Preliminary Investigation into the Association between Scoliosis and Hypoxia: A Retrospective Cohort Study on the Impact of Eliminating Hypoxic Factors on Scoliosis Outcomes
Abstract
1. Introduction
2. Patients and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSA | Obstructive Sleep Apnea |
| EOS | Early-onset scoliosis |
| HIF | Hypoxia-inducible factor |
| AT | Adenotonsillectomy |
| ICC | Intraclass correlation coefficients |
References
- Zaina, F.; Donzelli, S.; Negrini, S. Idiopathic Scoliosis: Novel Challenges for Researchers and Clinicians. Children 2023, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of Adolescent Idiopathic Scoliosis. J. Child. Orthop. 2013, 7, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Horng, M.-H.; Kuok, C.-P.; Fu, M.-J.; Lin, C.-J.; Sun, Y.-N. Cobb Angle Measurement of Spine from X-ray Images Using Convolutional Neural Network. Comput. Math. Methods Med. 2019, 2019, 6357171. [Google Scholar] [CrossRef]
- Bernstein, P.; Metzler, J.; Weinzierl, M.; Seifert, C.; Kisel, W.; Wacker, M. Radiographic Scoliosis Angle Estimation: Spline-Based Measurement Reveals Superior Reliability Compared to Traditional COBB Method. Eur. Spine J. 2020, 30, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Fadzan, M.; Bettany-Saltikov, J. Etiological Theories of Adolescent Idiopathic Scoliosis: Past and Present. Open Orthop. J. 2017, 11, 1466–1489. [Google Scholar] [CrossRef]
- Gorman, K.B.; Julien, C.; Moreau, A. The Genetic Epidemiology of Idiopathic Scoliosis. Eur. Spine J. 2012, 21, 1905–1919. [Google Scholar] [CrossRef]
- Dayer, R.; Haumont, T.; Belaieff, W.; Lascombes, P. Idiopathic Scoliosis: Etiological Concepts and Hypotheses. J. Child. Orthop. 2013, 7, 11–16. [Google Scholar] [CrossRef]
- Hawes, M.C.; O’Brien, J.P. The Transformation of Spinal Curvature into Spinal Deformity: Pathological Processes and Implications for Treatment. Scoliosis 2006, 1, 3. [Google Scholar] [CrossRef]
- Burwell, R.G.; Clark, E.M.; Dangerfield, P.H.; Moulton, A. Adolescent Idiopathic Scoliosis (AIS): A Multifactorial Cascade Concept for Pathogenesis and Embryonic Origin. Scoliosis Spinal Disord. 2016, 11, 1–7. [Google Scholar] [CrossRef]
- Shivan, M.; Tambe, A.D.; Millner, P.; Tsirikos, A.I. Adolescent Idiopathic Scoliosis. Bone Jt. J. 2022, 104-B, 915–921. [Google Scholar] [CrossRef]
- Erol, B.; Kusumi, K.; Lou, J.; Dormans, J. Etiology of Congenital Scoliosis. Univ. Pennsylvania Orthop. J. 2002, 15, 37–42. [Google Scholar]
- Farley, F.A.; Loder, R.T.; Nolan, B.T.; Dillon, M.T.; Frankenburg, E.P.; Kaciroti, N.A.; Miller, J.D.; Goldstein, S.A.; Hensinger, R.N. Mouse Model for Thoracic Congenital Scoliosis. J. Pediatr. Orthop. 2001, 21, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Rivard, C.H. Effects of Hypoxia on the Embryogenesis of Congenital Vertebral Malformations in the Mouse. Clin. Orthop. Relat. Res. 1986, 208, 126–130. [Google Scholar] [CrossRef]
- Tam, W.K.; Cheung, J.P.Y.; Koljonen, P.A.; Kwan, K.Y.H.; Cheung, K.M.C.; Leung, V.Y.L. Slow Twitch Paraspinal Muscle Dysregulation in Adolescent Idiopathic Scoliosis Exhibiting HIF-2α Misexpression. JOR Spıne 2022, 5, e1227. [Google Scholar] [CrossRef]
- Liu, S.-R.; Ren, D.; Wu, H.-T.; Yao, S.-Q.; Song, Z.-H.; Geng, L.-D.; Wang, P.-C. Reparative Effects of Chronic Intermittent Hypobaric Hypoxia Pre-Treatment on Intervertebral Disc Degeneration in Rats. Mol. Med. Rep. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Lafont, J.E. Lack of Oxygen in Articular Cartilage: Consequences for Chondrocyte Biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef]
- Li, J.; Tang, M.; Yang, G.; Wang, L.; Gao, Q.; Zhang, H. Muscle Injury Associated Elevated Oxidative Stress and Abnormal Myogenesis in Patients with Idiopathic Scoliosis. Int. J. Biol. Sci. 2019, 15, 2584–2595. [Google Scholar] [CrossRef]
- Shi, B.; Xu, L.; Mao, S.; Xu, L.; Liu, Z.; Sun, X.; Zhu, Z.; Qiu, Y. Abnormal PITX1 Gene Methylation in Adolescent Idiopathic Scoliosis: A Pilot Study. BMC Musculoskelet. Disord. 2018, 19, 1–6. [Google Scholar] [CrossRef]
- Suvarnan, L. The Role of Transcription Factor Pitx1 and Its Regulation by Hypoxia in Adolescent Idiopathic Scoliosis. Master’s Thesis, Université de Montréal, Montréal, QC, Canada, 2010. [Google Scholar]
- Nanduri, J.; Semenza, G.L.; Prabhakar, N.R. Epigenetic Changes by DNA Methylation in Chronic and Intermittent Hypoxia. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L1096–L1100. [Google Scholar] [CrossRef]
- Domany, K.A.; Dana, E.; Tauman, R.; Gut, G.; Greenfeld, M.; Yakir, B.-E.; Sivan, Y. Adenoidectomy for Obstructive Sleep Apnea in Children. J. Clin. Sleep Med. 2016, 12, 1285–1291. [Google Scholar] [CrossRef]
- Schupper, A.J.; Nation, J.; Pransky, S. Adenoidectomy in Children: What Is the Evidence and What Is Its Role? Curr. Otorhinolaryngol. Rep. 2018, 6, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zaffanello, M.; Ersu, R.H.; Nosetti, L.; Beretta, G.; Agosti, M.; Piacentini, G. Cardiac Implications of Adenotonsillar Hypertrophy and Obstructive Sleep Apnea in Pediatric Patients: A Comprehensive Systematic Review. Children 2024, 11, 208. [Google Scholar] [CrossRef] [PubMed]
- Abuhandan, M.; Bozkuş, F.; Demir, N.; Eren, E.; Koca, B.; Kadir Guler, O.; Selek, S. The Preoperative and Postoperative Oxidative Status of Children with Chronic Adenotonsillar Hypertrophy. La Clin. Ter. 2013, 164, e163–e167. [Google Scholar] [CrossRef]
- Yucel, K.; Aydin, I.; Erdem, S.S. Hypoxia Induced Factor-1α Levels in Patients Undergoing Adenoidectomy. Scand. J. Clin. Lab. Investig. 2020, 81, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, N.; Manduku, V.; Hlabangana, L.; Gong, W.; Moore, D.; Fancourt, N.; Madhi, S. Evaluation of paediatric chest X-ray quality in the Pneumonia Aetiology Research for Child Health (PERCH Study), a prospective international multicentre study in 7 developing countries. Wits J. Clin. Med. 2023, 5, 85–96. [Google Scholar]
- Pan, X.-X.; Huang, C.-A.; Lin, J.-L.; Zhang, Z.-J.; Shi, Y.-F.; Chen, B.-D.; Zhang, H.-W.; Dai, Z.-Y.; Yu, X.-P.; Wang, X.-Y. Prevalence of the Thoracic Scoliosis in Children and Adolescents Candidates for Strabismus Surgery: Results from a 1935-Patient Cross-Sectional Study in China. Eur. Spine J. 2020, 29, 786–793. [Google Scholar] [CrossRef]
- Kockara, N.; Ucpunar, H. The Value of Standard Chest Radiography in the Diagnosis of Scoliosis. Ann. Med. Res. 2019, 26, 190–193. [Google Scholar] [CrossRef]
- Oh, C.H.; Kim, C.G.; Lee, M.S.; Yoon, S.H.; Park, H.-C.; Park, C.O. Usefulness of Chest Radiographs for Scoliosis Screening: A Comparison with Thoraco-Lumbar Standing Radiographs. Yonsei Med. J. 2012, 53, 1183. [Google Scholar] [CrossRef]
- Parr, A.; Askin, G. Paediatric Scoliosis: Update on Assessment and Treatment. Aust. J. Gen. Pract. 2020, 49, 832–837. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Qi, S.; Zhang, X.; Li, S. Effect of Adenoid Hypertrophy on the Upper Airway and Craniomaxillofacial Region. Transl. Pediatr. 2021, 10, 2563–2572. [Google Scholar] [CrossRef]
- Pialoux, V.; Mounier, R.; Brown, A.D.; Steinback, C.D.; Rawling, J.M.; Poulin, M.J. Relationship between Oxidative Stress and HIF-1α MRNA during Sustained Hypoxia in Humans. Free Radic. Biol. Med. 2009, 46, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wang, M.; Xiao, Z.; Jiang, Z. Hypoxia Activates SUMO-1-HIF-1α Signaling Pathway to Upregulate Pro-Inflammatory Cytokines and Permeability in Human Tonsil Epithelial Cells. Life Sci. 2021, 276, 119432. [Google Scholar] [CrossRef] [PubMed]
- Fendri, K.; Patten, S.A.; Kaufman, G.N.; Zaouter, C.; Parent, S.; Grimard, G.; Edery, P.; Moldovan, F. Microarray Expression Profiling Identifies Genes with Altered Expression in Adolescent Idiopathic Scoliosis. Eur. Spine J. 2013, 22, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Y. PITX1 Plays Essential Functions in Cancer. Front. Oncol. 2023, 13, 1253238. [Google Scholar] [CrossRef]
- Mudie, S.; Bandarra, D.; Batie, M.; Biddlestone, J.; Moniz, S.; Ortmann, B.; Shmakova, A.; Rocha, S. PITX1, a Specificity Determinant in the HIF-1α-Mediated Transcriptional Response to Hypoxia. Cell Cycle 2014, 13, 3878–3891. [Google Scholar] [CrossRef]
- Wang, J.S.; Infante, C.R.; Park, S.; Menke, D.B. PITX1 Promotes Chondrogenesis and Myogenesis in Mouse Hindlimbs through Conserved Regulatory Targets. Dev. Biol. 2018, 434, 186–195. [Google Scholar] [CrossRef]
- Imanguli, M.; Ulualp, S.O. Risk Factors for Residual Obstructive Sleep Apnea after Adenotonsillectomy in Children. Laryngoscope 2016, 126, 2624–2629. [Google Scholar] [CrossRef]
- Geiger, Z.; Gupta, N. Adenoid Hypertrophy. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536984/ (accessed on 1 May 2023).
- Modi, H.N.; Suh, S.W.; Yang, J.H.; Hong, J.Y.; Venkatesh, K.; Muzaffar, N. Spontaneous Regression of Curve in Immature Idiopathic Scoliosis—Does Spinal Column Play a Role to Balance? An Observation with Literature Review. J. Orthop. Surg. Res. 2010, 5, 80. [Google Scholar] [CrossRef]
- Soucacos, P.N.; Zacharis, K.; Gelalis, J.; Soultanis, K.; Kalos, N.; Beris, A.; Xenakis, T.; Johnson, E.O. Assessment of Curve Progression in Idiopathic Scoliosis. Eur. Spine J. 1998, 7, 270–277. [Google Scholar] [CrossRef]
- Noshchenko, A. Predictors of Spine Deformity Progression in Adolescent Idiopathic Scoliosis: A Systematic Review with Meta-Analysis. World J. Orthop. 2015, 6, 537. [Google Scholar] [CrossRef]
- Lonstein, J.E.; Carlson, J.M. The Prediction of Curve Progression in Untreated Idiopathic Scoliosis during Growth. J. Bone Jt. Surg. 1984, 66, 1061–1071. [Google Scholar] [CrossRef]
- Karpe, P.; Killen, M.-C.; Fender, D. Scoliosis in Childhood. Surgery 2020, 38, 509–516. [Google Scholar] [CrossRef]
- Brooks, H.L.; Azen, S.P.; Gerberg, E.; Brooks, R.; Chan, L. Scoliosis: A prospective epidemiological study. J. Bone Jt. Surg. 1975, 57, 968–972. [Google Scholar] [CrossRef]
- Saran, S.; Saccomanno, S.; Viti, S.; Mastrapasqua, R.F.; Viti, G.; Giannotta, N.; Fioretti, P.; Lorenzini, E.; Raffaelli, L.; Levrini, L. Analysis of General Knowledge on Obstructive Sleep Apnea Syndrome (OSAS) among Italian Pediatricians. Children 2024, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Kotwicki, T.; Kinel, E.; Stryla, W.; Szulc, A. Discrepancy in Clinical versus Radiological Parameters Describing Deformity due to Brace Treatment for Moderate Idiopathic Scoliosis. Scoliosis 2007, 2, 18. [Google Scholar] [CrossRef]
- Su, X.; Dong, R.; Wen, Z.; Liu, Y. Reliability and Validity of Scoliosis Measurements Obtained with Surface Topography Techniques: A Systematic Review. J. Clin. Med. 2022, 11, 6998. [Google Scholar] [CrossRef]
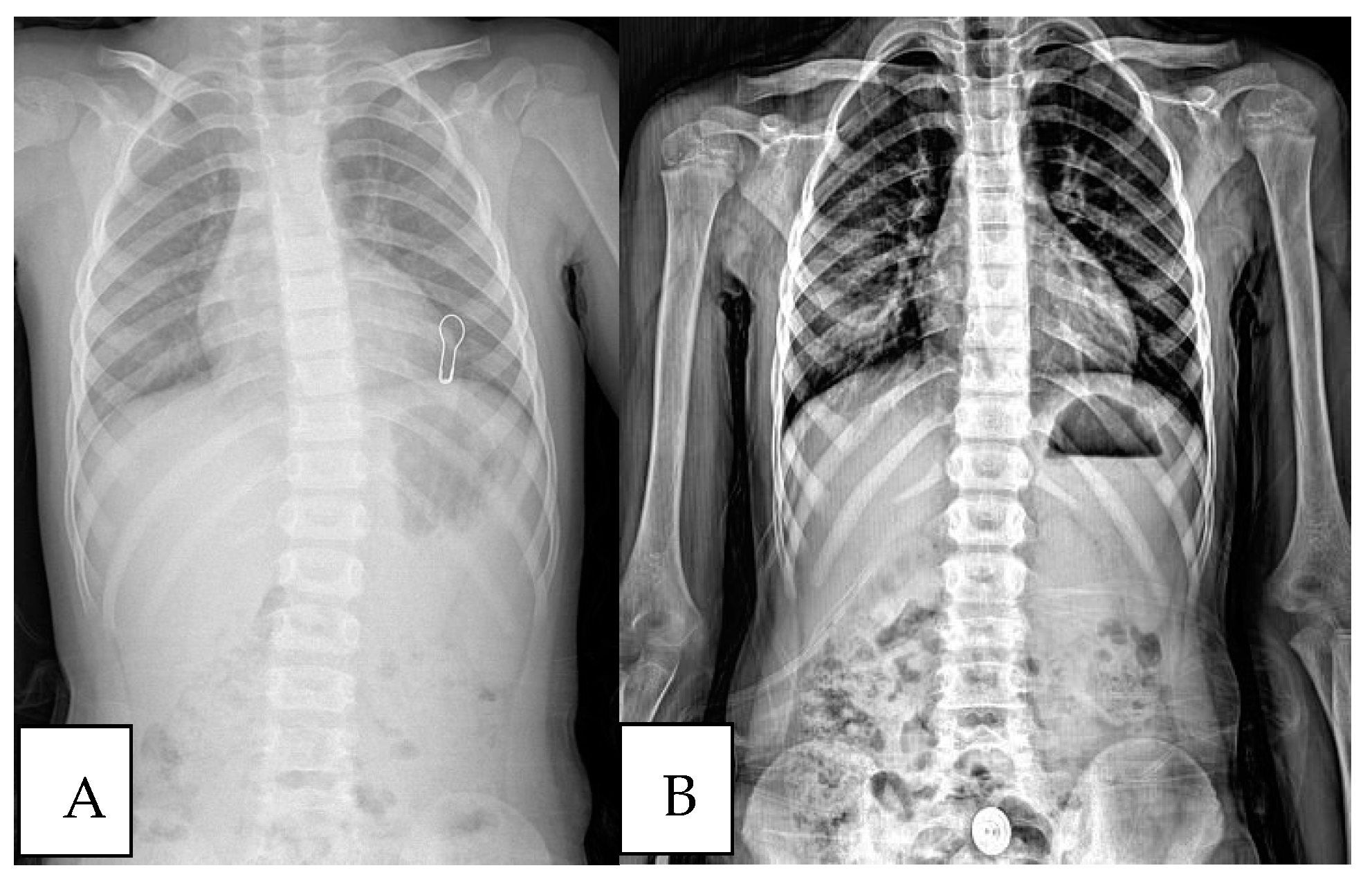
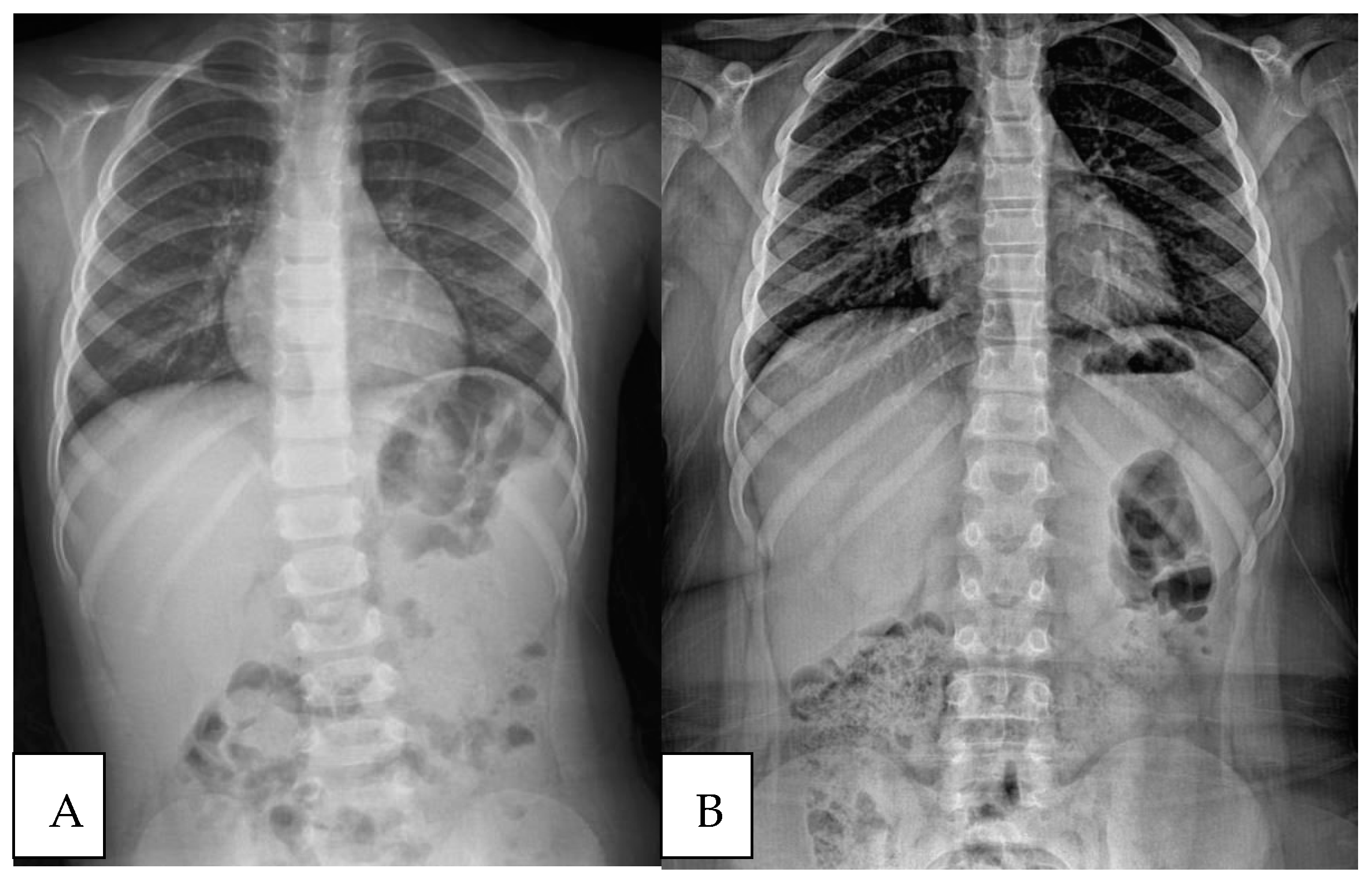
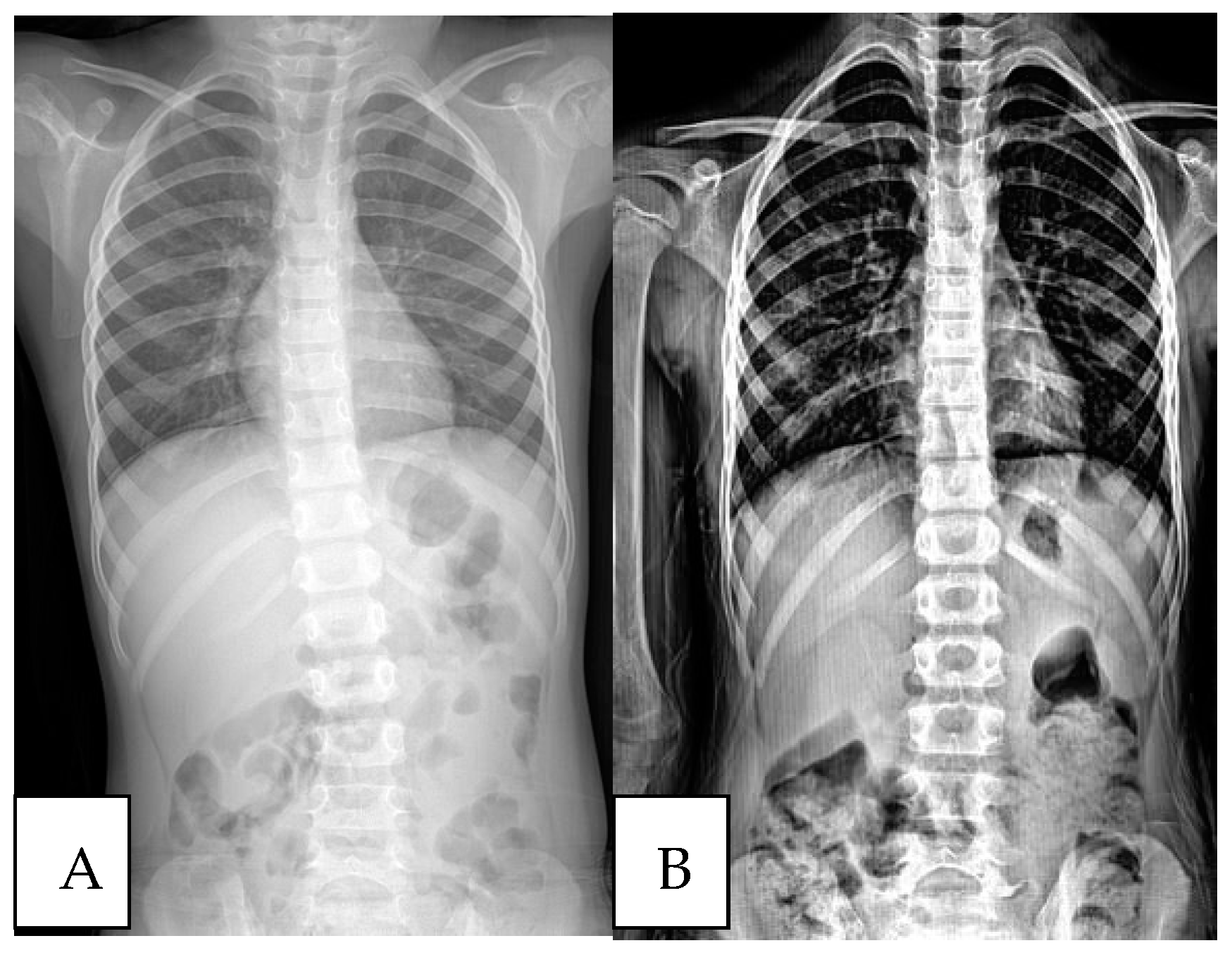
| Initial Assessment | Final Assessment | p-Value * | |
|---|---|---|---|
| Cobb Angle | 7 (5–17) | 3 (0–12) | <0.001 |
| Cobb Angle < 10° | 6.25 (5–9) | 0.50 (0–11) | <0.001 |
| Cobb Angle ≥ 10° | 12 (10–17) | 5 (0–12) | <0.001 |
| Final Assessment Cobb Angle | p-Value | |
|---|---|---|
| Age | r = 0.376 | 0.022 ** |
| Gender | ||
| Female | 3 (0–11) | 0.936 *** |
| Male | 3 (0–12) | |
| Follow-up time (months) | r = −0.168 | 0.320 ** |
| Final Assessment Cobb Angle | p-Value | |
|---|---|---|
| Age | r = 0.270 | 0.224 ** |
| Gender | ||
| Female | 0 (0–11) | 0.821 *** |
| Male | 1.50 (0–7) | |
| Follow-up time (months) | r = −0.044 | 0.845 ** |
| Final Assessment Cobb Angle | p-Value | |
|---|---|---|
| Age | r = 0.465 | 0.081 ** |
| Gender | ||
| Female | 4 (0–11) | 0.114 *** |
| Male | 10.50 (9–12) | |
| Follow-up time (months) | r = −0.231 | 0.407 ** |
| Cobb Angle < 10 | Cobb Angle ≥ 10 | p-Value | |
|---|---|---|---|
| Age | 6 (3–8) | 6 (3–8) | 0.915 *** |
| Gender | |||
| Female | 12 (54.5%) | 13 (86.7%) | 0.073 & |
| Male | 10 (45.5%) | 2 (13.3%) | |
| Follow-up time (months) | 47.50 (7–70) | 43 (6–78) | 0.366 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugur, F.; Topal, K.; Albayrak, M.; Taskin, R.; Topal, M. Preliminary Investigation into the Association between Scoliosis and Hypoxia: A Retrospective Cohort Study on the Impact of Eliminating Hypoxic Factors on Scoliosis Outcomes. Children 2024, 11, 1134. https://doi.org/10.3390/children11091134
Ugur F, Topal K, Albayrak M, Taskin R, Topal M. Preliminary Investigation into the Association between Scoliosis and Hypoxia: A Retrospective Cohort Study on the Impact of Eliminating Hypoxic Factors on Scoliosis Outcomes. Children. 2024; 11(9):1134. https://doi.org/10.3390/children11091134
Chicago/Turabian StyleUgur, Fatih, Kubra Topal, Mehmet Albayrak, Recep Taskin, and Murat Topal. 2024. "Preliminary Investigation into the Association between Scoliosis and Hypoxia: A Retrospective Cohort Study on the Impact of Eliminating Hypoxic Factors on Scoliosis Outcomes" Children 11, no. 9: 1134. https://doi.org/10.3390/children11091134
APA StyleUgur, F., Topal, K., Albayrak, M., Taskin, R., & Topal, M. (2024). Preliminary Investigation into the Association between Scoliosis and Hypoxia: A Retrospective Cohort Study on the Impact of Eliminating Hypoxic Factors on Scoliosis Outcomes. Children, 11(9), 1134. https://doi.org/10.3390/children11091134





