Abstract
Background: Neonatal surgical pathology presents highly technical complexity and few opportunities for training. Many of the neonatal surgical entities are not replicable in animal models. Realistic 3D models are a cost-effective and efficient alternative for training new generations of pediatric surgeons. Methods: We conceptualized, designed, and produced an anatomically realistic model for the open correction of jejunoileal atresia. We validated it with two groups of participants (experts and non-experts) through face, construct, and content validity questionnaires. Results: The model was validated by eleven experts and nine non-experts. The mean procedure time for the experts and non-experts groups was 41 and 42 min, respectively. Six non-experts and one expert did not complete the procedure by the designed time (45 min) (p = 0.02). The mean score of face validity was 3.1 out of 4. Regarding construct validity, we found statistically significant differences between groups for the correct calculation of the section length of the antimesenteric border (Nixon’s technique) (p < 0.01). Concerning content validity, the mean score was 3.3 out of 4 in the experts group and 3.4 out of 4 in the non-experts group. Conclusions: The present model is a realistic and low-cost valid option for training for open correction of jejunoileal atresia. Before drawing definitive conclusions, future studies with larger sample sizes and blinded validators are needed.
Keywords:
intestinal atresia; jejunoileal atresia; simulation; silicone; 3D; pediatric surgery; training; model; open surgery 1. Introduction
Pediatric Surgery is a highly complex surgical specialty that involves multiple organs, pathologies, and surgical procedures. In turn, the pediatric patient has unique differential characteristics, such as a lower homeostatic capacity and greater tissue fragility, which justifies the need for extreme delicacy and precision in the surgical act [1]. The training of new generations of pediatric surgeons is strongly conditioned by the possibility of acquiring and training in these complex and demanding technical competencies inherent to the specialty. While in many primary pathologies, the available volume is high and the learning curve is achievable [2], in other scenarios, the low prevalence of the disease conditions the training and learning possibilities.
Neonatal surgery, a significant part of the Pediatric Surgery specialty, exemplifies the above. Low European fertility [3] and recent remarkable improvements in prenatal diagnostic tools (which allow termination of pregnancy in cases of non-viability) [4] largely determine the volume of neonatal surgical pathology available. Lastly, the trend towards the use of minimally invasive techniques with complex and lengthy training curves (such as laparoscopy in congenital duodenal obstruction) [5] and the tendency to centralize cases in referral hospitals [6,7] make it difficult for new generations of residents to acquire the necessary surgical skills in these pathologies. Recent analyses confirm this decrease in index cases and highlight the need to reevaluate training programs and operative exposure in this specialty [8].
Given this situation, several options have been put forward to train new generations of pediatric surgeons: (1) The use of animal models. Multiple surgical training animal models presenting different pathologies have been previously reported. Notable pediatric examples are dismembered pyeloplasty surgery [9] and Swenson transanal endorectal pull-through [10]. Likewise, multiple animals are available depending on the required pathology and surgical anatomy [11]. Although animals have the great advantage of tissue realism and a mammal’s physiological and homeostatic conditions, they are expensive. Apart from that, many of the entities are not replicable in animal models because of their intrinsic characteristics (e.g., jejunoileal atresia, where there is a marked discordance in caliber between the intestinal ends, a situation that is not easy to replicate realistically in animal models). (2) The use of cadavers [12], which involves low availability, a high preparation cost, and a significant bioethical conflict, is not an acceptable resource for everyone. (3) Using simulation models built with different synthetic materials [13,14,15]. The marked industrialization and technological progression we are experiencing are contributing to lowering the design and production costs of these models and making their generalization for the training of pediatric surgeons possible. The most significant handicap of these models is, in many cases, the lack of realism. To the best of our knowledge, there is only one precedent in the literature regarding synthetic intestinal atresia models for the training of pediatric surgeons [16]. The present work aims to design, produce, and validate a low-cost and anatomically realistic model of neonatal jejunoileal atresia for the training of open corrective surgery by pediatric surgeons.
2. Methods
2.1. Conceptualization and Preliminary Design of the Model
For the initial design, necropsic, surgical, and prenatal radiological references of the small bowel (normal, dilated/obstructed, and obliterated/defunctionalized) were obtained [16,17,18,19], and a preliminary range of measures and diameters was established. Autodesk Fusion 360® (Autodesk, CA, USA) was used to design the model’s first iteration and establish an approximate proportional relationship between the two intestinal segments. Iterative adjustments and refinements were performed to ensure the model’s fidelity to neonatal bowel characteristics (Figure 1).
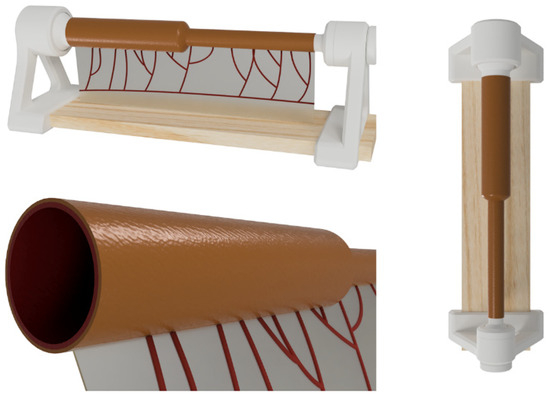
Figure 1.
Images corresponding to the design phase of the model, made in Autodesk Fusion 360® (Autodesk, CA, USA).
2.2. Model Production Methodology
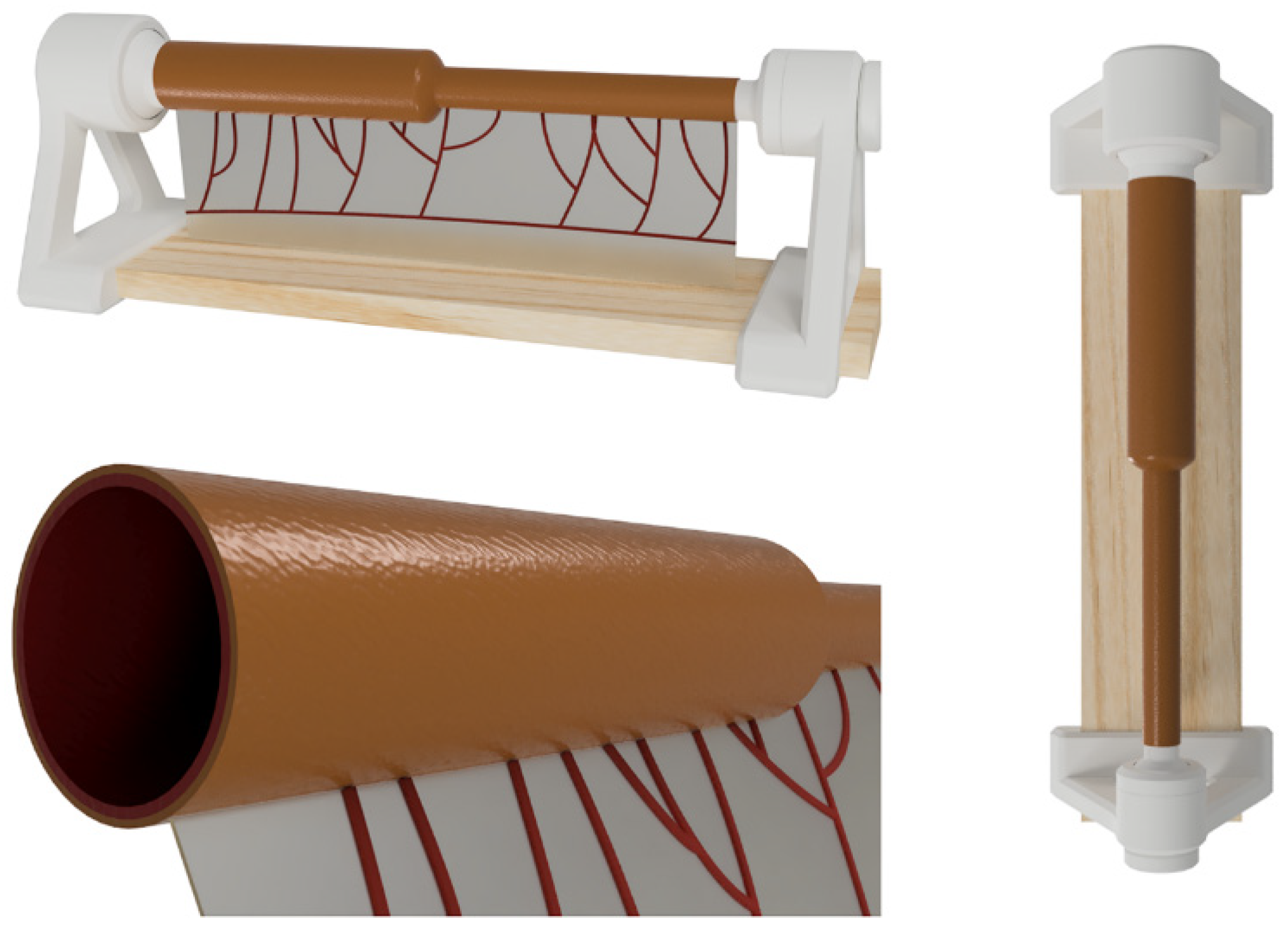
The simulator comprises an anatomical model of jejunoileal atresia with discordant ends and a vascularized mesentery. The model is supported by a specially designed stand for proper usage.
To simulate the mucosa inside the intestine, platinum silicone Eco-Flex 0030 (Smooth-On) with red dye and Silicone Thinner (Smooth-On) additive in a 1A:1B:0.2C ratio were used. The mixture was injected into 3D-printed PLA molds using a Prusa MK3S 3D printer (Prusa Research, Prague, Czech Republic). After a 4-h curing period, the molded cylinder representing atresia was covered with a slightly larger mold to create the serosa layer. This serosa layer, composed of 15 g of Eco-Flex 0030 with a rosy pigment, was cured and introduced into a third mold to add the mesentery to the model. The mesentery was created using 5 g of Eco-Flex 0030 with a red pigment. An arbitrary arboriform vascular pattern was reproduced to simulate real vasculature. Finally, the model was coated with Silicone Thinner, an oily coating, to provide a more realistic experience. A custom-designed stand was manufactured to secure the model, allowing adjustment of rotation and tension without requiring external assistance.
2.3. The Final Version of the Model
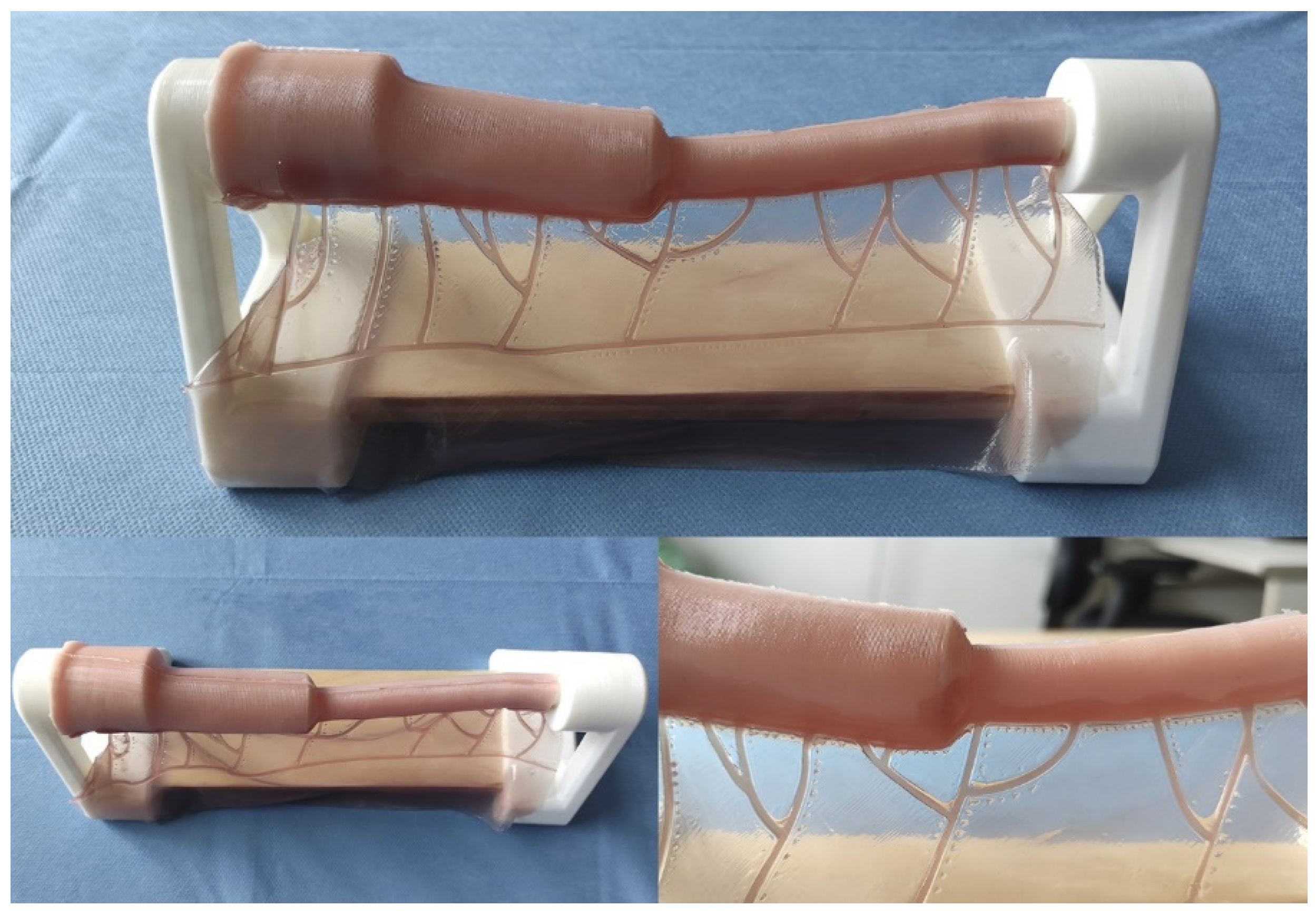
This model constitutes a low-cost and anatomically realistic representation of neonatal jejunoileal atresia with discordant ends, placed on a stand for optimal surgical technique positioning. The proximal intestinal segment (dilated) measures 12 cm in length and has a luminal diameter of 20 mm. It is composed of two platinum silicone layers of different hardness. The inner layer simulates the mucosal-muscular tissue and is 0.8 mm thick, while the outer layer simulates the serosa, is 0.3 mm thick, and is made of stiffer silicone. The distal intestinal segment (atretic) measures 12 cm in length and has a luminal diameter of 8 mm. It replicates the same layers and thicknesses of the proximal intestinal segment end. The simulator also includes a vascularized mesentery made of a thin silicone sheet, facilitating training in vascular control and bowel sectioning skills specific to this type of intervention (Figure 2 and Figure 3).
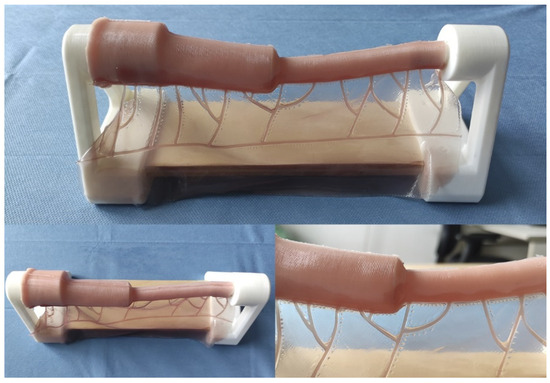
Figure 2.
Appearance of the final model.
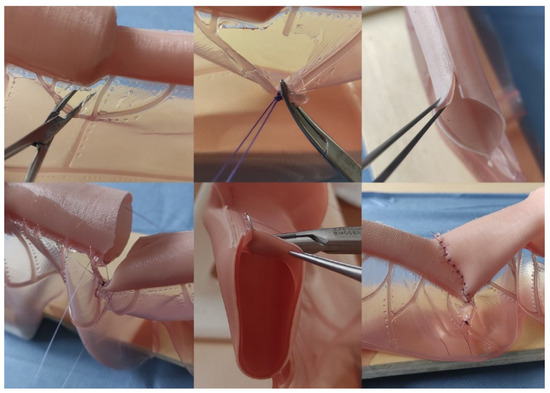
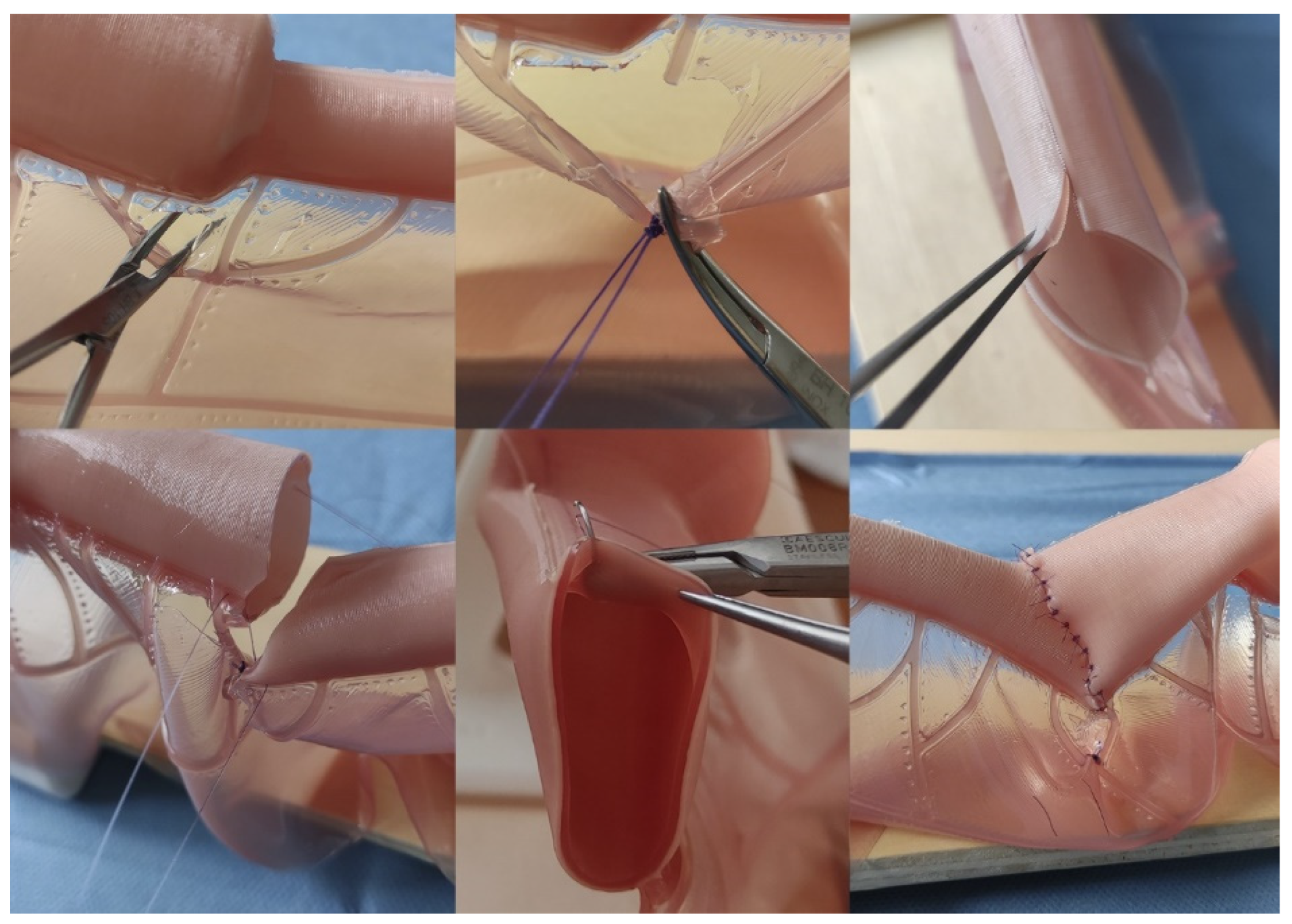
Figure 3.
Representative images of the intestinal atresia open correction performance on the model. Above Left, Center: dissection and ligation of mesenteric vessels; Above Right: distal (atretic) intestinal end after performing anastomotic congruence maneuvers (Benson and Nixon). Bottom Left: lateral references before anastomosis; Bottom Center: detail showing the bilayer structure of the intestinal model and how the needle only goes through the external layer (seromuscular); Bottom Right: Final aspect after the procedure.
The complete model’s production time is 4 h, and the replacement of parts (silicone) takes 3.5 h. The production cost, including materials, labor, and indirect costs (electricity consumption), is EUR 45 for the complete model (including the stand) and EUR 25.21 for the model without the stand. This price is estimated for individual handmade production. An industrialized production process would lower costs and provide greater uniformity, making the product more competitive.
This project and the previously reported model of type III esophageal atresia [13] belong to the SIMUPED® simulation development group.
2.4. Validation
A validation protocol was carried out using two groups of validators: experts (group 1) and non-experts (group 2). Group 1 comprised consultant pediatric surgeons who had performed the procedure on at least one previous occasion. Group 2 consisted of General or Pediatric Surgery residents in their second to fifth year of training. They had basic surgical skills but no specific training in neonatal surgical pathology or the surgical management of intestinal atresia.
Validation was conducted through a two-stage process. (1) First, an instructional video was presented to demonstrate the surgical procedure using the model (Supplementary Material S1). (2) Second, the procedure was performed under the direct visual supervision of two team collaborators (OEB and BPR) with continuous recording of the surgical field for reassessment. Participants did not see or manipulate the model before the validation procedure.
Specific questionnaires and checklists were developed to assess content, face, and construct validity. Each questionnaire included 10 items on content validity, 20 on face validity, and 21 on construct validity. All items were rated on a Likert scale from 1 (strongly disagree) to 4 (strongly agree).
2.5. Statistical Analysis
Continuous quantitative variables were expressed as mean (standard deviation). We used the Mann–Whitney U test to compare these variables. The statistical significance value was set at p = 0.05 (two-tailed). All analyses were performed in STATA 17.0 (StataCorp, LLC 4905 Lakeway Dr, College Station, TX 77845, USA)®.
3. Results
3.1. Construct Validity
The mean procedure time for the experts and non-experts groups was 41 (sd = 3.70) and 42 (sd = 5.15) minutes, respectively.
Two team collaborators completed a checklist regarding the construct validity questionnaire for all the experts (n = 11) and non-experts (n = 9). We found statistically significant differences in the proportion of participants who “Adequately calculates the section length of the antimesenteric border (Nixon technique)”, which was 100% in the expert group and 22.2% in the non-expert group (p = 0.01). Statistically significant differences were also found in the number of validators who completed the procedure within the established time, 90.9% in the expert group (n = 10) and 33% in the non-expert group (n = 3) (p = 0.02). Table 1 shows the comparison between groups for construct validity items.

Table 1.
Construct validity evaluation.
3.2. Face Validity
All the experts (n = 11) responded to the face validity questionnaire. The best-rated items were those concerning the simulation of anastomotic congruence techniques (Benson and Nixon), with an average score of 3.6 out of 4. The worst-rated item was “the model reproduces the surgical dimensions of a neonatal abdominal field”, with a mean score of 2.3 out of 4. The mean score of the face validity questionnaire was 3.1 out of 4 (sd = 0.4). Table 2 shows the mean score of each item in the face validity questionnaire.

Table 2.
Face validity questionnaire.
3.3. Content Validity
All the experts (n = 11) and non-experts (n = 9) responded to the content validity questionnaire. The best-rated item in the experts group was “This model helps the user understand the surgical technique”, with a mean score of 3.9 out of 4. The worst-rated item was “This model helps the user learn how to handle the neonatal bowel and mesenteric structures in a surgical context”, with a mean score of 2.7 out of 4. In the non-experts group, the best-rated items were “This model allows you to LEARN different surgical techniques”, “This model allows you to TRAIN different surgical techniques”, and “This model helps the user to be better prepared when performing corrective surgery for jejunoileal atresia in a neonate for the first time”, with a mean score of 3.6 out of 4. The worst-rated items were “This model allows to EVALUATE the user’s surgical technique” and “This model helps the user understand how intestinal tissue responds to being handled in surgery”, with a mean score of 3.1 out of 4. The mean score of the content validity questionnaire was 3.3 out of 4 for the experts (sd = 0.38) and 3.4 out of 4 for the non-experts (sd = 0.18). Table 3 shows the mean score of each item in the content validity questionnaire.

Table 3.
Content validity questionnaire.
4. Discussion
In the present work, we designed, produced, and validated a low-cost and anatomically realistic model of neonatal intestinal atresia with eleven experts and nine non-experts.
Since the literature in this regard is scarce [16], the most challenging aspect in the design and development of this model was the search for precise references of the anatomical calibers and measures. Pediatric surgeons’ participation in this phase was essential. In this regard, the publication of precise anatomical calibers and measures of the different neonatal pathologies may contribute to developing more realistic models in the future.
Correcting neonatal intestinal atresia requires specific surgical maneuvers, such as anastomotic congruence techniques. This validation study showed the highest scores in the items related to these maneuvers, demonstrating that this model is valid for learning and training. In our experience, the design of a tubular structure simulating the bowel is complex. It requires a delicate balance between the tube maintaining structural integrity and collapsing: excessive stiffness in the tube is unnatural, and too little stiffness collapses the interior and makes the practice equally difficult to perform. In our case, we achieved a reasonably realistic situation. However, after the anastomotic congruence maneuvers (Benson and Nixon), two small silicone apexes in the distal end remained, and they had to be sectioned before anastomosis. Figure 3 (above, right) illustrates these apexes (one of them is gripped by the clamp). Future designs with more realistic materials may solve this minor problem. Likewise, in the future, this model may allow new anastomotic congruency techniques to be designed and trained before they are tested in animals and before they are applied to humans.
One of this model’s most outstanding and innovative elements is the mesentery. Although the characteristics of the mesentery obtained a relatively low score in the face validity questionnaire, the construct validity evaluation showed differences between the two groups (i.e., “Ligates the mesenteric vessels without grasping any of them with the forceps”, which showed a proportion of 63.6% in the experts group and 22.2% in the non-experts group, p = 0.09). Despite the lack of statistical significance (attributable to the study’s limited sample size), this difference suggests that the experts were more able to manipulate delicate tissues than non-experts. Mesenteric surgical principles in the neonate are an integral part of the corrective procedure for neonatal intestinal atresia (both because of the tissue delicacy and these patients’ hemodynamic lability). Therefore, implementing the mesentery in this model constitutes a novelty and opens the way to new design lines.
Although the current trend is towards the development of minimally invasive surgery (MIS), experience in intestinal atresia is limited. In this context, it seems crucial that future specialists acquire the essential surgical skills of open surgery before progressing to MIS. Nevertheless, this model could be introduced in a simulated neonatal abdominal box to train the MIS technique.
Finally, we believe that the intestinal model we have produced (bilayer with differences in the hardness of each layer) allows for multiple types of intestinal suturing (seromuscular, total thickness …), which enriches the user’s training experience. We consider this a substantial improvement and difference from the existing precedent in the literature published by Takazawa et al. [16].
The construct validity showed exciting differences between groups on critical aspects of the surgical procedure (e.g., “Resects only the essential amount of affected intestine”, with 100% in the case of experts and 66.7% in the case of non-experts; p = 0.07). We attribute those items’ lack of statistical significance to the low sample size. The scarcity of pediatric surgeons and their broad geographical dispersion in Spain constituted essential difficulties when recruiting experts.
We believe using simulated models in Pediatric Surgery is promising for several reasons. The first is their low production cost and reproducibility. Animal models, which are expensive and may present some ethical conflicts, have an essential variability that may limit training conditions. Second, the required technology is available worldwide, which is particularly important in low- to middle-income countries. Third, it is easy to set up an individual practice.
Although this manuscript is confined to a pediatric surgical training model, simulation is relevant to all surgical specialties. Recent publications concerning training models in General Surgery [20], Obstetrics [21], and Vascular Surgery [22], among other specialties, attest to this. In our view, creating collaborative networks to share advances in the design and development of these models between specialties and to contribute to better tissue engineering and simulation is essential.
Lastly, new technical resources such as 3D simulation and virtual reality show enormous potential for surgical skills training. Applications such as Lap Mentor® or LapSim® have demonstrated enormous potential in acquiring surgical skills. These devices present a considerable advantage in haptic feedback and simulation of complex scenarios that cannot be easily simulated in synthetic or animal models [23]. Also, a significant advantage of these devices is the absence of consumable consumption and the possibility of reuse at no additional cost. Sustainability and ecology, which have been scarcely considered in surgery until now, are beginning to play a relevant role, and this aspect should be considered for future studies.
Concerning the strengths and limitations of this study, we acknowledge that the small sample size of both groups represents a significant limitation of this study. Furthermore, more complex validation systems (such as pressure sensors or leakage tests) would have provided more objective information. Also, the fact that the team collaborators who completed the construct validity questionnaire were not blinded to the type of participant (expert or non-expert) may have affected the results. Lastly, it should be considered that animal models present intrinsic advantages in terms of training that are not easily replicable, such as the acquisition of hemostasis skills. These represent a challenge for the future in this line of research. On the other hand, the methodological rigor in the study’s design and performance represents this work’s main strength.
In conclusion, we designed, created, and validated a low-cost, realistic model for training neonatal intestinal atresia open surgery. However, further studies with larger sample sizes and external validators blinded to the type of participants are needed before drawing definitive conclusions. Because simulators in Pediatric Surgery may contribute to better global care of children, especially neonates, this line of research should become a priority.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children11091109/s1, Video demonstration of the surgical correction of neonatal intestinal atresia in the model.
Author Contributions
J.A.M.: original idea; literature search; model conceptualization and design; study design; data curation and extraction; project administration; resources; writing—original draft; writing—review and editing; N.L.d.A.C.: model conceptualization and design; model production, project administration; resources; writing—original draft; writing—review and editing; N.M.C.: study design; data curation and extraction; formal analysis; project administration; resources; writing—original draft; writing—review and editing; B.P.P.R. and O.E.B.A.: model production and validation; analysis; resources; writing—original draft; writing—review and editing; F.J.P.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no specific grant from public, commercial, or not-for-profit funding agencies; none of the authors have external funding to declare.
Institutional Review Board Statement
The Research Ethics Committee of the University of Navarra approved this project on 22 December 2022 under code 2022.209.
Informed Consent Statement
All subjects involved in the study gave informed consent before participation. Written informed consent was also obtained from the participants to publish this paper.
Data Availability Statement
The data used to carry out this study are available upon request from the review authors due to personal reason.
Industrial Property
The present model is under industrial property registration and protection under the code U202330721.
Acknowledgments
We sincerely thank the following people for their generous and voluntary participation in validating this simulator; without their feedback and willingness, this project would never have been possible: Alvira Reyes Delgado, Paolo Bragagnini, Ainara González Esdera, Paulina Vargova, Yurema González Ruiz, Marina González Herrera, Mercedes Ruiz de Temiño Bravo, Carolina Corona Bellostas, Rafael Fernández Atuan, Raquel Ros Briones, Lucas Sabattel, Nuria Blanco, Sara Hernández-Martín, Elena Calleja Aguayo, Carlos Bardají Pascual, Helena Linero González, Iker Rodríguez Laguna, Cristian Fernández Romance, Carmen María Gálvez Estévez, and Marina Román Moleón.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tovar, J.A. Pediatric Surgery remains the only true General Surgery. Porto Biomed. J. 2017, 2, 143–144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cummins, C.B.; Bowen-Jallow, K.A.; Tran, S.; Radhakrishnan, R.S. Education of pediatric surgery residents over time: Examining 15 years of case logs. J. Pediatr. Surg. 2021, 56, 85–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ESHRE Capri Workshop Group. Europe the continent with the lowest fertility. Hum. Reprod. Update 2010, 16, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Raboei, E.H. The role of the pediatric surgeon in the perinatal multidisciplinary team. Eur. J. Pediatr. Surg. 2008, 18, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, X.; Wang, X.; Zhao, L.; Lv, Y.; Chen, K. Laparoscopic versus open repair of congenital duodenal obstruction: A systematic review and meta-analysis. Pediatr. Surg. Int. 2022, 38, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Lacher, M.; Barthlen, W.; Eckoldt, F.; Fitze, G.; Fuchs, J.; Hosie, S.; Kaiser, M.M.; Meyer, T.; Muensterer, O.J.; Reinshagen, K.; et al. Operative Volume of Newborn Surgery in German University Hospitals: High Volume Versus Low Volume Centers. Eur. J. Pediatr. Surg. 2022, 32, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sømme, S.; Shahi, N.; McLeod, L.; Torok, M.; McManus, B.; Ziegler, M.M. Neonatal surgery in low- vs. high-volume institutions: A KID inpatient database outcomes and cost study after repair of congenital diaphragmatic hernia, esophageal atresia, and gastroschisis. Pediatr. Surg. Int. 2019, 35, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, Z.; Cortez, A.R.; Potts, J.R., III; Tiao, G.M.; von Allmen, D.; Quillin, R.C., 3rd; Bondoc, A.J.; Garrison, A.P. 10 Year Analysis of Pediatric Surgery Fellowship Match and Operative Experience: Concerning Trends? Ann. Surg. 2023, 277, e475–e482. [Google Scholar] [CrossRef] [PubMed]
- Jhala, T.; Zundel, S.; Szavay, P. Surgical simulation of pediatric laparoscopic dismembered pyeloplasty: Reproducible high-fidelity animal-tissue model. J. Pediatr. Urol. 2021, 17, 833.e1–833.e4. [Google Scholar] [CrossRef] [PubMed]
- Gandra de Meira, M.L.; Buraschi Antunes, R.; de Oliveira Zani, V.; Dutra de Oliveira, G.; Generoso, D.; Ortolan, E.V.P.; Lourenção, P.L.T.A. Developing an Animal Model for Swenson Transanal Endorectal Pull-Through: A New Possibility for Training and Research Purposes. J. Investig. Surg. 2024, 37, 2376548. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Escolino, M.; Draghici, I.; Cerulo, M.; Farina, A.; De Pascale, T.; Cozzolino, S.; Settimi, A. Training Models in Pediatric Minimally Invasive Surgery: Rabbit Model Versus Porcine Model: A Comparative Study. J. Laparoendosc. Adv. Surg. Tech. A 2016, 26, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Miyano, G.; Tanaka, M.; Ikegami, M.; Kato, H.; Seo, S.; Ochi, T.; Koga, H.; Lane, G.J.; Takahashi, M.; et al. Cadaver Training for Minimally Invasive Pediatric Surgery: A Preliminary Report. J. Laparoendosc. Adv. Surg. Tech. A 2021, 31, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Arredondo Montero, J.; Pérez Riveros, B.P.; Bueso Asfura, O.E.; Martín-Calvo, N.; Pueyo, F.J.; López de Aguileta Castaño, N. Development and validation of a realistic type III esophageal atresia simulator for the training of pediatric surgeons. Pediatr. Surg. Int. 2024, 40, 251. [Google Scholar] [CrossRef] [PubMed]
- Joosten, M.; de Blaauw, I.; Botden, S.M. Validated simulation models in pediatric surgery: A review. J. Pediatr. Surg. 2022, 57, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Deonarain, A.R.; Harrison, R.V.; Gordon, K.A.; Looi, T.; Agur, A.M.R.; Estrada, M.; Wolter, N.E.; Propst, E.J. Synthetic Simulator for Surgical Training in Tracheostomy and Open Airway Surgery. Laryngoscope 2021, 131, E2378–E2386. [Google Scholar] [CrossRef] [PubMed]
- Takazawa, S.; Nishi, A.; Ishimaru, T.; Takahashi, M.; Sunouchi, T.; Kikuchi, K.; Koyama, R. Face and construct validity assessment of training models for intestinal anastomosis in low-birth-weight infants. Pediatr. Surg. Int. 2021, 37, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Evetts, A.A.; Shkrum, M.J.; Tugaleva, E. Postmortem Body and Organ Measurements in Neonates and Infants: A Review of Reference Resources Used by Ontario Pathologists (Part 1). Am. J. Forensic Med. Pathol. 2016, 37, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Evetts, A.M.; Shkrum, M.J.; Tugaleva, E. A New Reference Source for Postmortem Body Measurements and Organ Weights in Neonates and Infants: A Statistical Analysis Based on Sudden Death Classification (Part 2). Am. J. Forensic Med. Pathol. 2018, 39, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Lap, C.C.; Voskuilen, C.S.; Pistorius, L.R.; Mulder, E.J.H.; Visser, G.H.A.; Manten, G.T.R. Reference curves for the normal fetal small bowel and colon diameters; their usefulness in fetuses with suspected dilated bowel. J. Matern. Fetal Neonatal Med. 2020, 33, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Margonis, G.A.; Wang, J.J.; Yi, J.; Weng, X.; Lin, C.; Wang, W. Comparative Analysis of 2D and 3D Training Models for Emergency Appendectomy Among Surgical Residents: A Randomized Controlled Study. J. Gastrointest. Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Recker, F.; Schremmer, T.; Berg, C.; Schäfer, V.S.; Strizek, B.; Jimenez-Cruz, J. Advancement of 3D printing technology for the development of a training model in US-guided vesicoamniotic shunting for early LUTO therapy. Acta Obstet. Gynecol. Scand. 2024, 103, 1550–1557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amstutz, C.; Ilic, M.; Fontaine, N.; Siegenthaler, L.; Illi, J.; Haeberlin, A.; Zurbuchen, A.; Burger, J. Development of a patient-specific model of the human coronary system for percutaneous transluminal coronary angioplasty balloon catheter training and testing. Biomed. Eng. Online 2024, 23, 89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ntakakis, G.; Plomariti, C.; Frantzidis, C.; Antoniou, P.E.; Bamidis, P.D.; Tsoulfas, G. Exploring the use of virtual reality in surgical education. World J. Transplant. 2023, 13, 36–43. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).