Systematic Review on Microtia: Current Knowledge and Future Directions
Abstract
1. Introduction
2. Materials and Methods
3. Results
Data Collection and Screening
4. Discussion
4.1. General Description of Microtia
4.1.1. Development
4.1.2. Genetic Studies
4.1.3. Epidemiological Studies
4.1.4. Treatment and Tissue Engineering
4.2. Current Surgical Techniques in Clinical Practice, Efficacy, and Limitations
4.2.1. Use of Autologous Cartilage
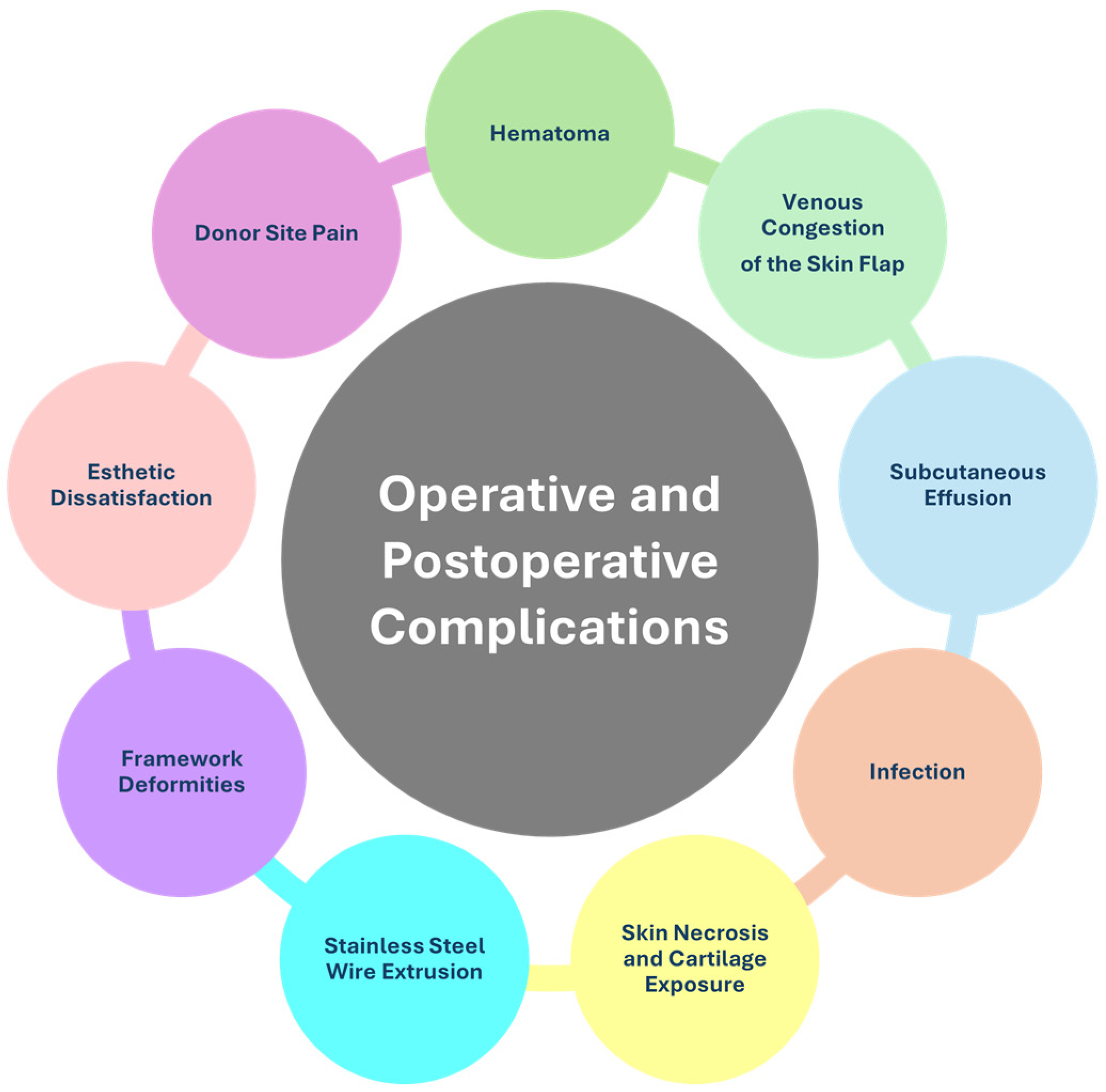
4.2.2. Operative and Postoperative Complications
Hematoma
Venous Congestion of the Skin Flap
Subcutaneous Effusion
Infection
Skin Necrosis and Cartilage Exposure
Stainless Steel Wire Extrusion
Framework Deformities
Esthetic Dissatisfaction
Donor Site Pain and Deformities
Excessive Hair on Reconstructed Skin
4.2.3. Innovative Surgical Techniques
Cartilage Molding
Cadaveric Material
Simultaneous Reconstruction with Transcutaneous Bone Conduction Implant
Innovative Sculpting and Shaping Strategies
Plates and Titanium Supports
Material Quantification
4.2.4. Surgical Advances for Esthetic Improvement
4.2.5. Tissue Expansion
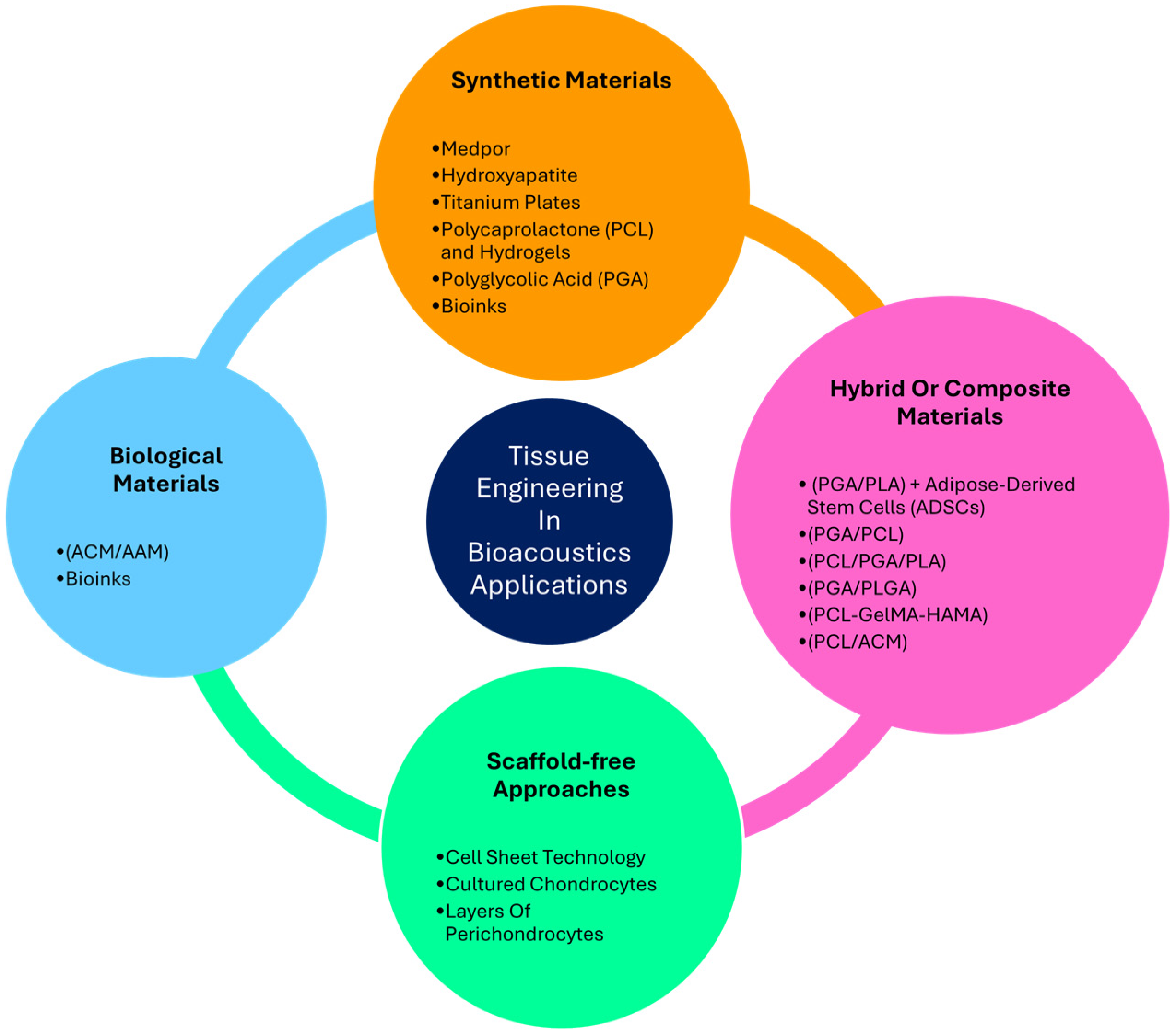
4.3. Tissue Engineering in Otology Application
4.3.1. Synthetic Materials
4.3.2. Hybrid or Composite Materials
4.3.3. Biological Materials
4.3.4. Scaffold-Free Approach
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Khan, N.; Zaki, D.P.; Brown, W.E.; Halaseh, F.F.; Willette, D.; Ziegler, M.; Athanasiou, K.A.; Widgerow, A.D. Tissue Engineering Auricular Cartilage: A Review of Auricular Cartilage Characteristics and Current Techniques for Auricular Reconstruction. J. Craniofacial Surg. 2024, 35, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Mastroiacovo, P.; Corchia, C.; Botto, L.D.; Lanni, R.; Zampino, G.; Fusco, D. Epidemiology and Genetics of Microtia-Anotia: A Registry Based Study on over One Million Births. J. Med. Genet. 1995, 32, 453–457. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, H.; Wang, Y.; Bi, S.; Zhou, K.; Li, H.; Zhou, C.; Wang, Y.; Wu, W.; Peng, B.; et al. The Application and Progress of Tissue Engineering and Biomaterial Scaffolds for Total Auricular Reconstruction in Microtia. Front. Bioeng. Biotechnol. 2023, 11, 1089031. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, D.; Fisch, P.; Moehrlen, U.; Finkielsztein, S.; Linder, T.; Zenobi-Wong, M.; Biedermann, T.; Klar, A.S. Combining Bioengineered Human Skin with Bioprinted Cartilage for Ear Reconstruction. Sci. Adv. 2023, 9, eadh1890. [Google Scholar] [CrossRef]
- Truong, M.T.; Liu, Y.-C.C.; Kohn, J.; Chinnadurai, S.; Zopf, D.A.; Tribble, M.; Tanner, P.B.; Sie, K.; Chang, K.W. Integrated Microtia and Aural Atresia Management. Front. Surg. 2022, 9, 944223. [Google Scholar] [CrossRef] [PubMed]
- Jakob, Y.; Kern, J.; Gvaramia, D.; Fisch, P.; Magritz, R.; Reutter, S.; Rotter, N. Suitability of Ex Vivo-Expanded Microtic Perichondrocytes for Auricular Reconstruction. Cells 2024, 13, 141. [Google Scholar] [CrossRef]
- Gardner, O.F.W.; Agabalyan, N.; Weil, B.; Ali, M.H.I.; Lowdell, M.W.; Bulstrode, N.W.; Ferretti, P. Human Platelet Lysate Enhances Proliferation but Not Chondrogenic Differentiation of Pediatric Mesenchymal Progenitors. Cytotherapy 2023, 25, 286–297. [Google Scholar] [CrossRef]
- Mukherjee, P.; Chung, J.; Cheng, K.; Gupta, R.; Haag, H.; Williams, Z.; Wallace, G. Invitro and Invivo Study of PCL-Hydrogel Scaffold to Advance Bioprinting Translation in Microtia Reconstruction. J. Craniofacial Surg. 2021, 32, 1931–1936. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, D.; Li, Y. Microtia Patients: Auricular Chondrocyte ECM Is Promoted by CGF through IGF-1 Activation of the IGF-1R/PI3K/AKT Pathway. J. Cell Physiol. 2019, 234, 21817–21824. [Google Scholar] [CrossRef]
- Shonka, D.C.; Livingston, W.J.; Kesser, B.W. The Jahrsdoerfer Grading Scale in Surgery to Repair Congenital Aural Atresia. Arch. Otolaryngol. Head. Neck Surg. 2008, 134, 873–877. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Li, J.; Shi, H.; Ma, S.; Wang, D.; Pan, B.; Xiao, R.; Jiang, H.; Liu, X. Decreased Tiam1-mediated Rac1 Activation Is Responsible for Impaired Directional Persistence of Chondrocyte Migration in Microtia. J. Cell Mol. Med. 2024, 28, e18443. [Google Scholar] [CrossRef] [PubMed]
- Otto, I.A.; Simone, A.; Malda, J.; Kon, M.; Breugem, C.C. Engaging Stakeholders in Bioprinting Research: Views and Concerns of Microtia Patients’ Parents on Bioprinted Auricular Cartilage. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 2239–2260. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, T.; Liu, R.; Wang, C.; Jiang, H.; Sun, H. Congenital Microtia Patients: The Genetically Engineered Exosomes Released from Porous Gelatin Methacryloyl Hydrogel for Downstream Small RNA Profiling, Functional Modulation of Microtia Chondrocytes and Tissue-Engineered Ear Cartilage Regeneration. J. Nanobiotechnol. 2022, 20, 164. [Google Scholar] [CrossRef]
- Padilla-Cabello, J.; Moral-Munoz, J.A.; Santisteban-Espejo, A.; Velez-Estevez, A.; Cobo, M.J.; Martin-Piedra, M.A. Analysis of Cognitive Framework and Biomedical Translation of Tissue Engineering in Otolaryngology. Sci. Rep. 2023, 13, 13492. [Google Scholar] [CrossRef]
- McDaniel, S.L.; Hollatz, A.J.; Branstad, A.M.; Gaskill, M.M.; Fox, C.A.; Harrison, M.M. Tissue-Specific DNA Replication Defects in Drosophila Melanogaster Caused by a Meier-Gorlin Syndrome Mutation in Orc4. Genetics 2020, 214, 355–367. [Google Scholar] [CrossRef]
- Guo, F.; Lin, L.; Yu, X.; Song, Y.; Yang, Q.; He, L.; Pan, B.; Jiang, H. Classification of the Concha-Type Microtia and Their New Suitable Treatment Strategies without Autogenous Costal Cartilage Grafting. Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109801. [Google Scholar] [CrossRef]
- Lam, D.D.; Hegde, N.V.; Patel, D.D.; Lakeland, D.L.; Guardino, N.; Kochhar, A.; Mariani, F.V. Histological Assessment of Microtia Cartilage, a Potential Source of Autograft Tissue in Ear Reconstruction. J. Anat. 2024, 245, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, L. Mass Spectrometric Characterization of Metabolites in Ear Cartilage. Ann. Plast. Surg. 2020, 85, 76–82. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Ma, R.; Zhu, X.; Wang, R.; He, L.; Shu, M. Multi-Omics Analysis of a Case of Congenital Microtia Reveals Aldob and Oxidative Stress Associated with Microtia Etiology. Orphanet J. Rare Dis. 2024, 19, 218. [Google Scholar] [CrossRef]
- Dong, W.; Jiang, H.; He, L.; Pan, B.; Lin, L.; Song, Y.; Yang, Q. Protein Profile of Ear Auricle Cartilage and the Important Role of ITGB1/PTK2 in Microtia. Int. J. Pediatr. Otorhinolaryngol. 2020, 137, 110235. [Google Scholar] [CrossRef]
- Yang, R.; Chu, H.; Yue, H.; Mishina, Y.; Zhang, Z.; Liu, H.; Li, B. BMP Signaling Maintains Auricular Chondrocyte Identity and Prevents Microtia Development by Inhibiting Protein Kinase A. eLife 2024, 12, RP91883. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Lin, L.; Yang, Q.; Zhang, L. Differential Expression of Noncoding RNAs Revealed Enhancer RNA AC016735.2 as a Potential Pathogenic Marker of Congenital Microtia Patients. J. Craniofacial Surg. 2024, 35, 787–793. [Google Scholar] [CrossRef]
- Zielinska, D.; Yosef, H.K.; Zollitsch, T.; Kern, J.; Jakob, Y.; Gvaramia, D.; Rotter, N.; Pontiggia, L.; Moehrlen, U.; Biedermann, T.; et al. Characterization of Distinct Chondrogenic Cell Populations of Patients Suffering from Microtia Using Single-Cell Micro-Raman Spectroscopy. Biomedicines 2023, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.F.; O’Toole, G.; Sabbagh, W.; Szarko, M.; Butler, P.E. Comparison of the Compressive Mechanical Properties of Auricular and Costal Cartilage from Patients with Microtia. J. Biomech. 2020, 103, 109688. [Google Scholar] [CrossRef]
- Park, C. Total Rebuilding of the Ear after Unsatisfactory Initial Microtia Reconstruction: 30-Year Experience Using Autogenous Costal Cartilage Framework. J. Plast. Reconstr. Aesthetic Surg. 2023, 86, 174–182. [Google Scholar] [CrossRef]
- Asirova, G.; Wynands, J.; Frolov, S.; Almeida, D.; Davydenko, P.; Madina, S. Optimal Surgical Timing for Ear Reconstruction with Autologous Cartilage: Analysis of the Computed Tomography Scan Characteristics of the Ribs. J. Plast. Reconstr. Aesthet. Surg. 2024, 88, 15–23. [Google Scholar] [CrossRef]
- Kim, A.; Moon, J.; Lim, S.Y.; Oh, K.S. The Interchondral Joints of Thorax in Microtia Surgery: Classification and Fabrication Strategies. Ann. Plast. Surg. 2021, 87, 98–104. [Google Scholar] [CrossRef]
- Ladani, P.S.; Valand, R.; Sailer, H. Ear Reconstruction Using Autologus Costal Cartilage: A Steep Learning Curve. J. Maxillofac. Oral. Surg. 2019, 18, 371–377. [Google Scholar] [CrossRef]
- Luo, K.; Chen, Z.; Jiang, Z.; Cai, S.; Zhou, Y.; Cui, W.; Sheng, Y.; Lin, Y.; Chen, Y.; Cai, Z. Ear Reconstruction Stage I: Minor Modifications in Sculpting the Auricle Support Using the 7th and 8th Costal Cartilages. J. Plast. Reconstr. Aesthet. Surg. 2023, 84, 357–364. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, G.; Liu, W.; Wang, N.; Liu, Z. Different Methods of Fabricating Cartilaginous Ear Framework in Children With Microtia According to the Length of the Eighth Costal Cartilage Intraoperatively. J. Craniofacial Surg. 2019, 30, 1425–1429. [Google Scholar] [CrossRef]
- Xing, W.; Qian, J.; Wang, B.; Wang, Y.; Hu, J.; Zhang, Q. Auricular Reconstruction with Modified Expanded Two-Flap Method in Goldenhar Syndrome: 7-Year Experiences. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110228. [Google Scholar] [CrossRef]
- Mao, X.; Li, X.; Jia, J.; Kang, D.; Miao, Y.; Lu, Z.; Hu, Z. Validity and Reliability of Three-dimensional Costal Cartilage Imaging for Donor-site Assessment and Clinical Application in Microtia Reconstruction Patients: A Prospective Study of 22 Cases. Clin. Otolaryngol. 2020, 45, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, R.; Zhang, Q.; Xu, Z.; Xu, F.; Li, Y.; Sun, N.; Wang, C. A Novel Method of Naturally Contouring the Reconstructed Ear. Plast. Reconstr. Surg. 2014, 133, 1168–1174. [Google Scholar] [CrossRef]
- Yang, J.; Lin, L.; Zhang, Y.; Wang, Y.; Cui, L.; He, L. The Regenerated Tissue at the Donor Site After Costal Cartilage Harvest for Auricular Reconstruction. J. Craniofacial Surg. 2019, 30, e490–e494. [Google Scholar] [CrossRef]
- Saha, S.; Singh, A.; Mohammad, A.; Chauhan, S.; Chinta, K.; Singhal, M. Don’t Play It by Ear: Technical Considerations to Optimize Outcome and Procedural Safety of Congenital Microtia Reconstruction in a Dextrocardia Patient With Situs Inversus Totalis, Butterfly Vertebra, and Hemivertebra. Eplasty 2023, 23, e57. [Google Scholar]
- Sun, H.; Huang, Q.; Dong, W.; Yang, Q. Differences between Bilateral Costal Cartilage in Patients with Microtia: A Retrospective Study Using Three-Dimensional Imaging. Plast. Reconstr. Surg. 2022, 149, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Vu, D.D.; Ngo, L.M.; Tran, H.T.T. Anatomical Variant of the Superficial Temporal Artery in Temporoparietal Fascia Flap for Microtia Reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2024, 91, 105–110. [Google Scholar] [CrossRef]
- Sun, P.; Wang, C.; Luan, F.; Pan, B. Comparison of Auricle Reconstruction with Expanded Flaps and Auricle Reconstruction with Non-Expanded Flaps in Patients with Microtia: A Meta-Analysis. Ear Nose Throat J. 2024, 103, NP351–NP359. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Martinez, J.D.C.; Sanabria, R.; González, L.V.; Díaz-Báez, D. Postoperative Safety and Satisfaction in Patients With Microtia. J. Oral. Maxillofac. Surg. 2021, 79, 472.e1–472.e9. [Google Scholar] [CrossRef]
- Sharma, R.K.; DeSisto, N.G.; Ortiz, A.S.; Landeen, K.C.; Yang, S.F.; Stephan, S.J.; Patel, P.N. Early Postoperative Complications in Microtia Reconstruction: An Analysis of the NSQIP-P Database. Laryngoscope 2024, 134, 1214–1219. [Google Scholar] [CrossRef]
- Xu, Z.; Li, T.; Zhang, R.; Zhang, Q.; Xu, F.; Li, D.; Li, Y.; Chen, X. Strategies for the Treatment of Auricular Complications after the First Stage of Autologous Cartilage Microtia Reconstruction. Plast. Reconstr. Surg. 2022, 150, 157e–167e. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, T.; Wang, B.; Zhang, Q. Management Strategy of Local Subcutaneous Effusion After Auricle Reconstruction. Ear Nose Throat J. 2023, 102, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sasaki, M.; Oshima, J.; Nishijima, A.; Aihara, Y.; Sekido, M. Salvaging Exposed Microtia Cartilage Framework with Negative Pressure Wound Therapy. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1355–1401. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, M.; Fukui, K.; Yoshino, K.; Noguchi, M.; Murakami, R. Salvage of Ear Framework Exposure Following Autologous Microtia Reconstruction: Repair Strategy for Each Location of Exposure. Cleft Palate Craniofacial J. 2023, 60, 1172–1175. [Google Scholar] [CrossRef]
- Tuang, G.J.; Mansor, M.; Abdullah, A. An Unusual Delayed Complication of Rib Graft Microtia Reconstruction After Two Decades: A Case Report. Indian. J. Otolaryngol. Head. Neck Surg. 2022, 74, 3671–3674. [Google Scholar] [CrossRef]
- Yue, X.; Jiang, H.; Pan, B.; He, L.; Dong, W.; Yang, Q. Secondary Surgery for the Unsatisfactory Auricle after Auricular Reconstruction. Int. J. Pediatr. Otorhinolaryngol. 2022, 154, 111043. [Google Scholar] [CrossRef]
- Reinisch, J.F.; van Hövell Tot Westerflier, C.V.A.; Gould, D.J.; Tahiri, Y.T. Secondary Salvage of the Unsatisfactory Microtia Reconstruction. Plast. Reconstr. Surg. 2020, 145, 1252–1261. [Google Scholar] [CrossRef]
- Xiang, G.; Chen, C.; Chen, K.; Liu, Q.; Sun, X.; Huang, Y.; Huang, L.; Jin, J.; Shang, J.; Yang, D. Comparing the Analgesic Effects Between the Pre- and Post-Costal Cartilage Harvest Cohorts Using Ultrasound-Guided Deep Serratus Anterior Plane Block in Children with Microtia Undergoing Auricular Reconstruction: A Randomized Clinical Trial. Aesthetic Plast. Surg. 2024, 48, 1846–1854. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, X.; Yu, X.; Pan, B. Free Dermofat Grafting for Chest Deformity in Microtia Reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2023, 82, 130–136. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Feng, Y.; Huo, M.; Lin, L.; Zhang, L. Utilization of Intense Pulsed Light for Hair Removal in Pediatric Auricular Reconstruction Using Tissue Expander: A Retrospective Cohort Study. Medicine 2023, 102, e33736. [Google Scholar] [CrossRef]
- Yang, Q.; Qiang, S.; Fan, X.; Guo, S.Z.; Yin, Y.; Li, T.; Dang, H.; Dong, L.W.; Song, B.Q. Clinical Application of Long-Pulsed 800-Nm Diode Laser Depilation Technology on Microtia Reconstruction in 965 Patients. Aesthetic Plast. Surg. 2024, 48, 2155–2161. [Google Scholar] [CrossRef]
- Wei, J.; Baptista-Hon, D.T.; Wang, Z.; Li, G.; Herrler, T.; Dai, C.; Liu, K.; Yu, B.; Chen, X.; Yang, M.; et al. Bioengineered Human Tissue Regeneration and Repair Using Endogenous Stem Cells. Cell Rep. Med. 2023, 4, 101156. [Google Scholar] [CrossRef]
- Palacios, J.F.; Hazkour, N.; Robinson, E.; Swami, P.; Smith, L.; Grande, D.; Bastidas, N. Primary Ear Reconstruction Using Cadaveric Costal Cartilage. Ann. Plast. Surg. 2023, 90, S547–S551. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-C.; Wallace, C.G.; Wai-Yee Ho, V.; Wu, C.-M.; Chen, H.-Y.; Chen, Z.-C. Simultaneous Auricular Reconstruction and Transcutaneous Bone Conduction Device Implantation in Patients with Microtia. J. Formos. Med. Assoc. 2019, 118, 1202–1210. [Google Scholar] [CrossRef]
- Nishiyama, T.; Hayashi, S.; Oishi, N. A Novel Auricular Prosthesis Which Incorporates a Cartilage Conduction Hearing Aid Based on 3D Data Processing Technique: A Preclinical Evaluation. Eur. Arch. Otorhinolaryngol. 2022, 279, 3741–3744. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Sun, P.; Pan, B.; Jiang, H. Subtractive Thinking: A Novel Combined Application of Antihelix Reconstruction and Outer Helix Reconstruction to Treat Mild Cases of Type I to II Conchal Microtia. J. Plast. Reconstr. Aesthet. Surg. 2023, 84, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Xu, Z.; Zhang, R.; Zhang, Q.; Xu, F.; Chen, X. New Strategies for Base Frame Fabrication in Microtia Reconstruction. Sci. Rep. 2021, 11, 15947. [Google Scholar] [CrossRef]
- Park, H.Y.; Lee, K.-T.; Kim, E.-J.; Oh, K.S. Reconstruction of Congenital Microtia after Ear Canaloplasty Using V-Y Advancement of a Temporal Triangular Flap. Arch. Plast. Surg. 2021, 48, 614–621. [Google Scholar] [CrossRef]
- Sun, P.; Lu, M.; Wang, C.; Pan, B.; Jiang, H. Clinical Observations of a Surgical Method Comprising a Combination of Cross Flap and Autologous Auricular Cartilage Transplantation in the Treatment of Type I to III Congenital Concha-Type Microtia. Ear Nose Throat J. 2024, 103, NP60–NP66. [Google Scholar] [CrossRef]
- Lese, I.; Aldabbas, M.; Mazeed, A.S.; Bulstrode, N.W. Lop Ear to Conchal Microtia. Ann. Plast. Surg. 2022, 88, 188–194. [Google Scholar] [CrossRef]
- Lesta-Compagnucci, L.; Arámburo-García, R.; Galaso-Trujillo, J.R.; Apellaniz-Campo, A.G.; Pérez-Caro, E. Morbidity in Patients With Separation of Cartilaginous Framework: Temporoparietal Fascia Flap and Treatment With Dermal Regeneration Template. J. Craniofacial Surg. 2020, 31, 107–109. [Google Scholar] [CrossRef]
- Li, D.; Sun, J.; Zhang, R.; Xu, Z.; Zhang, Q.; Xu, F.; Li, Y.; Chen, X. Firm Elevation of the Auricle in Reconstruction of Microtia with a Retroauricular Fascial Flap Wrapping Two Titanium Plate Struts. J. Plast. Reconstr. Aesthet. Surg. 2023, 83, 134–140. [Google Scholar] [CrossRef]
- Li, C.-L.; Xie, Y.-Z.; He, A.; Liu, N.-H.; Cui, C.-X.; Chen, Y.; Fu, Y.; Zhang, T. Quantitative Framework Fabrication with Autogenous Costal Cartilage in Microtia Reconstruction. Laryngoscope 2024, 134, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Banda, C.H.; Narushima, M.; Mitsui, K.; Danno, K.; Fujita, M.; Furuya, M.; Karakawa, R.; Ogishima, S.; Ishiura, R. Posterior Auricular Artery Free Flap Reconstruction of the Retroauricular Sulcus in Microtia Repair. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2349–2357. [Google Scholar] [CrossRef]
- Mazeed, A.S.; O’Hara, J.; Bulstrode, N.W. Modification of the Cartilaginous Framework for Autologous Ear Reconstruction: Construction of a Stable Complete Ring Framework with Grander Highs and Lows. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Wang, M.; Li, Q.; Zhang, Q.; Zhou, X. A Modified Crescent Cartilage Block for Improving the Retroauricular Contour of the Reconstructed Ear: A Retrospective Cohort Study. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 1324–1330. [Google Scholar] [CrossRef]
- Ma, T.; Li, L.; Wang, X.; Zhang, Z. Auricular Reconstruction in Adult Patients With Unprepared Congenital Microtia: A Single Institution’s Experience. Ann. Plast. Surg. 2022, 89, 395–399. [Google Scholar] [CrossRef]
- Parri, F.; Alonso, V.; Albert, A.; Bejarano, M.; Vicario, F.; Rubio-Palau, J. Auricular Reconstructive Surgery Improvement to the Firmin Technique for Placing an Earring. Cleft Palate Craniofacial J. 2019, 56, 1260–1262. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, J.S.; Lee, J.W.; Choi, K.Y.; Yang, J.D.; Chung, H.Y.; Cho, B.C. Correction of Microtia with Constriction Features Using a Superficial Temporal Fascial Flap Combined with a Rib Cartilage Graft. Arch. Plast. Surg. 2020, 47, 317–323. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, R.; Zhang, Q.; Xu, F.; Li, D.; Li, Y. New Strategies for Tragus and Antitragus Complex Fabrication in Lobule-Type Microtia Reconstruction. Plast. Reconstr. Surg. 2019, 144, 913–921. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Li, D.; Zhang, R.; Zhang, Q.; Xu, F.; Chen, X. Strategies for Ear Elevation and the Treatment of Relevant Complications in Autologous Cartilage Microtia Reconstruction. Sci. Rep. 2022, 12, 13536. [Google Scholar] [CrossRef] [PubMed]
- Ibrahiem, S.M.S. Concha-Type Microtia: New Surgical Incision. Aesthet. Surg. J. 2023, 43, NP815–NP822. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Ding, W.; Ouyang, H.; Long, X.; Fu, A.; Liu, X. Reconstruction of Concha-Type Microtia Using a Delayed Postauricular Skin Flap. Plast. Reconstr. Surg. 2024, 153, 407e–410e. [Google Scholar] [CrossRef]
- Greene, A.K.; Sudduth, C.L. Elevation of Costal Cartilage Ear Construct for Microtia Using a V-Y Scalp Flap. J. Craniofacial Surg. 2020, 31, 1467–1468. [Google Scholar] [CrossRef] [PubMed]
- Quang, L.X.; Linh, T.N.T.; Ha, V.T.H.; Quyen, L.V.V.; Ngoc, T.L.H.; Dung, N.T.; Nga, N.T.T.; Chen, Y.-C.; Hung, S.-H.; Dang, L.H. A Two-Flap Combination for Auricular Elevation in Microtia Reconstruction. Plast. Reconstr. Surg. 2023, 151, 991e–1001e. [Google Scholar] [CrossRef]
- Li, D.; Zhang, R.; Xu, Z.; Zhang, Q.; Xu, F.; Li, Y.; Chen, X.; Hou, R. Ear Reconstruction: Empirical Data of 406 Cases of Carving the Convex Structures of the Framework. Laryngoscope 2023, 133, 569–575. [Google Scholar] [CrossRef]
- Wang, B.; Guo, R.; Li, Q.; Ou, Y.; Hu, J.; Wang, Y.; Zhang, Q.; Liu, T. A Novel Two-Stage Strategy Combing Tissue Expansion and Nagata`s Technique for Total Auricular Reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, J.; Wang, B.; Wang, Y.; Kang, C.; Zhang, Q. Total Auricular Reconstruction Using a Single Extended Postauricular Flap Without Skin Grafting in Two Stages: Experiences of 106 Cases. Aesthetic Plast. Surg. 2020, 44, 365–372. [Google Scholar] [CrossRef]
- Liang, J.; Cao, T.; Wang, Y.; Wang, B.; Qian, J.; Chen, Q.; Zhang, Q. A Modified Tissue Expander Method for Ear Reconstruction in Patients with Excessively Insufficient Postauricular Skin. Ear Nose Throat J. 2023, 102, NP449–NP456. [Google Scholar] [CrossRef]
- Landau, S.; Szklanny, A.A.; Machour, M.; Kaplan, B.; Shandalov, Y.; Redenski, I.; Beckerman, M.; Harari-Steinberg, O.; Zavin, J.; Karni-Katovitch, O.; et al. Human-Engineered Auricular Reconstruction (HEAR) by 3D-Printed Molding with Human-Derived Auricular and Costal Chondrocytes and Adipose-Derived Mesenchymal Stem Cells. Biofabrication 2021, 14, 015010. [Google Scholar] [CrossRef]
- Badawy, M.S.; Elshahat, A. Retroposition of the Vestigial Cartilage in Patients With Microtia: A Novel Technique to Enhance Projection of the Reconstructed Ear. J. Craniofacial Surg. 2022, 33, 1197–1200. [Google Scholar] [CrossRef]
- Guo, R.; Ying, J.; Yuan, X.; Xi, T.; Xiong, J.; Jiang, H. Novel Method for High-Density Porous Polyethylene Ear Reconstruction Stent Remodeling to Achieve High Satisfactory Outcomes. J. Cosmet. Dermatol. 2022, 21, 3486–3493. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Ng, L.-S.; Chang, C.-S.; Lu, T.-C.; Chen, N.-H.; Chen, Z.-C. Pursuing Mirror Image Reconstruction in Unilateral Microtia: Customizing Auricular Framework by Application of Three-Dimensional Imaging and Three-Dimensional Printing. Plast. Reconstr. Surg. 2017, 139, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, L.; Xie, C.; Wei, Y.; Huang, C.; Wang, Y.; Zhou, J.; Jia, C.; Junlin, L. Current Status of Auricular Reconstruction Strategy Development. J. Craniofacial Surg. 2024, 35, 984–992. [Google Scholar] [CrossRef]
- Joo, O.Y.; Kim, T.H.; Kim, Y.S.; Roh, T.S.; Lee, E.-J.; Shim, J.-H.; Cho, H.W.; Yun, I.S. Fabrication of 3D-Printed Implant for Two-Stage Ear Reconstruction Surgery and Its Clinical Application. Yonsei Med. J. 2023, 64, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Melgar-Lesmes, P.; Bosch, O.; Zubajlo, R.; Molins, G.; Comfort, S.; Luque-Saavedra, A.; López-Moya, M.; García-Polite, F.; Parri Ferrandis, F.J.; Rogers, C.; et al. Optimization of 3D Autologous Chondrocyte-Seeded Polyglycolic Acid Scaffolds to Mimic Human Ear Cartilage. Biomater. Sci. 2023, 11, 3695–3708. [Google Scholar] [CrossRef]
- Velasquillo, C.; Melgarejo-Ramírez, Y.; García-López, J.; Gutiérrez-Gómez, C.; Lecona, H.; González-Torres, M.; Sánchez-Betancourt, J.I.; Ibarra, C.; Lee, S.J.; Yoo, J.J. Remaining Microtia Tissue as a Source for 3D Bioprinted Elastic Cartilage Tissue Constructs, Potential Use for Surgical Microtia Reconstruction. Cell Tissue Bank. 2024, 25, 571–582. [Google Scholar] [CrossRef]
- Casarin, M.; Todesco, M.; Fontanella, C.G.; Morlacco, A.; Dal Moro, F.; Bagno, A. Hybrid Materials for Tissue Repair and Replacement: Another Frontier in Biomaterial Exploitation Focusing on Cardiovascular and Urological Fields. Processes 2023, 11, 2013. [Google Scholar] [CrossRef]
- Hirano, N.; Kusuhara, H.; Sueyoshi, Y.; Teramura, T.; Murthy, A.; Asamura, S.; Isogai, N.; Jacquet, R.D.; Landis, W.J. Ethanol Treatment of NanoPGA/PCL Composite Scaffolds Enhances Human Chondrocyte Development in the Cellular Microenvironment of Tissue-Engineered Auricle Constructs. PLoS ONE 2021, 16, e0253149. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Li, X.; Wang, K.; Huang, Z.; Wang, Q.; Fu, X.; Jiang, H.; Pan, B.; Xiao, R. Co-Culture of RhoA-Overexpressed Microtia Chondrocytes and Adipose-Derived Stem Cells in the Construction of Tissue-Engineered Ear-Shaped Cartilage. Stem Cells 2024, 42, 554–566. [Google Scholar] [CrossRef]
- Moon, J.; Lee, J.; Oh, K.S.; Lim, S.Y. Usefulness of Resorbable Plate in Auricular Elevation in Two-Stage Microtia Reconstruction. Int. J. Pediatr. Otorhinolaryngol. 2023, 171, 111646. [Google Scholar] [CrossRef]
- Gentilin, E.; Simoni, E.; Albertin, G.; Candito, M.R.; Sandrin, D.; Romanato, F.; Martini, A.; Nicolai, P.; Astolfi, L. Characterization of a Decellularized Rat Larynx: Comparison between Microscopy Techniques. Ann. Anat.—Anat. Anz. 2023, 245, 152020. [Google Scholar] [CrossRef]
- Todesco, M.; Imran, S.J.; Fortunato, T.M.; Sandrin, D.; Borile, G.; Romanato, F.; Casarin, M.; Giuggioli, G.; Conte, F.; Marchesan, M.; et al. A New Detergent for the Effective Decellularization of Bovine and Porcine Pericardia. Biomimetics 2022, 7, 104. [Google Scholar] [CrossRef]
- Casarin, M.; Fortunato, T.M.; Imran, S.J.; Todesco, M.; Sandrin, D.; Marchesan, M.; Gerosa, G.; Romanato, F.; Bagno, A.; Dal Moro, F.; et al. Preliminary In Vitro Assessment of Decellularized Porcine Descending Aorta for Clinical Purposes. J. Funct. Biomater. 2023, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Casarin, M.; Fortunato, T.M.; Imran, S.; Todesco, M.; Sandrin, D.; Borile, G.; Toniolo, I.; Marchesan, M.; Gerosa, G.; Bagno, A.; et al. Porcine Small Intestinal Submucosa (SIS) as a Suitable Scaffold for the Creation of a Tissue-Engineered Urinary Conduit: Decellularization, Biomechanical and Biocompatibility Characterization Using New Approaches. Int. J. Mol. Sci. 2022, 23, 2826. [Google Scholar] [CrossRef]
- Childs, R.D.; Nakao, H.; Isogai, N.; Murthy, A.; Landis, W.J. An Analytical Study of Neocartilage from Microtia and Otoplasty Surgical Remnants: A Possible Application for BMP7 in Microtia Development and Regeneration. PLoS ONE 2020, 15, e0234650. [Google Scholar] [CrossRef]
- Yue, H.; Pathak, J.L.; Zou, R.; Qin, L.; Liao, T.; Hu, Y.; Kuang, W.; Zhou, L. Fabrication of Chondrocytes/Chondrocyte-Microtissues Laden Fibrin Gel Auricular Scaffold for Microtia Reconstruction. J. Biomater. Appl. 2021, 35, 838–848. [Google Scholar] [CrossRef]
- Donnelly, H.; Kurjan, A.; Yong, L.Y.; Xiao, Y.; Lemgruber, L.; West, C.; Salmeron-Sanchez, M.; Dalby, M.J. Fibronectin Matrix Assembly and TGFβ1 Presentation for Chondrogenesis of Patient Derived Pericytes for Microtia Repair. Biomater. Adv. 2023, 148, 213370. [Google Scholar] [CrossRef]
- Ziegler, M.E.; Sorensen, A.M.; Banyard, D.A.; Evans, G.R.D.; Widgerow, A.D. Improving In Vitro Cartilage Generation by Co-Culturing Adipose-Derived Stem Cells and Chondrocytes on an Allograft Adipose Matrix Framework. Plast. Reconstr. Surg. 2021, 147, 87–99. [Google Scholar] [CrossRef]
- Uto, S.; Hikita, A.; Sakamoto, T.; Mori, D.; Yano, F.; Ohba, S.; Saito, T.; Takato, T.; Hoshi, K. Ear Cartilage Reconstruction Combining Induced Pluripotent Stem Cell-Derived Cartilage and Three-Dimensional Shape-Memory Scaffold. Tissue Eng. Part A 2021, 27, 604–617. [Google Scholar] [CrossRef]
- Zucchelli, E.; Birchall, M.; Bulstrode, N.W.; Ferretti, P. Modeling Normal and Pathological Ear Cartilage in Vitro Using Somatic Stem Cells in Three-Dimensional Culture. Front. Cell Dev. Biol. 2020, 8, 666. [Google Scholar] [CrossRef]
- Sakahara, D.; Yanaga, H.; Noto, M.; Fujimoto, T.; Imai, K. Long-Term Clinical Results of Two-Stage Total Ear Reconstruction of Microtia Using Autologous Cell-Engineered Chondrocytes. Plast. Reconstr. Surg. 2023, 151, 282e–287e. [Google Scholar] [CrossRef] [PubMed]
- Enomura, M.; Murata, S.; Terado, Y.; Tanaka, M.; Kobayashi, S.; Oba, T.; Kagimoto, S.; Yabuki, Y.; Morita, K.; Uemura, T.; et al. Development of a Method for Scaffold-Free Elastic Cartilage Creation. Int. J. Mol. Sci. 2020, 21, 8496. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellies, F.; Fracaro, S.; Marioni, G.; Trotta, A.; Todesco, M.; Casarin, M.; Bagno, A.; Zanoletti, E.; Albertin, G.; Astolfi, L. Systematic Review on Microtia: Current Knowledge and Future Directions. Children 2025, 12, 411. https://doi.org/10.3390/children12040411
Hellies F, Fracaro S, Marioni G, Trotta A, Todesco M, Casarin M, Bagno A, Zanoletti E, Albertin G, Astolfi L. Systematic Review on Microtia: Current Knowledge and Future Directions. Children. 2025; 12(4):411. https://doi.org/10.3390/children12040411
Chicago/Turabian StyleHellies, Filippo, Silvia Fracaro, Gino Marioni, Annalisa Trotta, Martina Todesco, Martina Casarin, Andrea Bagno, Elisabetta Zanoletti, Giovanna Albertin, and Laura Astolfi. 2025. "Systematic Review on Microtia: Current Knowledge and Future Directions" Children 12, no. 4: 411. https://doi.org/10.3390/children12040411
APA StyleHellies, F., Fracaro, S., Marioni, G., Trotta, A., Todesco, M., Casarin, M., Bagno, A., Zanoletti, E., Albertin, G., & Astolfi, L. (2025). Systematic Review on Microtia: Current Knowledge and Future Directions. Children, 12(4), 411. https://doi.org/10.3390/children12040411










