] [CrossRef] [PubMed]
| Sex (Female/Male) | n/n | 23/6 |
|---|---|---|
| Age at treatment onset, months | Mean (SD) | 5.2 (4.9) |
| Median (IQR) | 4.2 (1.6–7) | |
| IHs classification, n/n total (%) | Superficial | 14/29 (48) |
| Deep | 5/29 (17) | |
| Combined | 10/29 (35) | |
| Problems in pregnancy, n/n total (%) | Twins | 3/29 (10.3) |
| Preterm birth | 7/29 (24) | |
| Maternal or placental problems | 12/29 (41.4) | |
| Indication for propranolol, n/n total (%) | Functional damage | 7/29 (24) |
| Ulceration | 9/29 (31) | |
| Risk of cosmetic permanent damage | 13/29 (45) |
| Sex | Age at Treatment Onset (Months) | Ambulatory Visit: Sleep Disturbance | Questionnaire Assessment: Sleep Disturbance | Additional Declarations | Measures Taken | Causality Assessment (Naranjo’s Algorithm) |
|---|---|---|---|---|---|---|
| Female | 4.5 | Yes | No | ≥3 awakenings/night on most nights, average nocturnal sleep onset time ≥20 min. | Definitive discontinuation of pharmacological therapy after 5 months of treatment. The adverse event was unchanged after drug discontinuation. | Possible |
| Female | 24.2 | Yes | Yes | ≥3 awakenings per night on most nights, average nocturnal sleep onset time ≥20 min. | Definitive discontinuation of pharmacological therapy after 6 months of treatment (IH not yet resolved). The adverse event resolved after drug discontinuation. | Probable |
| Female | 3.5 | No | Yes | Mild | None | Probable |
| Female | 11.6 | No | Yes | Mild | None | Probable |
| Female | 10.8 | No | Yes | ≥3 awakenings per night on most nights with an average nocturnal sleep onset time of ≥20 min | None | Probable |
| Female | 3.7 | No | Yes | The average nocturnal sleep onset time and the duration of wakefulness during the night are prolonged (30 min and 60 min, respectively), with the presence of sudden awakenings accompanied by crying, daytime irritability, and fatigue. | None | Probable |
| Female | 14.4 | Yes | Yes | Daytime irritability | Early and definitive treatment discontinuation (after 1.5 months from initiation), adverse events resolved after drug discontinuations. | Probable |
| Male | 1.8 | No | Yes | Daytime irritability | None | Probable |
| Male | 5.8 | Yes | Yes | Daytime irritability; sleep disturbance (≥3 awakenings per night, average nocturnal sleep onset time ≥20 min). | None | Probable |
| Female | 2.8 | No | Yes | Unconfirmed sleep disturbance and daytime irritability. | None | Doubtful |
| Female | 2.6 | No | Yes | Unconfirmed sleep disturbance and daytime irritability. | None | Doubtful |
| Female | 19.9 | Yes | Yes | Sleep disturbances with ≥3 awakenings per night. | Melatonin treatment. Continuation of therapy with propranolol at total dose. The adverse event was resolved without drug discontinuation. | Possible |
| Female | 4.9 | No | Yes | With hyperhidrosis, the average nocturnal sleep onset time is prolonged to approximately 60 min. | None | Probable |
| Female | 2.1 | No | Yes | Excessive daytime sleepiness and irritability. | Temporary discontinuation of treatment. The adverse event was unchanged at drug discontinuation. | Possible |
| Male | 3.6 | No | Yes | Excessive daytime sleepiness and irritability. | None | Probable |
| Female | 1.3 | No | Yes | Psychomotor agitation during the day, as well as recurrent episodes of bronchospasm; prolonged average nocturnal sleep onset time (approximately 60 min) and ≥3 awakenings per night. | Continuation of therapy at the same dose due to the localisation in the oral cavity with a high risk of complications. | Probable |
| Male | 8.7 | Yes | No | Nocturnal sleep disturbance; daytime fatigue and irritability. | Early and definitive discontinuation of therapy after 2.6 months from the start of treatment, with symptoms reported as fully resolved. | Probable |
| N° | Patient Enrolment | Follow-Up (8 Weeks) | Stop Follow-Up (24 Weeks) | |||
|---|---|---|---|---|---|---|
| O.V. | Q | O.V. | Q | O.V. | Q | |
| Sleep dist. n/n total (%) | 0/29 | (-) | 3/29 (10%) | 10/29 (34%) | 3/10 (30%) | 7/10 (70%) |
| Trial effect n/n total | (-) | (-) | 2/10 | 3/7 | ||
| positive | 1/10 | 2/7 | ||||
| negative | 1/10 | 1/7 | ||||
| not performed | 8/10 | 4/7 | ||||
| Confounders Category | Sleep Disturbances with Possible Confounders | Sleep Disturbances without Possible Confounders | p-Value (Chi-Square Test) |
|---|---|---|---|
| n/n Total (%) | n/n Total (%) | ||
| Onset in “sleep regression” phases: | 12/17 (71%) | 5/12 (42%) | 0.110 |
| “4–5 months” | 5/17 | ||
| “9–11 months” | 4/17 | ||
| “12–14 months” | 2/17 | ||
| “24–25 months” | 1/17 | ||
| Regular time for falling asleep | 13/23 (57%) | 4/6 (67%) | 0.65 |
| To take milk as a preparatory sleep routine | 11/22 (50%) | 6/7 (86%) | 0.09 |
| To fall asleep cradled or in parent’s arms or with physical contact | 15/19 (79%) | 2/10 (20%) | 0.002 |
| To fall asleep with a pacifier | 10/15 (67%) | 7/14 (50%) | 0.36 |
| To fall asleep, take milk | 8/15 (53%) | 9/14 (64%) | 0.54 |
| To fall asleep with electronic objects switched on in the room | 4/8 (50%) | 13/21 (32%) | 0.56 |
| Room-sharing with parents | 13/19 (68%) | 4/10 (40%) | 0.13 |
| Bed-sharing with parents | 14/18 (78%) | 3/11 (27%) | 0.007 |
| Actively cradled or breastfed or moved to parents’ bed at nocturnal awakening | 12/16 (75%) | 5/13 (38%) | 0.046 |
| First-degree family history of sleep disturbances | 3/4 (75%) | 14/25 (56%) | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opri, F.; Opri, R.; Zaffanello, M.; Rigotti, E. Assessing Response Rates and Sleep Disorder Prevalence: Insights from a Propranolol Treatment Study for Infantile Haemangiomas. Children 2024, 11, 1086. https://doi.org/10.3390/children11091086
Opri F, Opri R, Zaffanello M, Rigotti E. Assessing Response Rates and Sleep Disorder Prevalence: Insights from a Propranolol Treatment Study for Infantile Haemangiomas. Children. 2024; 11(9):1086. https://doi.org/10.3390/children11091086
Chicago/Turabian StyleOpri, Francesca, Roberta Opri, Marco Zaffanello, and Erika Rigotti. 2024. "Assessing Response Rates and Sleep Disorder Prevalence: Insights from a Propranolol Treatment Study for Infantile Haemangiomas" Children 11, no. 9: 1086. https://doi.org/10.3390/children11091086
APA StyleOpri, F., Opri, R., Zaffanello, M., & Rigotti, E. (2024). Assessing Response Rates and Sleep Disorder Prevalence: Insights from a Propranolol Treatment Study for Infantile Haemangiomas. Children, 11(9), 1086. https://doi.org/10.3390/children11091086








