Are the Risk Factors for Bronchopulmonary Dysplasia and Retinopathy of Prematurity in Very Low-Birth-Weight Infants the Same?
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Participants
2.1.1. Source of Data
2.1.2. Inclusion Criteria
- (1)
- Birth weight < 1500 g and GA ≤ 32 weeks.
- (2)
- Born in the obstetrics department of our hospital and transferred to the NICU for treatment after birth.
- (3)
- Survival to a postmenstrual age (PMA) of 36 weeks.
2.1.3. Exclusion Criteria
- (1)
- Severe congenital malformations, such as congenital lung diseases (e.g., congenital pulmonary hypoplasia, pulmonary sequestration, congenital bronchopulmonary cysts, transparent lungs, and congenital pulmonary arteriovenous fistulas), congenital heart diseases (e.g., pulmonary valve stenosis, aortic valve stenosis, and tetralogy of Fallot), or other systemic malformations.
- (2)
- Incomplete medical history prior to PMA of 36 weeks due to factors such as death, discontinuation of treatment, self-discharge, or transfer to other hospitals for surgical treatment.
2.2. Diagnosis and Grouping
2.3. Data Collection and Definitions
2.4. Statistical Analysis
3. Results
3.1. Enrollment of Study Participants
3.2. Pairwise Comparisons Among the Four Groups
3.2.1. Comparison of Maternal Pregnancy and Birth Conditions
3.2.2. Comparison of Postnatal Diseases and Treatment Conditions
3.3. Independent Risk Factor Analysis for BPD and ROP
3.3.1. Univariate Analysis of BPD Group vs. Non-BPD Group
3.3.2. Multivariate Analysis of BPD
3.3.3. Univariate Analysis of ROP Group vs. Non-ROP Group
3.3.4. Multivariate Analysis of ROP
4. Discussion
4.1. Common Risk Factors for BPD and ROP
4.2. Differences in Risk Factors for BPD and ROP
4.3. Interaction Between BPD and ROP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPD | bronchopulmonary dysplasia |
| GA | gestational age |
| hsPDA | hemodynamically significant patent ductus arteriosus |
| NICU | neonatal intensive care unit |
| NRDS | neonatal respiratory distress syndrome |
| PMA | postmenstrual age |
| PPHN | persistent pulmonary hypertension of the newborn |
| ROP | retinopathy of prematurity |
| SGA | small for gestational age |
| VLBW | very low birth weight |
References
- Khan, S.I.; Ryu, W.Y.; Wood, E.H.; Moshfeghi, D.M.; Shah, J.K.; Lambert, S.R. Retinopathy of prematurity treatment trends from 2003 to 2020 in the United States. Ophthalmology 2022, 129, 1216–1218. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Yang, J.; Cao, B. Predictive value of neonatal complications for poor prognosis at corrected age of 12 months in very low birth weight preterm infants. Chin. J. Pediatr. 2017, 55, 608–612. [Google Scholar]
- Bae, S.P.; Shin, S.H.; Yoon, Y.M.; Kim, E.K.; Kim, H.S. Association of severe retinopathy of prematurity and bronchopulmonary dysplasia with adverse neurodevelopmental outcomes in preterm infants without severe brain injury. Brain Sci. 2021, 11, 699. [Google Scholar] [CrossRef]
- Dammann, O.; Hartnett, M.E.; Stahl, A. Retinopathy of prematurity. Dev. Med. Child Neurol. 2023, 65, 625–631. [Google Scholar] [CrossRef]
- Stark, A.; Dammann, C.; Nielsen, H.C.; Volpe, M.V. A Pathogenic relationship of bronchopulmonary dysplasia and retinopathy of prematurity? A review of angiogenic mediators in both diseases. Front. Pediatr. 2018, 6, 125. [Google Scholar] [CrossRef]
- Wickramasinghe, L.C.; van Wijngaarden, P.; Tsantikos, E.; Hibbs, M.L. The immunological link between neonatal lung and eye disease. Clin. Transl. Immunol. 2021, 10, e1322. [Google Scholar] [CrossRef]
- Wickramasinghe, L.C.; Lau, M.; Deliyanti, D.; Gottschalk, T.A.; van Wijngaarden, P.; Talia, D.; Johnson, C.; Wilkinson-Berka, J.L.; Tsantikos, E.; Hibbs, M.L. Lung and eye disease develop concurrently in supplemental oxygen-exposed neonatal mice. Am. J. Pathol. 2020, 190, 1801–1812. [Google Scholar] [CrossRef]
- Podraza, W.; Michalczuk, B.; Jezierska, K.; Domek, H.; Kordek, A.; Łoniewska, B.; Modrzejewska, M.; Kot, J. Correlation of retinopathy of prematurity with bronchopulmonary dysplasia. Open Med. 2018, 13, 67–73. [Google Scholar] [CrossRef]
- Li, C.W.; Ao, D.; Li, Y.D.; Chen, H.X. Research progress on risk factors for retinopathy of prematurity in preterm infants. Zhongwai Yixue Yanjiu (Chin. Foreign Med. Res.) 2024, 22, 180–184. [Google Scholar]
- Huang, J.; Shen, W.; Wu, F.; Mao, J.; Liu, L.; Chang, Y.; Zhang, R.; Ye, X.; Qiu, Y.; Ma, L.; et al. Risk factors for severe bronchopulmonary dysplasia in a Chinese cohort of very preterm infants. Saudi Med. J. 2024, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary dysplasia: Executive summary of a workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.F.; Quinn, G.E.; Fielder, A.R.; Ostmo, S.R.; Paul Chan, R.V.; Berrocal, A.; Binenbaum, G.; Blair, M.; Peter Campbell, J.; Capone, A.; et al. International Classification of Retinopathy of Prematurity, Third Edition. Ophthalmology 2021, 128, e51–e68. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.L.; Noori, S. What is a hemodynamically significant PDA in preterm infants? Congenit. Heart Dis. 2019, 14, 21–26. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Blazon, M.N.; Rezar-Dreindl, S.; Wassermann, L.; Neumayer, T.; Berger, A.; Stifter, E. Retinopathy of prematurity: Incidence, risk factors, and treatment outcomes in a tertiary care center. J. Clin. Med. 2024, 13, 6926. [Google Scholar] [CrossRef]
- Carlo, W.A.; Higgins, R.D. Optimum oxygen therapy to prevent retinopathy of prematurity. Expert Rev. Ophthalmol. 2010, 5, 583–585. [Google Scholar] [CrossRef]
- Berger, J.; Mehta, P.; Bucholz, E.; Dziura, J.; Bhandari, V. Impact of early extubation and reintubation on the incidence of bronchopulmonary dysplasia in neonates. Am. J. Perinatol. 2014, 31, 1063–1072. [Google Scholar] [CrossRef]
- Jensen, E.A.; DeMauro, S.B.; Kornhauser, M.; Aghai, Z.H.; Greenspan, J.S.; Dysart, K.C. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015, 169, 1011–1017. [Google Scholar] [CrossRef]
- Glenn, T.; Fischer, L.; Markowski, A.; Carr, C.B.; Malay, S.; Hibbs, A.M. Complicated Intubations Are Associated with Bronchopulmonary Dysplasia in Very Low Birth Weight Infants. Am. J. Perinatol. 2023, 40, 1245–1252. [Google Scholar] [CrossRef]
- Protsyk, O.; García Serrano, J.L. Mechanical ventilation, retinal avascularity and rate of vascularisation: A triad of predictors for retinopathy of prematurity treatment. J. Pers. Med. 2024, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Li, L.L.; Li, R.; Zeng, Y.Y. Analysis of clinical factors associated with retinopathy of prematurity and bronchopulmonary dysplasia in preterm infants. Chin. J. Fundus Dis. 2020, 36, 600–604. [Google Scholar]
- Kim, S.H.; Han, Y.S.; Chun, J.; Lee, M.H.; Sung, T.J. Risk factors that affect the degree of bronchopulmonary dysplasia: Comparison by severity in the same gestational age. PLoS ONE 2020, 15, e0235901. [Google Scholar] [CrossRef] [PubMed]
- Sucasas Alonso, A.; Pértega Diaz, S.; Sáez Soto, R.; Avila-Alvarez, A. Epidemiology and risk factors for bronchopulmonary dysplasia in preterm infants born at or less than 32 weeks of gestation. An. Pediatr. (Engl. Ed.) 2022, 96, 242–251. [Google Scholar] [CrossRef]
- Glaser, K.; Härtel, C.; Klingenberg, C.; Herting, E.; Fortmann, M.I.; Speer, C.P.; Stensvold, H.J.; Huncikova, Z.; Rønnestad, A.E.; Nentwich, M.M.; et al. Neonatal sepsis episodes and retinopathy of prematurity in very preterm infants. JAMA Netw. Open 2024, 7, e2423933. [Google Scholar] [CrossRef]
- Dammann, O.; Rivera, J.C.; Chemtob, S. The prenatal phase of retinopathy of prematurity. Acta Paediatr. 2021, 110, 2521–2528. [Google Scholar] [CrossRef]
- Jung, E.H.; Moon, G.Y. The incidence and risk factors of retinopathy of prematurity in South Korea: A nationwide cohort study. Medicine 2024, 103, e38080. [Google Scholar] [CrossRef]
- Chang, J.W. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS ONE 2019, 14, e0219934. [Google Scholar] [CrossRef]
- Procianoy, R.S.; Garcia-Prats, J.A.; Hittner, H.M.; Adams, J.M.; Rudolph, A.J. An association between retinopathy of prematurity and intraventricular hemorrhage in very low birth weight infants. Acta Paediatr. Scand. 1981, 70, 473–477. [Google Scholar] [CrossRef]
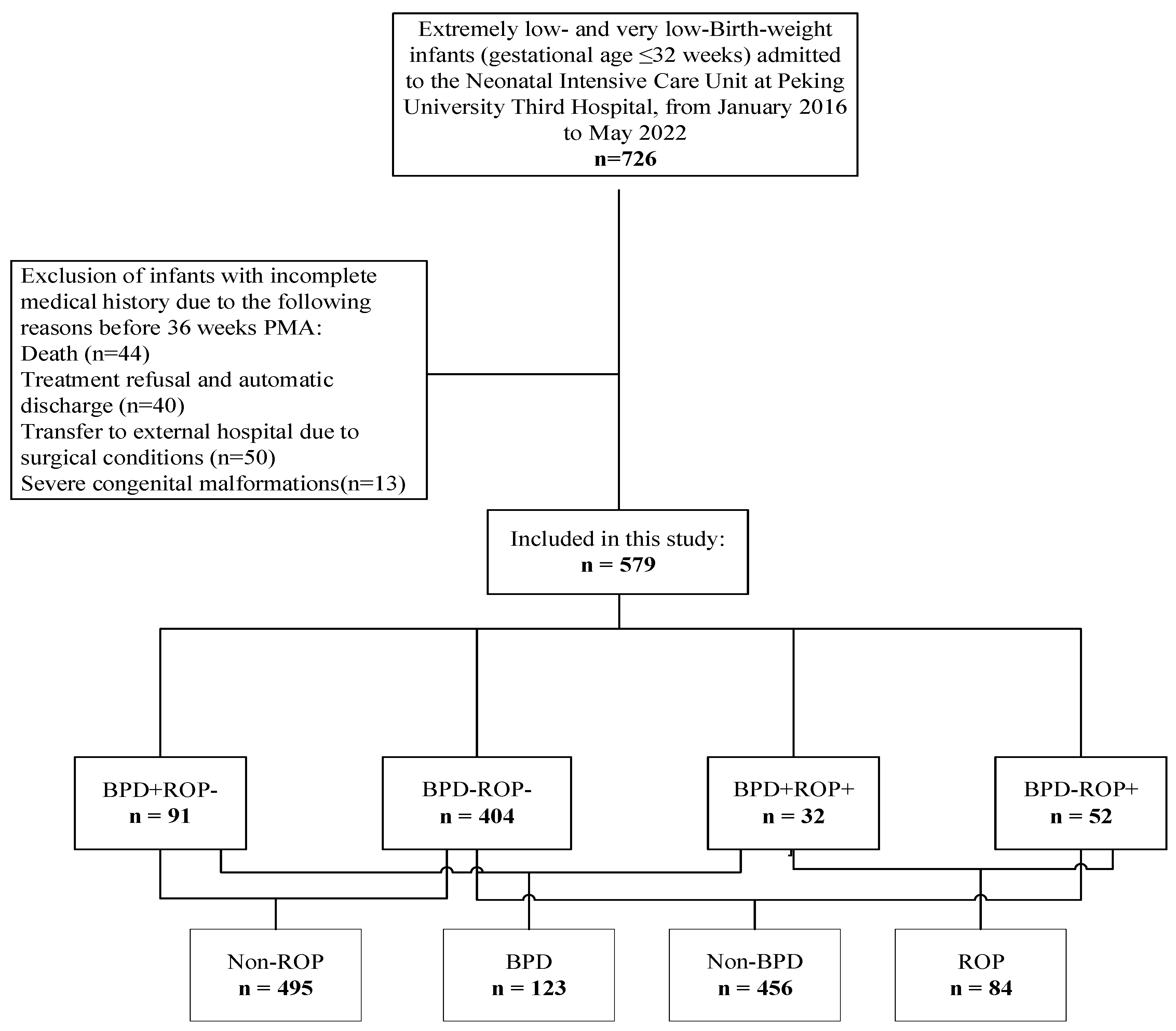






| Variable | BPD-ROP- (n = 404) | BPD+ROP+ (n = 32) | BPD+ROP- (n = 91) | BPD-ROP+ (n = 52) | χ2/Z | p-Value |
|---|---|---|---|---|---|---|
| Male sex | 203 (50.25%) | 20 (62.5%) | 56 (61.54%) | 22 (42.31%) | 7.185 | 0.066 |
| Gestational age (weeks) | 30.05 (29, 31) | 26.95 (26.3, 28) | 29.2 (28.2, 30.05) | 29.2 (28.25, 30) | 72.823 | <0.001 |
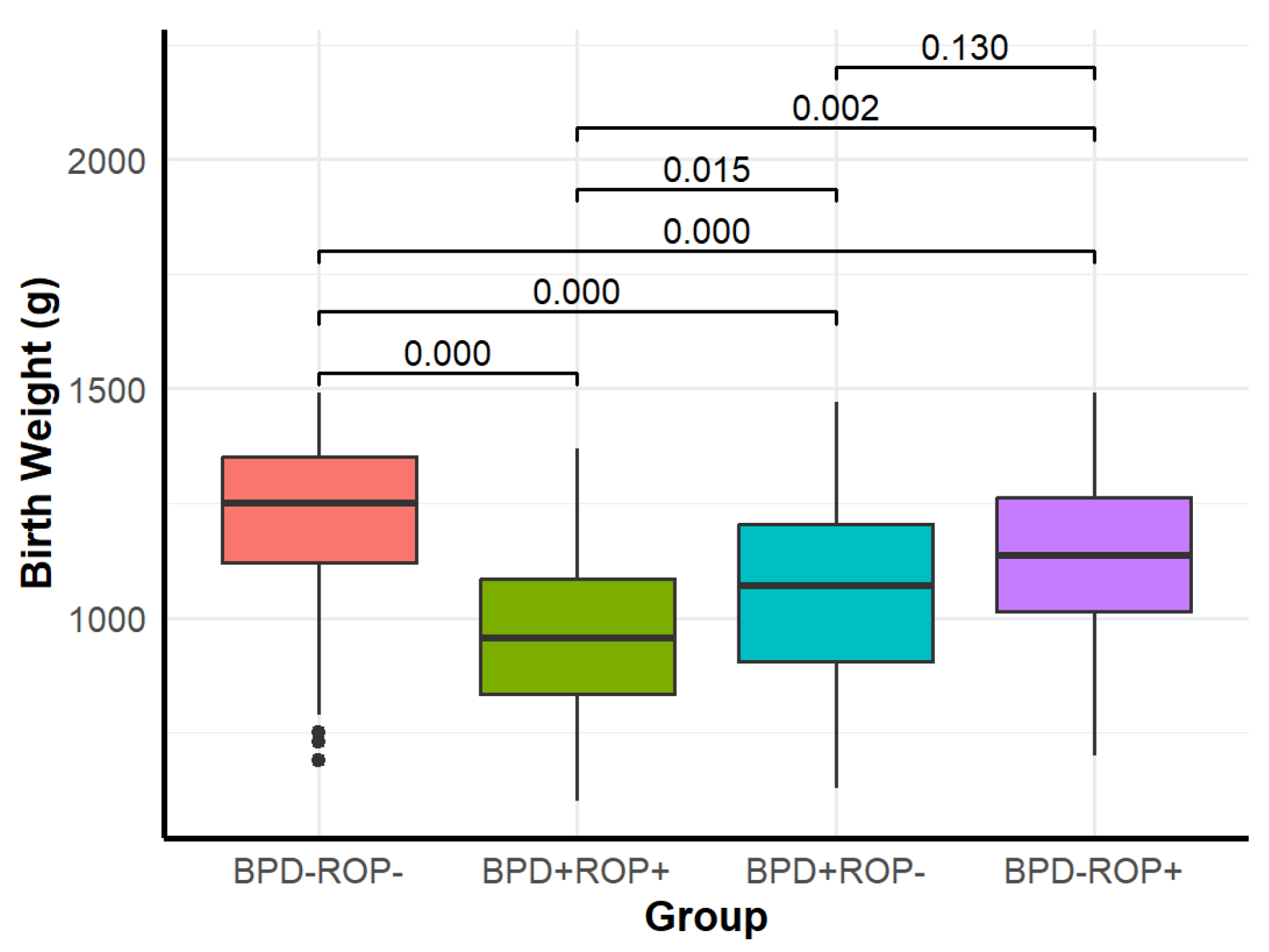
| Birth weight (g) | 1250 (1120, 1352.5) | 955 (835, 1085) | 1070 (905, 1205) | 1135 (1015, 1262.5) | 88.722 | <0.001 |
| SGA | 32 (7.92%) | 1 (3.12%) | 20 (21.98%) | 5 (9.62%) | 18.108 | <0.001 |
| Antenatal corticosteroids | 302 (74.75%) | 16 (50%) | 68 (74.73%) | 36 (69.23%) | 9.7 | 0.021 |
| Maternal preeclampsia | 151 (37.38%) | 10 (31.25%) | 29 (31.87%) | 16 (30.77%) | 1.903 | 0.593 |
| Premature rupture of membranes > 18 h | 86 (21.29%) | 5 (15.62%) | 23 (25.27%) | 11 (21.15%) | 1.43 | 0.698 |
| 1 min Apgar score ≤ 7 | 77 (19.06%) | 16 (50%) | 27 (29.67%) | 14 (26.92%) | 19.362 | <0.001 |
| Endotracheal intubation during resuscitation | 62 (15.35%) | 18 (56.25%) | 33 (36.26%) | 11 (21.15%) | 43.837 | <0.001 |
| NRDS | 157 (38.86%) | 26 (81.25%) | 57 (62.64%) | 27 (51.92%) | 35.155 | <0.001 |
| hsPDA | 137 (33.9%) | 24 (75%) | 48 (52.7%) | 20 (38.5%) | 28.849 | <0.001 |
| PPHN | 26 (6.44%) | 3 (9.38%) | 14 (15.38%) | 1 (1.92%) | 11.164 | 0.011 |
| Pulmonary hemorrhage | 9 (2.23%) | 4 (12.5%) | 9 (9.89%) | 2 (3.85%) | 16.931 | 0.001 |
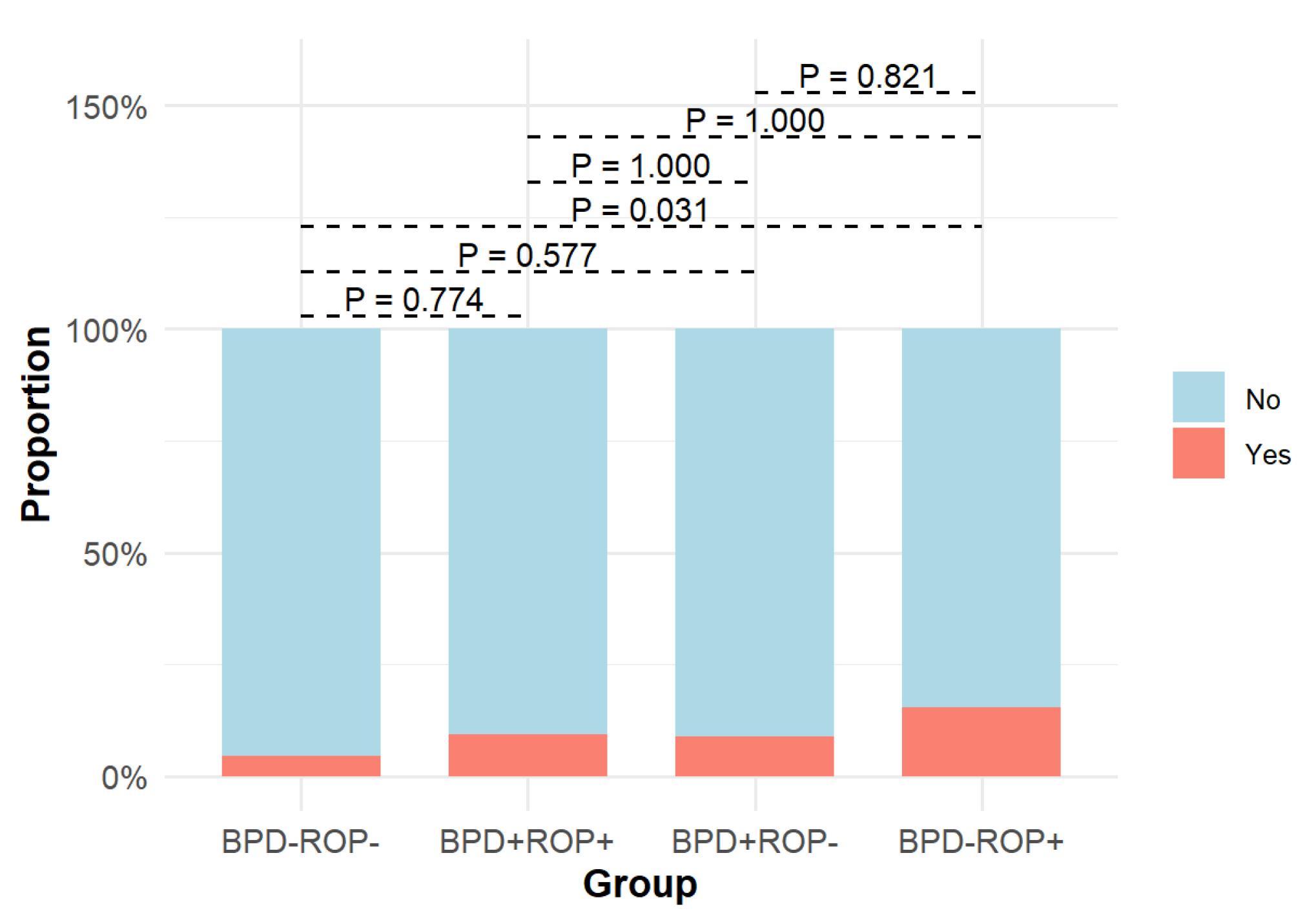
| Intraventricular hemorrhage (grade ≥ 3) | 18 (4.46%) | 3 (9.38%) | 8 (8.79%) | 8 (15.38%) | 10.914 | 0.012 |
| Early-onset sepsis | 8 (1.98%) | 1 (3.12%) | 1 (1.1%) | 3 (5.77%) | - | 0.218 |
| Intrauterine infectious pneumonia | 68 (16.83%) | 6 (18.75%) | 20 (21.98%) | 9 (17.31%) | 1.374 | 0.712 |
| Hospital-acquired pneumonia | 122 (30.2%) | 22 (68.75%) | 55 (60.44%) | 20 (38.46%) | 42.804 | <0.001 |
| Late-onset sepsis | 62 (15.35%) | 7 (21.88%) | 27 (29.67%) | 3 (5.77%) | 16.245 | 0.001 |
| Mechanical ventilation time (h) | 0 (0, 0) | 132 (18.5, 390) | 80 (0, 180) | 0 (0, 72) | 120.498 | <0.001 |
| Total oxygen therapy time (h) | 624 (323, 897.75) | 1740 (1548, 1920) | 1296 (1140, 1560) | 924 (669.25, 1110) | 220.831 | <0.001 |
| Variable | Non-BPD (n = 456) | BPD (n = 123) | χ2/Z | p-Value |
|---|---|---|---|---|
| Male sex | 231 (50.66%) | 47 (38.21%) | 6.012 | 0.014 |
| Gestational age (weeks) | 30 (28.6, 31) | 28.6 (27.35, 30) | 6.391 | <0.001 |
| Birth weight (g) | 1240 (1100, 1350) | 1050 (875, 1175) | 8.396 | <0.001 |
| SGA | 37 (8.11%) | 21 (17.07%) | 8.626 | 0.003 |
| Antenatal corticosteroids | 338 (74.12%) | 84 (68.29%) | 1.666 | 0.197 |
| Maternal preeclampsia | 167 (36.62%) | 39 (31.71%) | 1.021 | 0.312 |
| Premature rupture of membranes > 18 h | 97 (21.27%) | 28 (22.76%) | 0.127 | 0.721 |
| 1 min Apgar score ≤ 7 | 91 (19.96%) | 43 (34.96%) | 12.259 | <0.001 |
| Endotracheal intubation during resuscitation | 73 (16.01%) | 51 (41.46%) | 37.295 | <0.001 |
| NRDS | 37.295 | <0.001 | 28.691 | <0.001 |
| hsPDA | 157 (34.43%) | 72 (58.54%) | 23.546 | <0.001 |
| PPHN | 27 (5.92%) | 17 (13.82%) | 8.610 | 0.003 |
| Pulmonary hemorrhage | 11 (2.41%) | 13 (10.57%) | 16.221 | <0.001 |
| Intraventricular hemorrhage (grade ≥ 3) | 26 (5.7%) | 11 (8.94%) | 1.701 | 0.192 |
| Early-onset sepsis | 11 (2.41%) | 2 (1.63%) | 0.032 | 0.858 |
| Intrauterine infectious pneumonia | 77 (16.89%) | 26 (21.14%) | 1.198 | 0.274 |
| Hospital-acquired pneumonia | 142 (31.14%) | 77 (62.6%) | 40.771 | <0.001 |
| Late-onset sepsis | 65 (14.25%) | 34 (27.64%) | 12.249 | <0.001 |
| Mechanical ventilation time (h) | 0 (0, 19.25) | 96 (0, 216) | −10.698 | <0.001 |
| Total oxygen therapy time (h) | 672 (336, 936) | 1392 (1200, 1728) | −14.241 | <0.001 |
| ROP | 52 (11.4%) | 32 (26.02%) | 16.677 | <0.001 |
| Variable | β | SE | Wald | p-Value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Constant | −56.710 | 7.003 | −8.098 | <0.001 | ||
| Gestational age (weeks) | 1.490 | 0.200 | 7.438 | <0.001 | 4.44 | 3–6.57 |
| Endotracheal intubation during resuscitation | 0.854 | 0.391 | 2.184 | 0.029 | 2.35 | 1.09–5.05 |
| Mechanical ventilation time ≥7 days | 0.010 | 0.001 | 9.279 | <0.001 | 1.01 | 1.01–1.01 |
| Total oxygen therapy time (h) | 1.141 | 0.455 | 2.509 | 0.012 | 3.13 | 1.28–7.64 |
| Variable | Non-ROP (n = 495) | ROP (n = 84) | χ2/Z | p-Value |
|---|---|---|---|---|
| Male sex | 236 (47.68%) | 42 (50%) | 0.155 | 0.694 |
| Gestational age (weeks) | 29.6 (28.6, 30.6) | 28.4 (26.58, 29.4) | 6.506 | <0.001 |
| Birth weight (g) | 1220 (1080, 1350) | 1080 (877.5, 1210) | 5.605 | <0.001 |
| SGA | 52 (10.51%) | 6 (7.14%) | 0.901 | 0.343 |
| Antenatal corticosteroids | 370 (74.75%) | 52 (61.9%) | 5.993 | 0.014 |
| Maternal preeclampsia | 180 (36.36%) | 26 (30.95%) | 0.917 | 0.338 |
| Premature rupture of membranes > 18 h | 109 (22.02%) | 16 (19.05%) | 0.375 | 0.54 |
| 1 min Apgar score ≤ 7 | 104 (21.01%) | 30 (35.71%) | 8.729 | 0.003 |
| Endotracheal intubation during resuscitation | 95 (19.19%) | 29 (34.52%) | 10.030 | 0.002 |
| NRDS | 214 (43.23%) | 53 (63.1%) | 11.402 | 0.001 |
| hsPDA | 185 (37.37%) | 44 (52.38%) | 6.765 | 0.009 |
| PPHN | 40 (8.08%) | 4 (4.76%) | 1.127 | 0.289 |
| Pulmonary hemorrhage | 18 (3.64%) | 6 (7.14%) | 1.427 | 0.232 |
| Intraventricular hemorrhage (grade ≥ 3) | 26 (5.25%) | 11 (13.1%) | 7.384 | 0.007 |
| Early-onset sepsis | 9 (1.82%) | 4 (4.76%) | 1.653 | 0.199 |
| Intrauterine infectious pneumonia | 88 (17.78%) | 15 (17.86%) | 0.000 | 0.986 |
| Hospital-acquired pneumonia | 177 (35.76%) | 42 (50%) | 6.194 | 0.013 |
| Late-onset sepsis | 89 (17.98%) | 10 (11.9%) | 1.870 | 0.172 |
| Mechanical ventilation time (h) | 0 (0, 48) | 22 (0, 126) | −4.209 | <0.001 |
| Total oxygen therapy time (h) | 724 (407, 1102) | 1152 (851.25, 1656) | −6.532 | <0.001 |
| BPD | 91 (18.38%) | 32 (38.1%) | 16.677 | <0.001 |
| Variable | β | SE | Wald | p-Value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Constant | 9.476 | 3.154 | 3.004 | 0.003 | ||
| GA (weeks) | −0.412 | 0.103 | −3.994 | <0.001 | 0.66 | 0.54–0.81 |
| Total oxygen therapy time (h) | 0.017 | 0.007 | 2.383 | 0.017 | 1.02 | 1–1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, J.; Zhang, J.; Yu, Y.; Zhang, H.; Han, T. Are the Risk Factors for Bronchopulmonary Dysplasia and Retinopathy of Prematurity in Very Low-Birth-Weight Infants the Same? Children 2025, 12, 509. https://doi.org/10.3390/children12040509
Wu H, Zhang J, Zhang J, Yu Y, Zhang H, Han T. Are the Risk Factors for Bronchopulmonary Dysplasia and Retinopathy of Prematurity in Very Low-Birth-Weight Infants the Same? Children. 2025; 12(4):509. https://doi.org/10.3390/children12040509
Chicago/Turabian StyleWu, Hui, Juan Zhang, Jing Zhang, Yanhong Yu, Hua Zhang, and Tongyan Han. 2025. "Are the Risk Factors for Bronchopulmonary Dysplasia and Retinopathy of Prematurity in Very Low-Birth-Weight Infants the Same?" Children 12, no. 4: 509. https://doi.org/10.3390/children12040509
APA StyleWu, H., Zhang, J., Zhang, J., Yu, Y., Zhang, H., & Han, T. (2025). Are the Risk Factors for Bronchopulmonary Dysplasia and Retinopathy of Prematurity in Very Low-Birth-Weight Infants the Same? Children, 12(4), 509. https://doi.org/10.3390/children12040509






