Changes in Protein Levels during the Storage and Warming of Breast Milk in a Domestic Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Milk Collection, Storage, and Heating Procedures
2.3. Analytical Methods for Determining Total Protein Level
2.4. Statistical Analysis
3. Results
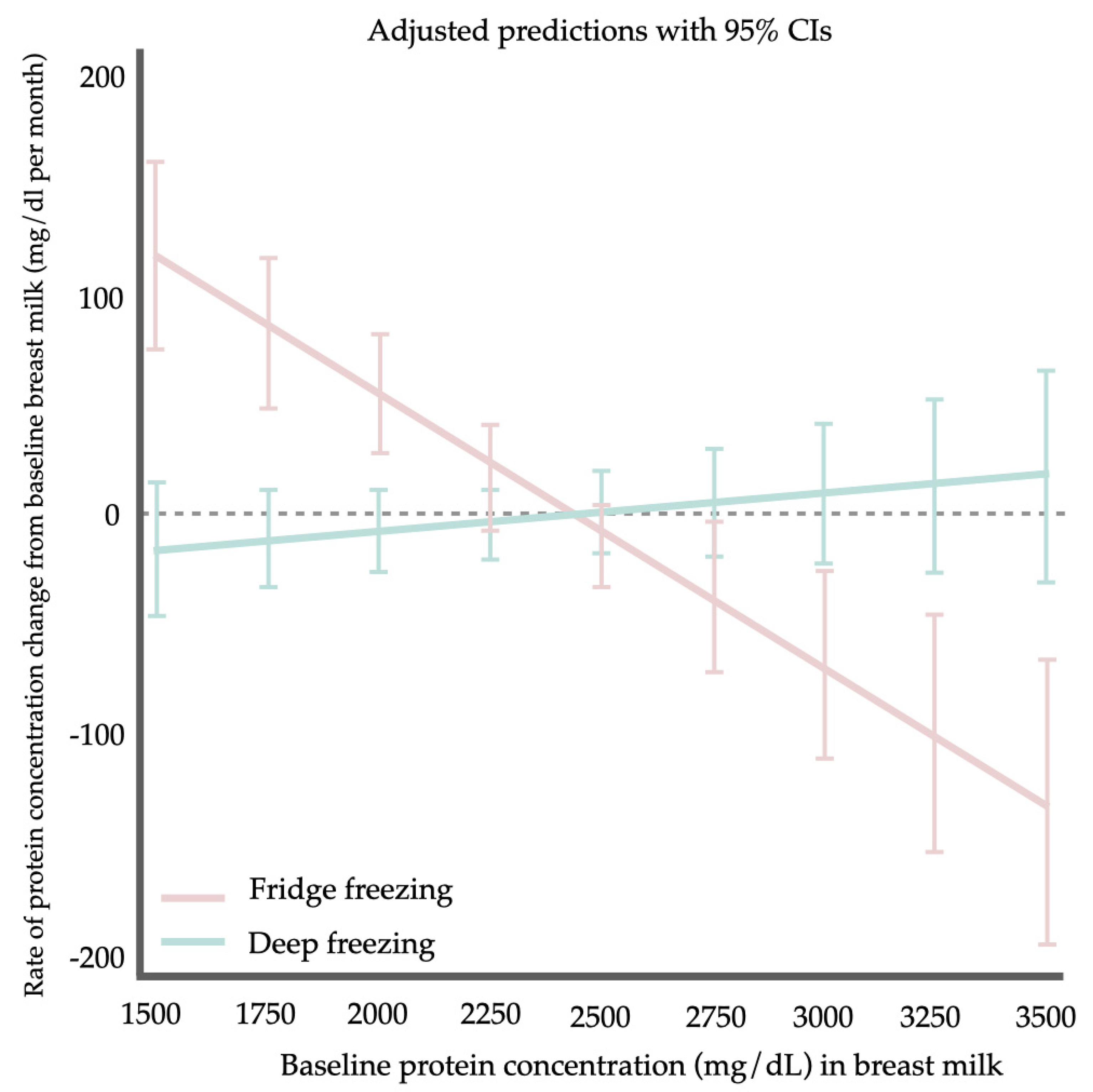
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Donovan, S.M. Human Milk Proteins: Composition and Physiological Significance. Nestle Nutr. Inst. Workshop Ser. 2019, 90, 93–101. [Google Scholar] [CrossRef]
- Meek, J.Y.; Noble, L. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.Y.; Kim, S.Y. Human Breast Milk Composition and Function in Human Health: From Nutritional Components to Microbiome and MicroRNAs. Nutrients 2021, 13, 3094. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Ö.; Çınar, N. The effects of freezing and thawing on mature human milk’s contains: A systematic review. Midwifery 2023, 118, 103519. [Google Scholar] [CrossRef] [PubMed]
- Kasiati; Budi Pertami, S.; Utomo, A.; Arifah, S. Stability of lactoferrin and lysozyme in human milk at various temperatures and duration of storage. Mal. J. Nutr. 2021, 27, 271–278. [Google Scholar] [CrossRef]
- Boyce, J.O.; Reilly, S.; Skeat, J.; Cahir, P. ABM Clinical Protocol #17: Guidelines for Breastfeeding Infants with Cleft Lip, Cleft Palate, or Cleft Lip and Palate-Revised 2019. Breastfeed. Med. 2019, 14, 437–444. [Google Scholar] [CrossRef]
- Ferri, R.L.; Rosen-Carole, C.B.; Jackson, J.; Carreno-Rijo, E.; Greenberg, K.B. ABM Clinical Protocol #33: Lactation Care for Lesbian, Gay, Bisexual, Transgender, Queer, Questioning, Plus Patients. Breastfeed. Med. 2020, 15, 284–293. [Google Scholar] [CrossRef]
- Hernández-Aguilar, M.T.; Bartick, M.; Schreck, P.; Harrel, C. ABM Clinical Protocol #7: Model Maternity Policy Supportive of Breastfeeding. Breastfeed. Med. 2018, 13, 559–574. [Google Scholar] [CrossRef]
- Thomas, J.; Marinelli, K.; Hennessy, M. ABM Clinical Protocol #16: Breastfeeding the Hypotonic Infant. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2007, 2, 112–118. [Google Scholar] [CrossRef]
- Center of Disease Control and Prevention. Storage and Preparation of Breast Milk. Available online: https://www.cdc.gov/breastfeeding/pdf/preparation-of-breast-milk_H.pdf (accessed on 5 December 2023).
- Eglash, A.; Simon, L. ABM Clinical Protocol #8: Human Milk Storage Information for Home Use for Full-Term Infants, Revised 2017. Breastfeed. Med. 2017, 12, 390–395. [Google Scholar] [CrossRef]
- Australian Breastfeeding Association. Storing Expressed Breastmilk. Available online: https://www.breastfeeding.asn.au/resources/storing-ebm (accessed on 5 December 2023).
- DiMaggio, D. Tips for Freezing & Refrigerating Breast Milk. Available online: https://www.healthychildren.org/English/ages-stages/baby/breastfeeding/Pages/Storing-and-Preparing-Expressed-Breast-Milk.aspx?_gl=1*6eziqi*_ga*NjE1ODA1OTguMTcwMTczNTc3Mg..*_ga_FD9D3XZVQQ*MTcwMTczNTc3Mi4xLjAuMTcwMTczNTc4MC4wLjAuMA (accessed on 5 December 2023).
- Ministry of Public Health (MOPH) of Thailand. The Personal Child Health Record Version. 2020. Available online: https://anamai.moph.go.th/th (accessed on 15 July 2024).
- Qu, J.; Zhang, L.; Yin, L.; Liu, J.; Sun, Z.; Zhou, P. Changes in bioactive proteins and serum proteome of human milk under different frozen storage. Food Chem. 2021, 352, 129436. [Google Scholar] [CrossRef] [PubMed]
- Uygur, O.; Yalaz, M.; Can, N.; Koroglu, O.A.; Kultursay, N. Preterm Infants May Better Tolerate Feeds at Temperatures Closer to Freshly Expressed Breast Milk: A Randomized Controlled Trial. Breastfeed. Med. 2019, 14, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Chen, C.H.; Fang, L.J.; Tsai, C.R.; Chang, Y.C.; Wang, T.M. Influence of prolonged storage process, pasteurization, and heat treatment on biologically-active human milk proteins. Pediatr. Neonatol. 2013, 54, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Moro, G.E.; Tonetto, P.; De Nisi, G.; Ambruzzi, A.M.; Biasini, A.; Profeti, C.; Gagliardi, L.; Salvatori, G.; Bertino, E. Recommendations for the establishment and operation of a donor human milk bank. Nutr. Rev. 2023, 81, 1–28. [Google Scholar] [CrossRef]
- Kamizake, N.K.K.; Gonçalves, M.M.; Zaia, C.T.B.V.; Zaia, D.A.M. Determination of total proteins in cow milk powder samples: A comparative study between the Kjeldahl method and spectrophotometric methods. J. Food Compos. Anal. 2003, 16, 507–516. [Google Scholar] [CrossRef]
- Warakaulle, S.; Mohamed, H.; Ranasinghe, M.; Shah, I.; Yanyang, X.; Chen, G.; Ayyash, M.M.; Vincent, D.; Kamal-Eldin, A. Advancement of milk protein analysis: From determination of total proteins to their identification and quantification by proteomic approaches. J. Food Compos. Anal. 2024, 126, 105854. [Google Scholar] [CrossRef]
- Verulkar, S.; Arunima, S. Comparative analysis of different protein estimation methods. J. Pharm. Innov. 2022, 11, 2091–2095. [Google Scholar] [CrossRef]
- Akinbi, H.; Meinzen-Derr, J.; Auer, C.; Ma, Y.; Pullum, D.; Kusano, R.; Reszka, K.J.; Zimmerly, K. Alterations in the host defense properties of human milk following prolonged storage or pasteurization. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 347–352. [Google Scholar] [CrossRef]
- Meng, T.; Perrin, M.T.; Allen, J.C.; Osborne, J.; Jones, F.; Fogleman, A.D. Storage of Unfed and Leftover Pasteurized Human Milk. Breastfeed. Med. 2016, 11, 538–543. [Google Scholar] [CrossRef]
- Paulaviciene, I.J.; Liubsys, A.; Eidukaite, A.; Molyte, A.; Tamuliene, L.; Usonis, V. The Effect of Prolonged Freezing and Holder Pasteurization on the Macronutrient and Bioactive Protein Compositions of Human Milk. Breastfeed. Med. 2020, 15, 583–588. [Google Scholar] [CrossRef]
- Ahrabi, A.F.; Handa, D.; Codipilly, C.N.; Shah, S.; Williams, J.E.; McGuire, M.A.; Potak, D.; Aharon, G.G.; Schanler, R.J. Effects of Extended Freezer Storage on the Integrity of Human Milk. J. Pediatr. 2016, 177, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Handa, D.; Ahrabi, A.F.; Codipilly, C.N.; Shah, S.; Ruff, S.; Potak, D.; Williams, J.E.; McGuire, M.A.; Schanler, R.J. Do thawing and warming affect the integrity of human milk? J. Perinatol. 2014, 34, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Siviroj, P.; Ruangsuriya, J.; Phanpong, C.; Sirikul, W.; Ongprasert, K. Comparison of Effects of Storage at Different Temperatures in a Refrigerator, Upright Freezer on Top of Refrigerator, and Deep Freezer on the Immunoglobulin A Concentration and Lysozyme Activity of Human Milk. Int. J. Environ. Res. Public Health 2022, 19, 13203. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Siviroj, P.; Ruangsuriya, J.; Yousaibua, N.; Ongprasert, K. Effects of the thawing rate and heating temperature on immunoglobulin A and lysozyme activity in human milk. Int. Breastfeed. J. 2022, 17, 52. [Google Scholar] [CrossRef]
- Binder, C.; Baumgartner-Parzer, S.; Gard, L.I.; Berger, A.; Thajer, A. Human Milk Processing and Its Effect on Protein and Leptin Concentrations. Nutrients 2023, 15, 347. [Google Scholar] [CrossRef]
- Eduard, M.; Silvia, S.; Danilmary, S.; Alfonso, B.; Marlon, R.; Marcela, V. Changes in Protein Composition of Mature Breast Milk During Storage by Freezing. Pediatría 2010, 37, 187–194. [Google Scholar]
- Yochpaz, S.; Mimouni, F.B.; Mandel, D.; Lubetzky, R.; Marom, R. Effect of Freezing and Thawing on Human Milk Macronutrients and Energy Composition: A Systematic Review and Meta-Analysis. Breastfeed. Med. 2020, 15, 559–562. [Google Scholar] [CrossRef]
- Blanco, A. Proteins. In Medical Biochemistry; Blanco, A., Blanco, G., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 21–71. [Google Scholar]
- Păduraru, L.; Zonda, G.I.; Avasiloaiei, A.L.; Moscalu, M.; Dimitriu, D.C.; Stamatin, M. Influence of refrigeration or freezing on human milk macronutrients and energy content in early lactation: Results from a tertiary centre survey. Paediatr. Child Health 2019, 24, 250–257. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef]
- Qian, F.; Sun, J.; Cao, D.; Tuo, Y.; Jiang, S.; Mu, G. Experimental and Modelling Study of the Denaturation of Milk Protein by Heat Treatment. Korean J. Food Sci. Anim. Resour. 2017, 37, 44–51. [Google Scholar] [CrossRef]
- Stefanou, C.R.; Szosland-Fałtyn, A.; Bartodziejska, B. Survey of Domestic Refrigerator Storage Temperatures in Poland for Use as a QMRA Tool for Exposure Assessment. Int. J. Environ. Res. Public Health 2023, 20, 2924. [Google Scholar] [CrossRef]
- Ovca, A.; Škufca, T.; Jevšnik, M. Temperatures and storage conditions in domestic refrigerators—Slovenian scenario. Food Control 2021, 123, 107715. [Google Scholar] [CrossRef]
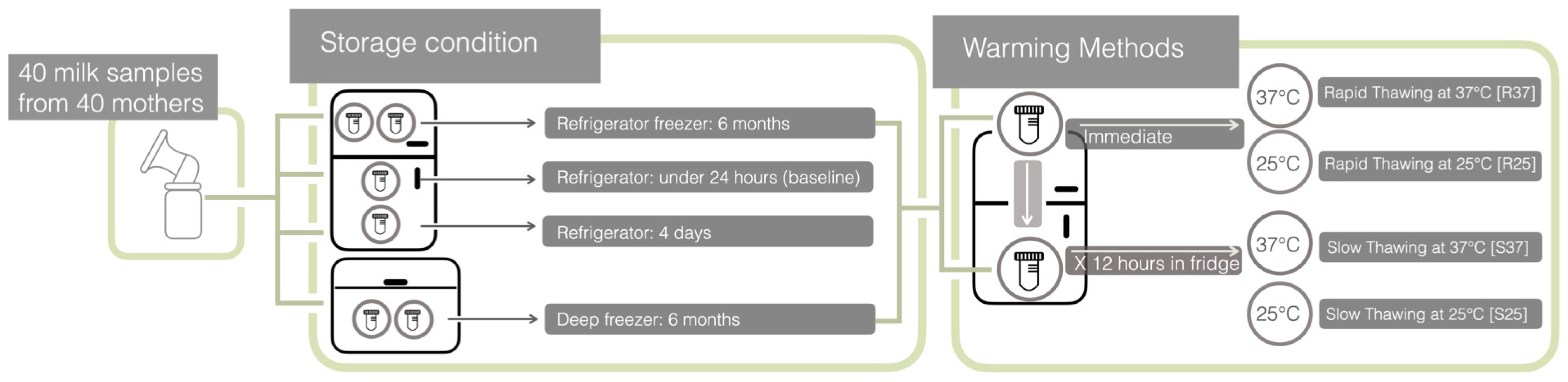
| Total Protein Levels | Baseline Sample | Stored in a Refrigerator (2 °C) for 4 Days | Thawing Techniques and Temperature | Frozen for 6 Months | ||||
|---|---|---|---|---|---|---|---|---|
| Rapid | Slow | Refrigerator Freezer (–16.7 °C) | Deep Freezer (–22.3 °C) | |||||
| (25 °C) | (37 °C) | (25 °C) | (37 °C) | |||||
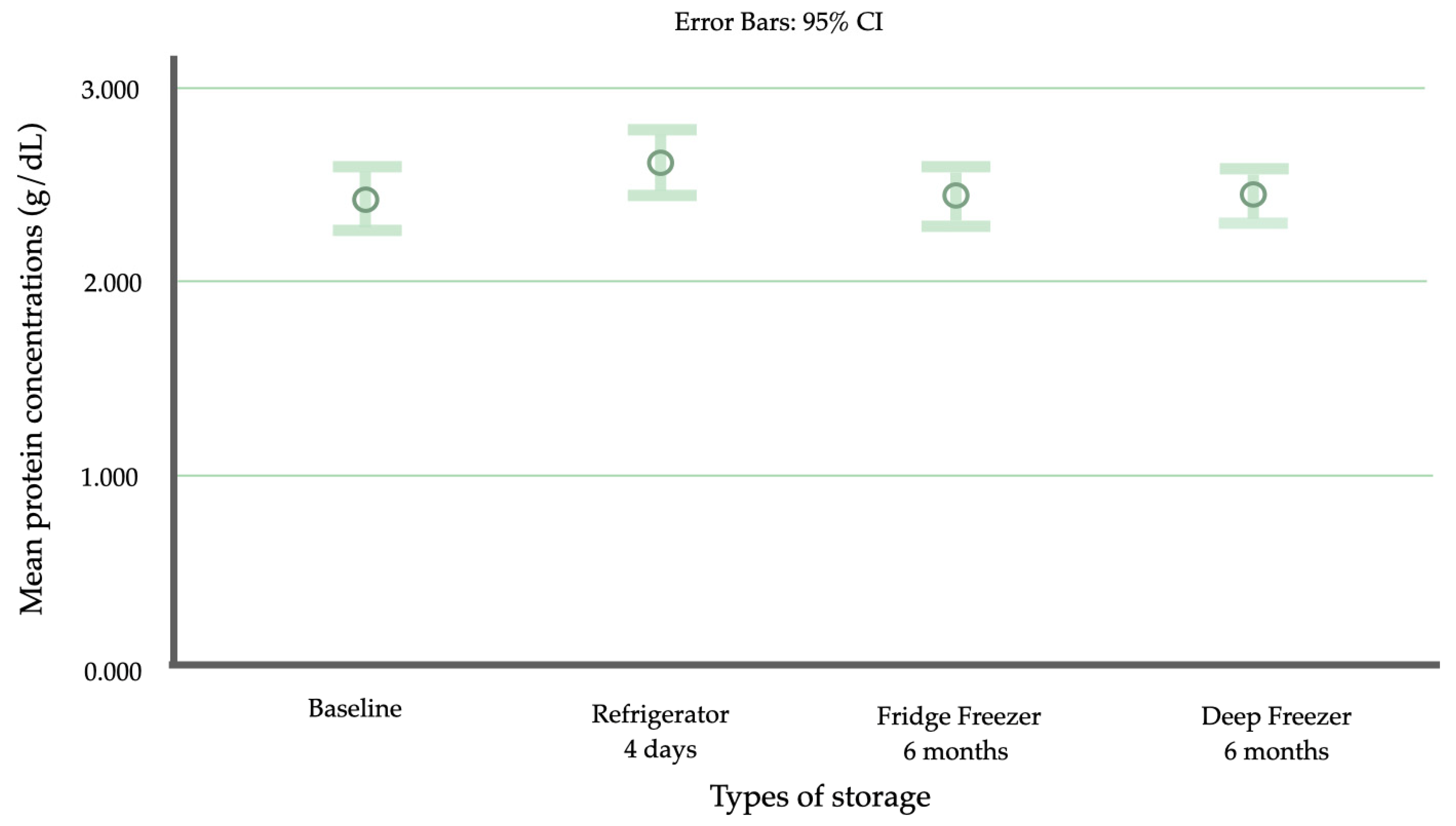
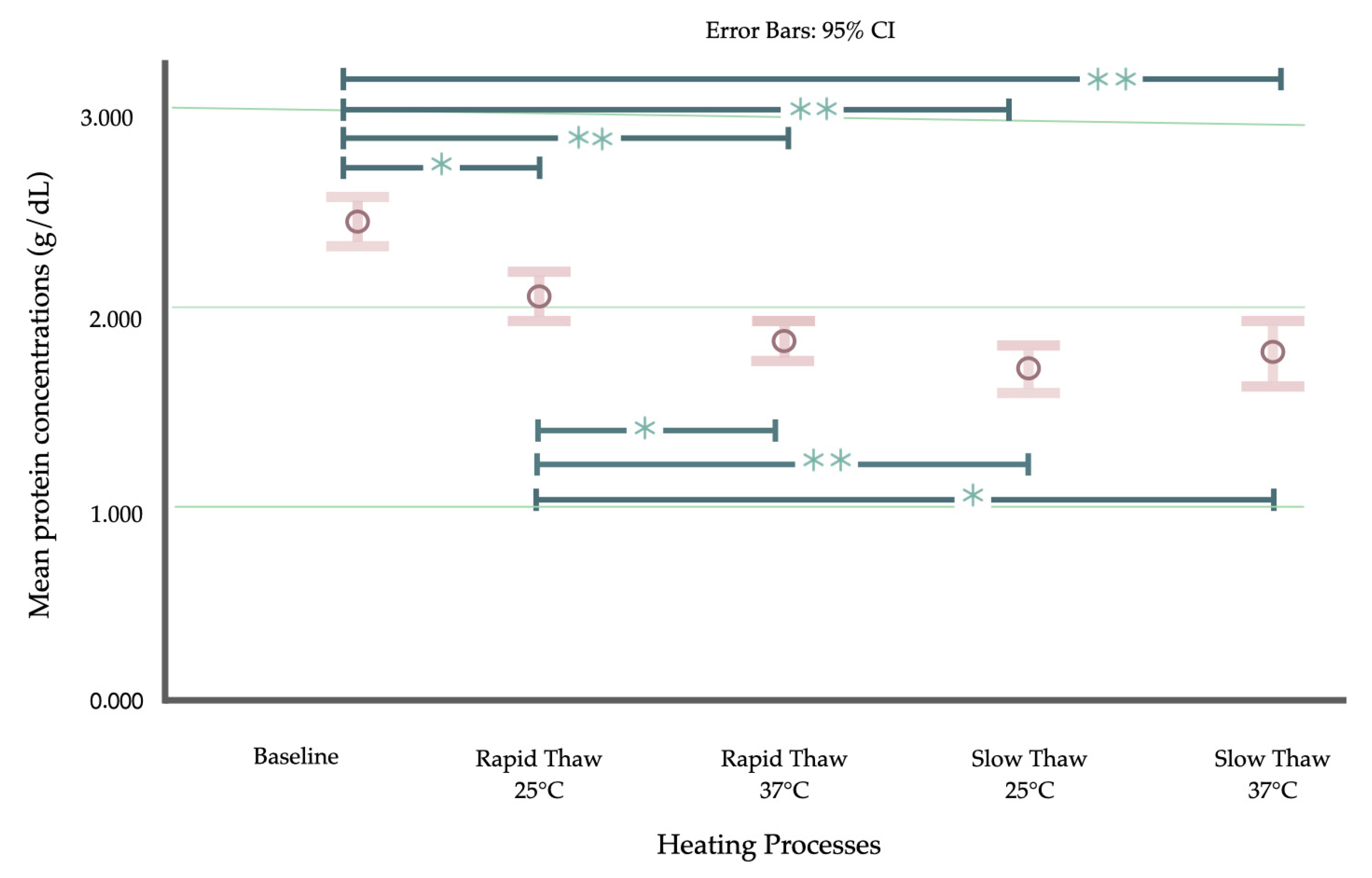
| Mean ± SD (95% CI) | 2.409 ± 0.518 (2.243 to 2.574) | 2.612 ± 0.509 (2.449 to 2.775) | 2.130 ± 0.375 (2.010 to 2.250) | 1.919 ± 0.288 (1.827 to 2.011) | 1.782 ± 0.335 (1.675 to 1.889) | 1.859 ± 0.500 (1.699 to 2.019) | 2.441 ± 0.467 (2.291 to 2.590) | 2.441 ± 0.411 (2.311 to 2.573) |
| Minimum–Maximum | 1.306–3.731 | 1.762–3.825 | 1.51–3.13 | 1.45–2.74 | 1.22–2.59 | 1.09–3.04 | 1.696–4.203 | 1.536–3.391 |
| Percentile (25th, 75th) | 2.257 (2.097, 2.718) | 2.587 (2.214, 3.004) | 2.158 | 1.887 | 1.796 | 1.841 | 2.384 (2.157, 2.725) | 2.424 (2.206, 2.739) |
| Year/Author | Sample Size/Type | Current LabCrop Method Protein | Baseline | Intervention | Effect | ||
|---|---|---|---|---|---|---|---|
| Storage | Thawing | Warming Temperature | |||||
| 2023/Binder [29] | 136 samples | The Miris BM Analyser (Miris AB, Sweden) | Stored at +4 °C to +6 °C for a max. of 48 h | Freezing at −21 °C to −27 °C for a max. of 12 h | Thawing +4 °C to +6 °C over 12 h | Warmed at 40 °C | No significant change in the protein concentration between baseline and frozen samples |
| 2020/Paulaviciene [24] | 42 samples | The Miris BM Analyser (Miris AB, Sweden) | Fresh | Freezing at −40 °C for up to 10 months of (pasteurized milk (62.5 °C for 30 min)) | NS | Warmed at 40 °C | Freezing and holder pasteurization did not decrease the BM protein concentration in frozen samples compared to baseline samples |
| 2018/Paduraru [33] | 90 samples (60 and 30 from mothers of preterm and full-term infants) | The Miris BM Analyser (Miris AB, Sweden) | Analyzed within 2 h after expression | Refrigerated at 4 °C for up to 72 h and frozen at −20 °C for up to 12 weeks | NS | Warmed at 40 °C | Milk frozen for more than 2 weeks contained less BM protein than milk refrigerated for up to 72 h |
| 2016/Ahrabi [25] | 40 samples | Quick Start Bradford Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA) | Stored at −80 °C until analysis | Samples were removed from the freezer (−20 °C) at 1, 3, 6, and 9 months and kept at −80 °C until analyzed | NS | NS | Freezing for up to 9 months did not affect the total BM protein concentration in frozen samples compared to baseline samples |
| 2016/Meng [23] | Pasteurized milk from 5 mothers | The bicinchoninic acid assay (PI23227; Fisher Scientific, Waltham, MA, USA). | Stored at room temperature for 12 h | Refrigerated at 4 °C for 7 days | All samples went through two freeze/thaw cycles before analysis | NS | A significant decrease in the BM protein concentration during 12 h at room temperature at 24 °C of storage (p = 0.02). No significant change for storage at 4 °C for 7 days |
| 2014/Handa [26] | 40 samples | A modified Quickstart Bradford Protein Assay (Bio-Rad, Hercules, CA, USA) | A baseline sample was stored at −80 °C | Stored for 7 days at −20 °C. then removed and kept at −80 °C until analyzed | 1. The temperature for TW was set to 37 °C, during which the milk went through a thawing period of 10 min, followed by an additional 10 min warming phase. 2. The waterless warmer technique: Based on the quantity of milk in the container, the equipment established the time limit automatically | No differences were detected between the waterless technique and the TW technique | |
| 2010/Eduard [30] | 31 samples | Lowry method | A baseline sample was analyzed as the basal control on day 0 | Freezing at −20 °C for 15, 30, 60, and 90 days. | NS | NS | Freezing for up to 3 months did not affect the total BM protein concentration in frozen samples compared to baseline samples |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siviroj, P.; Ruangsuriya, J.; Ongprasert, K. Changes in Protein Levels during the Storage and Warming of Breast Milk in a Domestic Environment. Children 2024, 11, 1133. https://doi.org/10.3390/children11091133
Siviroj P, Ruangsuriya J, Ongprasert K. Changes in Protein Levels during the Storage and Warming of Breast Milk in a Domestic Environment. Children. 2024; 11(9):1133. https://doi.org/10.3390/children11091133
Chicago/Turabian StyleSiviroj, Penprapa, Jetsada Ruangsuriya, and Krongporn Ongprasert. 2024. "Changes in Protein Levels during the Storage and Warming of Breast Milk in a Domestic Environment" Children 11, no. 9: 1133. https://doi.org/10.3390/children11091133
APA StyleSiviroj, P., Ruangsuriya, J., & Ongprasert, K. (2024). Changes in Protein Levels during the Storage and Warming of Breast Milk in a Domestic Environment. Children, 11(9), 1133. https://doi.org/10.3390/children11091133








