Associations of Prenatal Socioeconomic Status and Childhood Working Memory: A Structural Equation Modeling Approach
Abstract
1. Introduction
2. Methods
2.1. Data
2.2. Measures
2.3. Current SES
2.4. Working Memory Tasks
2.5. Cambridge Neuropsychological Test Automated Battery (CANTAB) Spatial Working Memory (CSWM) [54,55,56]
2.6. Delayed Matching to Sample (DMTS)
2.7. Incremental Repeated Acquisition (IRA)
2.8. Covariates
2.9. Developing a Measurement Model for Childhood WM
2.10. Associations Between Prenatal SES and WM
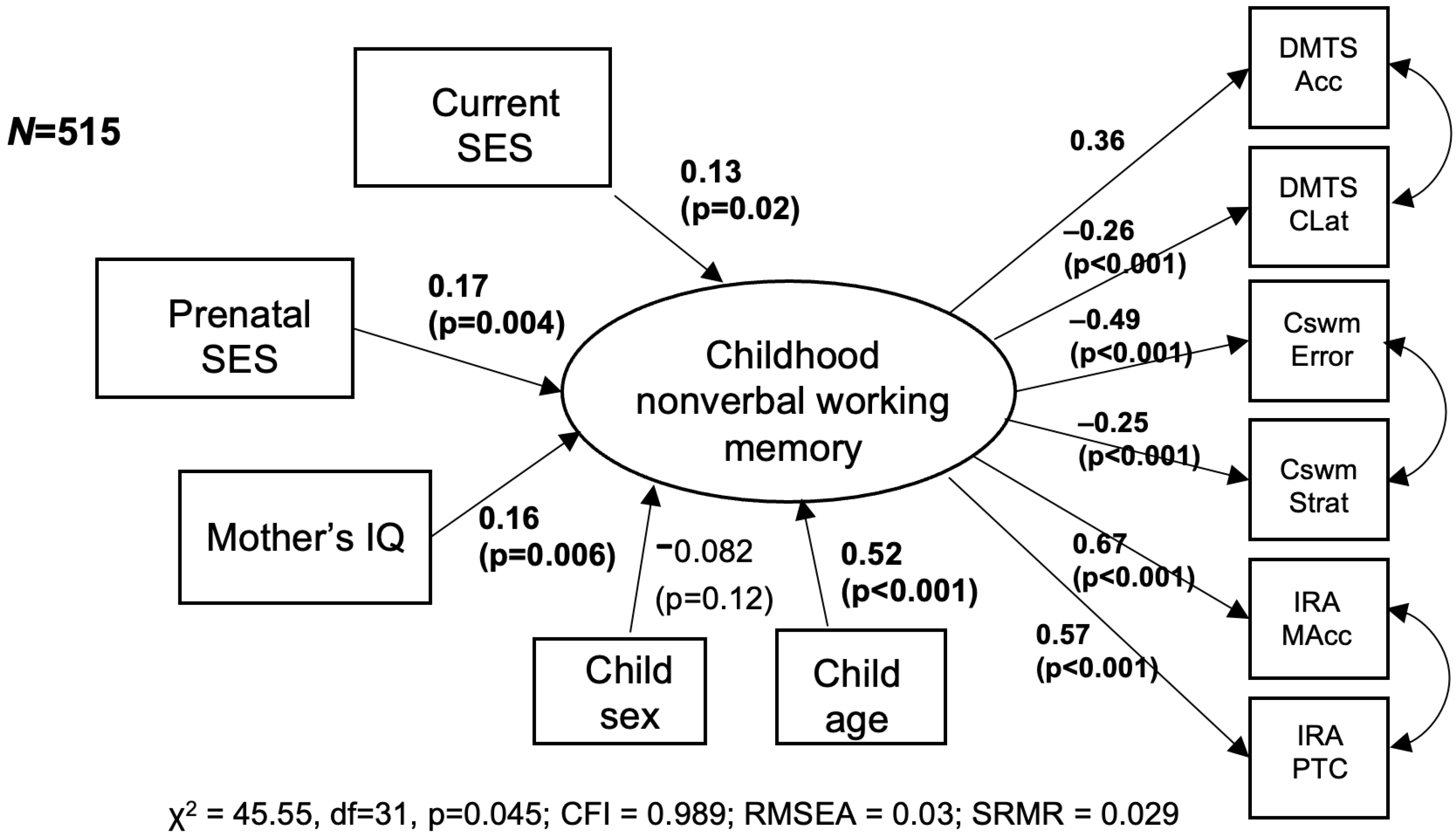
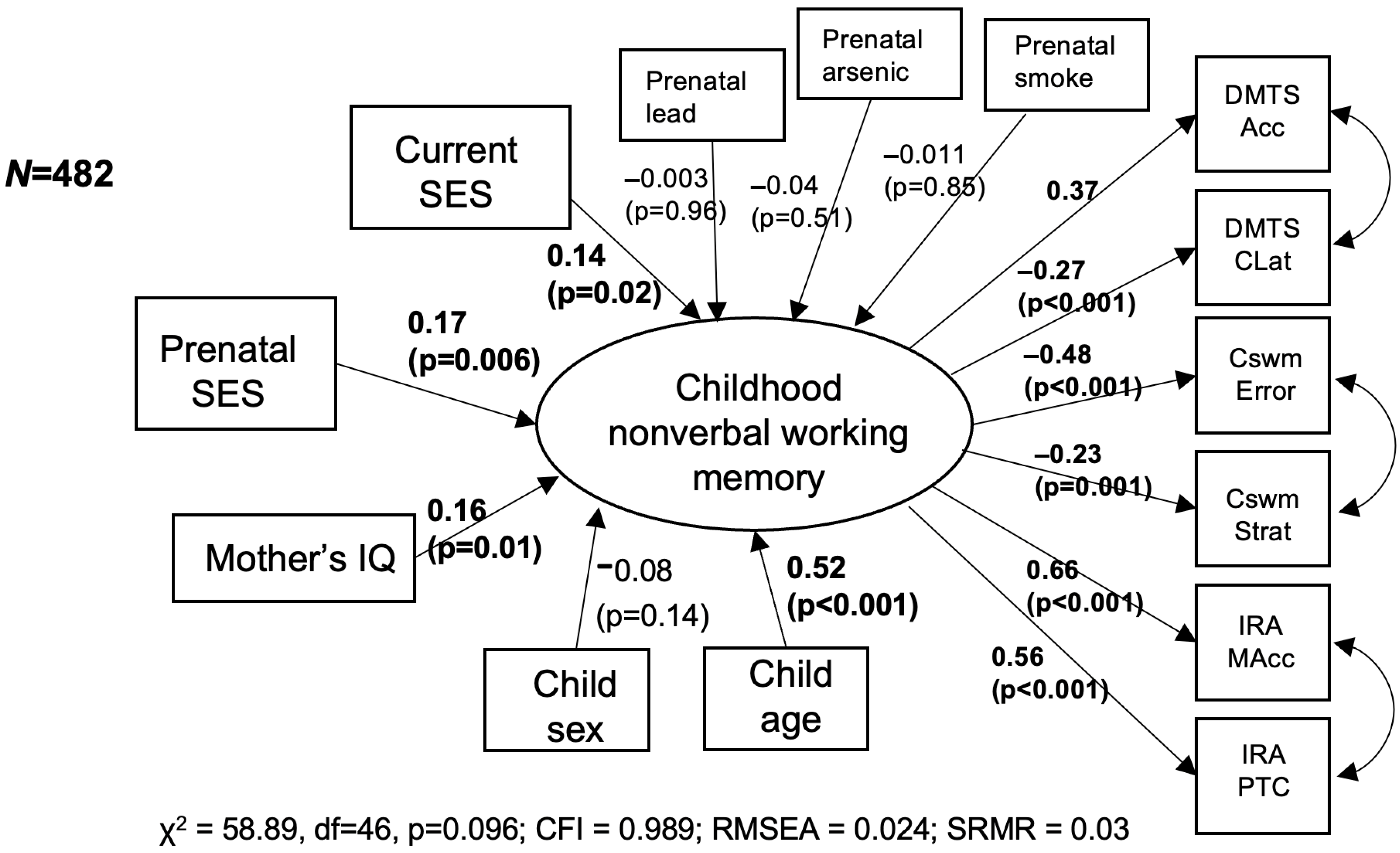
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spann, M.N.; Bansal, R.; Hao, X.; Rosen, T.S.; Peterson, B.S. Prenatal socioeconomic status and social support are associated with neonatal brain morphology, toddler language and psychiatric symptoms. Child Neuropsychol. 2020, 26, 170–188. [Google Scholar] [CrossRef]
- Hung, G.C.; Hahn, J.; Alamiri, B.; Buka, S.L.; Goldstein, J.M.; Laird, N.; Nelson, C.A.; Smoller, J.W.; Gilman, S.E. Socioeconomic disadvantage and neural development from infancy through early childhood. Int. J. Epidemiol. 2015, 44, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Farah, M.J.; Meaney, M.J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2011, 11, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef]
- Best, J.R.; Miller, P.H. A developmental perspective on executive function. Child Dev. 2010, 81, 1641–1660. [Google Scholar] [CrossRef]
- Garon, N.; Bryson, S.E.; Smith, I.M. Executive function in preschoolers: A review using an integrative framework. Psychol. Bull. 2008, 134, 31–60. [Google Scholar] [CrossRef]
- Hughes, C.; Ensor, R.; Wilson, A.; Graham, A. Tracking executive function across the transition to school: A latent variable approach. Dev. Neuropsychol. 2010, 35, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, M.; Dolan, C.V.; van der Molen, M.W. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia 2006, 44, 2017–2036. [Google Scholar] [CrossRef]
- Lawson, G.M.; Farah, M.J. Executive Function as a Mediator Between SES and Academic Achievement Throughout Childhood. Int. J. Behav. Dev. 2017, 41, 94–104. [Google Scholar] [CrossRef]
- O’Hearn, K.; Asato, M.; Ordaz, S.; Luna, B. Neurodevelopment and executive function in autism. Dev. Psychopathol. 2008, 20, 1103–1132. [Google Scholar] [CrossRef]
- Simanowski, S.; Krajewski, K. Specific Preschool Executive Functions Predict Unique Aspects of Mathematics Development: A 3-Year Longitudinal Study. Child Dev. 2019, 90, 544–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.K.; Chan, S.K.; Leung, P.W.; Liu, W.S.; Leung, F.L.; Ng, R. Components and developmental differences of executive functioning for school-aged children. Dev. Neuropsychol. 2011, 36, 319–337. [Google Scholar] [CrossRef]
- Lawson, G.M.; Hook, C.J.; Farah, M.J. A meta-analysis of the relationship between socioeconomic status and executive function performance among children. Dev. Sci. 2018, 21, e12529. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.A.; Cubbin, C.; Egerter, S.; Chideya, S.; Marchi, K.S.; Metzler, M.; Posner, S. Socioeconomic status in health research: One size does not fit all. JAMA 2005, 14, 2879–2888. [Google Scholar] [CrossRef] [PubMed]
- AMAI NSE 8×7 Rule Update. AMAI. 2011. Available online: https://amai.org/NSE/ (accessed on 10 April 2021).
- Duncan, G.J.; Magnuson, K. Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 377–386. [Google Scholar] [CrossRef]
- Willoughby, M.T.; Wirth, R.J.; Blair, C.B.; Family Life Project Investigators. Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychol. Assess. 2012, 24, 418–431. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Karr, J.E.; Areshenkoff, C.N.; Rast, P.; Hofer, S.M.; Iverson, G.L.; Garcia-Barrera, M.A. The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychol. Bull. 2018, 144, 1147–1185. [Google Scholar] [CrossRef]
- Wiebe, S.A.; Espy, K.A.; Charak, D. Using confirmatory factor analysis to understand executive control in preschool children: I. Latent structure. Dev. Psychol. 2008, 44, 575–587. [Google Scholar] [CrossRef]
- Willoughby, M.T.; Pek, J.; Blair, C. Measuring executive function in early childhood: A focus on maximal reliability and the derivation of short forms. Psychol. Assess. 2013, 25, 664–670. [Google Scholar] [CrossRef]
- Seo, J.; Lee, B.K.; Jin, S.U.; Jang, K.E.; Park, J.W.; Kim, Y.T.; Park, S.J.; Jeong, K.S.; Park, J.; Kim, A.; et al. Altered executive function in the lead-exposed brain: A functional magnetic resonance imaging study. Neurotoxicology 2015, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Baggetta, P.; Alexander, P.A. Conceptualization and operationalization of executive function. Mind Brain Educ. 2016, 10, 10–33. [Google Scholar] [CrossRef]
- Chan, R.C.; Shum, D.; Toulopoulou, T.; Chen, E.Y. Assessment of executive functions: Review of instruments and identification of critical issues. Arch. Clin. Neuropsychol. 2008, 23, 201–216. [Google Scholar] [CrossRef]
- Ethier, A.A.; Muckle, G.; Jacobson, S.W.; Ayotte, P.; Jacobson, J.L.; Saint-Amour, D. Assessing new dimensions of attentional functions in children prenatally exposed to environmental contaminants using an adapted Posner paradigm. Neurotoxicol. Teratol. 2015, 51, 27–34. [Google Scholar] [CrossRef]
- Hong, S.B.; Im, M.H.; Kim, J.W.; Park, E.J.; Shin, M.S.; Kim, B.N.; Yoo, H.J.; Cho, I.H.; Bhang, S.Y.; Hong, Y.C.; et al. Environmental lead exposure and attention deficit/hyperactivity disorder symptom domains in a community sample of South Korean school-age children. Environ. Health Perspect. 2015, 123, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Last, B.S.; Lawson, G.M.; Breiner, K.; Steinberg, L.; Farah, M.J. Childhood socioeconomic status and executive function in childhood and beyond. PLoS ONE 2018, 13, e0202964. [Google Scholar] [CrossRef]
- Putnick, D.L.; Bornstein, M.H. Measurement Invariance Conventions and Reporting: The State of the Art and Future Directions for Psychological Research. Dev. Rev. 2016, 41, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Rabin, L.A.; Barr, W.B.; Burton, L.A. Assessment practices of clinical neuropsychologists in the United States and Canada: A survey of INS, NAN, and APA Division 40 members. Arch. Clin. Neuropsychol. 2005, 20, 33–65. [Google Scholar] [CrossRef]
- Rabin, L.A.; Paolillo, E.; Barr, W.B. Stability in Test-Usage Practices of Clinical Neuropsychologists in the United States and Canada Over a 10-Year Period: A Follow-Up Survey of INS and NAN Members. Arch. Clin. Neuropsychol. 2016, 31, 206–230. [Google Scholar] [CrossRef]
- Stewart, P.W.; Sargent, D.M.; Reihman, J.; Gump, B.B.; Lonky, E.; Darvill, T.; Hicks, H.; Pagano, J. Response inhibition during Differential Reinforcement of Low Rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environ. Health Perspect. 2006, 114, 1923–1929. [Google Scholar] [CrossRef]
- Wu, N.; Chen, Y.; Yang, J.; Li, F. Childhood Obesity and Academic Performance: The Role of Working Memory. Front. Psychol. 2017, 8, 611. [Google Scholar] [CrossRef]
- Simms, N.K.; Frausel, R.R.; Richland, L.E. Working memory predicts children’s analogical reasoning. J. Exp. Child Psychol. 2018, 166, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Nouwens, S.; Groen, M.A.; Verhoeven, L. How working memory relates to children’s reading comprehension: The importance of domain-specificity in storage and processing. Read. Writ. 2017, 30, 105–120. [Google Scholar] [CrossRef]
- Michel, E.; Molitor, S.; Schneider, W. Executive Functions and Fine Motor Skills in Kindergarten as Predictors of Arithmetic Skills in Elementary School. Dev. Neuropsychol. 2020, 45, 367–379. [Google Scholar] [CrossRef]
- Raghubar, K.P.; Barnes, M.A.; Hecht, S.A. Working memory and mathematics: A review of developmental, individual difference, and cognitive approaches. Learn. Individ. Differ. 2010, 20, 110–122. [Google Scholar] [CrossRef]
- Gathercole, S.E.; Pickering, S.J.; Knight, C.; Stegmann, Z. Working memory skills and educational attainment: Evidence from national curriculum assessments at 7 and 14 years of age. Appl. Cogn. Psychol. 2004, 18, 1–16. [Google Scholar] [CrossRef]
- Cain, K.; Oakhill, J.; Bryant, P. Children’s reading comprehension ability: Concurrent prediction by working memory, verbal ability, and component skills. J. Educ. Psychol. 2004, 96, 31. [Google Scholar] [CrossRef]
- Dumontheil, I.; Klingberg, T. Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb. Cortex 2012, 22, 1078–1085. [Google Scholar] [CrossRef]
- St Clair-Thompson, H.L.; Gathercole, S.E. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Q. J. Exp. Psychol. 2006, 59, 745–759. [Google Scholar] [CrossRef]
- Fitzpatrick, C.; Archambault, I.; Janosz, M.; Pagani, L.S. Early childhood working memory forecasts high school dropout risk. Intelligence 2015, 53, 160–165. [Google Scholar] [CrossRef]
- Miller, M.; Nevado-Montenegro, A.J.; Hinshaw, S.P. Childhood executive function continues to predict outcomes in young adult females with and without childhood-diagnosed ADHD. J. Abnorm. Child Psychol. 2012, 40, 657–668. [Google Scholar] [CrossRef]
- Takahashi, N.; Nishimura, T.; Harada, T.; Okumura, A.; Iwabuchi, T.; Rahman, S.; Kuwabara, H.; Takagai, S.; Nomura, Y.; Takei, N.; et al. Association Between Genetic Risks for Obesity and Working Memory in Children. Front. Neurosci. 2021, 15, 749230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, W.; Jiang, F.; Wu, F.; Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Fan, X.; Wei, X.; et al. Associations among body mass index, working memory performance, gray matter volume, and brain activation in healthy children. Cereb. Cortex 2023, 33, 6335–6344. [Google Scholar] [CrossRef]
- Bathelt, J.; Gathercole, S.E.; Johnson, A.; Astle, D.E. Differences in brain morphology and working memory capacity across childhood. Dev. Sci. 2018, 21, e12579. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.; Hilton, K.A.; Place, M.; Alloway, T.P.; Elliott, J.G.; Gathercole, S.E. Children with low working memory and children with ADHD: Same or different? Front. Hum. Neurosci. 2014, 8, 976. [Google Scholar] [CrossRef] [PubMed]
- Martinussen, R.; Hayden, J.; Hogg-Johnson, S.; Tannock, R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 377–384. [Google Scholar] [CrossRef]
- Smith-Spark, J.H.; Fisk, J.E. Working memory functioning in developmental dyslexia. Memory 2007, 15, 34–56. [Google Scholar] [CrossRef]
- Rotzer, S.; Loenneker, T.; Kucian, K.; Martin, E.; Klaver, P.; von Aster, M. Dysfunctional neural network of spatial working memory contributes to developmental dyscalculia. Neuropsychologia 2009, 47, 2859–2865. [Google Scholar] [CrossRef]
- Szucs, D.; Devine, A.; Soltesz, F.; Nobes, A.; Gabriel, F. Developmental dyscalculia is related to visuo-spatial memory and inhibition impairment. Cortex 2013, 49, 2674–2688. [Google Scholar] [CrossRef]
- Wang, M.; Wei, J.; Dou, Y.; Wang, Y.; Fan, H.; Yan, Y.; Du, Y.; Zhao, L.; Wang, Q.; Yang, X.; et al. Differential association between childhood trauma subtypes and neurocognitive performance in adults with major depression. BMC Psychiatry 2024, 24, 773. [Google Scholar] [CrossRef]
- Testa, R.; Bennett, P.; Ponsford, J. Factor analysis of nineteen executive function tests in a healthy adult population. Arch. Clin. Neuropsychol. 2012, 27, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P. Research on Individual Differences in Executive Functions: Implications for the Bilingual Advantage Hypothesis. Linguist. Approaches Biling. 2016, 6, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W.; James, M.; Owen, A.M.; Sahakian, B.J.; McInnes, L.; Rabbit, P. Cambridge neuropsychological test automated battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dementia 1994, 5, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, B.J.; Owen, A.M. Computerized assessment in neuropsychiatry using Cantab—Discussion paper. J. R. Soc. Med. 1992, 85, 399–402. [Google Scholar]
- Goghari, V.M.; Brett, C.; Tabraham, P.; Johns, L.; Valmaggia, L.; Broome, M.; Woolley, J.; Bramon, E.; Howes, O.; Byrne, M.; et al. Spatial working memory ability in individuals at ultra high risk for psychosis. J. Psychiatr. Res. 2014, 50, 100–105. [Google Scholar] [CrossRef][Green Version]
- Chelonis, J.J.; Cox, A.R.; Karr, M.J.; Prunty, P.K.; Baldwin, R.L.; Paule, M.G. Comparison of delayed matching-to-sample performance in monkeys and children. Behav. Process. 2014, 101, 261–268. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Chelonis, J.J.; Prunty, P.K.; Paule, M.G. The use of an incremental repeated acquisition task to assess learning in children. Behav. Process. 2012, 91, 103–114. [Google Scholar] [CrossRef]
- Tucker, L.; Lewis, C. A reliability coefficient for maximum likelihood factor analysis. Psychometrika 1973, 38, 1–10. [Google Scholar] [CrossRef]
- Kline, R.B. Principles and Practive of Structural Equation Modeling, 4th ed.; The Guilford Press: New York, NY, USA, 2015. [Google Scholar]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Jackson, C.H. Multi-state models for panel data: The msm package for R. J. Stat. Softw. 2011, 38, 1–28. [Google Scholar] [CrossRef]
- Behrens, G.; Winkler, T.W.; Gorski, M.; Leitzmann, M.F.; Heid, I.M. To stratify of not to stratify: Power considerations for population-based genome-wide association studies of quantitative traits. Genet. Epidemiol. 2011, 35, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Hackman, D.A.; Betancourt, L.M.; Gallop, R.; Romer, D.; Brodsky, N.L.; Hurt, H.; Farah, M.J. Mapping the trajectory of socioeconomic disparity in working memory: Parental and neighborhood factors. Child Dev. 2014, 85, 1433–1445. [Google Scholar] [CrossRef]
- St John, A.M.; Kibbe, M.; Tarullo, A.R. A systematic assessment of socioeconomic status and executive functioning in early childhood. J. Exp. Child Psychol. 2019, 178, 352–368. [Google Scholar] [CrossRef]
- Nishio, H.; Kasuga, S.; Ushijima, M.; Harada, Y. Prenatal stress and postnatal development of neonatal rats—Sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int. J. Dev. Neurosci. 2001, 19, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vallee, M.; Maccari, S.; Dellu, F.; Simon, H.; Le Moal, M.; Mayo, W. Long-term effects of prenatal stress and postnatal hadling on age-related glucocorticoid secretion and cognitive performance: A longitudinal study in the rat. Eur. J. Neurosci. 1999, 11, 2906–2916. [Google Scholar] [CrossRef] [PubMed]
- Yaka, R.; Salomon, S.; Matzner, H.; Weinstock, M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav. Brain Res. 2007, 179, 126–132. [Google Scholar] [CrossRef]
- Lopez-Ojeda, W.; Hurley, R.A. Sexual Dimorphism in Brain Development: Influence on Affective Disorders. J. Neuropsychiatry Clin. Neurosci. 2021, 33, A4-85. [Google Scholar] [CrossRef]
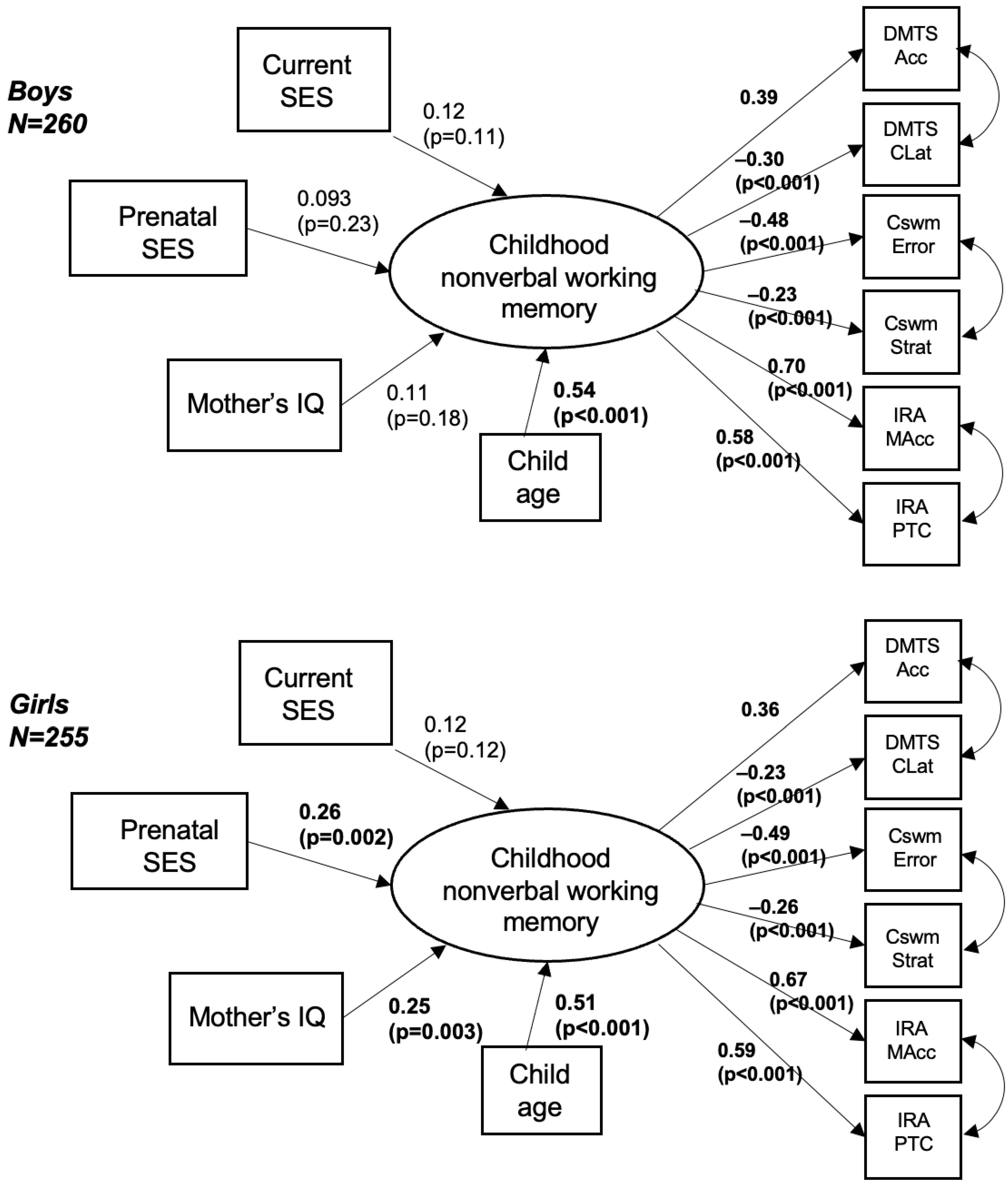
| Boys | Girls | p-Value | |
|---|---|---|---|
| Sample size (N) | 260 | 255 | |
| DMTS overall accuracy (median [IQR]) | 86.27 [81.82, 91.23] | 87.50 [82.03, 91.75] | 0.155 |
| CANTAB SWM between search errors | 63.00 [55.75, 71.00] | 65.00 [58.00, 71.00] | 0.449 |
| CANTAB SWM strategy | 39.00 [37.00, 41.00] | 40.00 [38.00, 41.00] | 0.013 |
| DMTS overall correct choice latency time | 3.08 [2.45, 4.12] | 2.87 [2.34, 3.78] | 0.022 |
| IRA memory accuracy | 72.32 [51.43, 82.10] | 69.94 [47.09, 81.05] | 0.118 |
| IRA percent of task complete | 100.00 [66.67, 100.00] | 100.00 [66.67, 100.00] | 0.119 |
| Age (years) | 6.56 [6.30, 7.07] | 6.56 [6.29, 7.01] | 0.761 |
| Prenatal SES (%) | 0.235 | ||
| E (lowest) | 27 (10.4) | 23 (9.0) | |
| D | 115 (44.2) | 112 (43.9) | |
| D+ | 57 (21.9) | 63 (24.7) | |
| C | 36 (13.8) | 34 (13.3) | |
| C+ | 25 (9.6) | 18 (7.1) | |
| A/B (highest) | 0 (0.0) | 5 (2.0) | |
| Mother’s IQ (median [IQR]) | 86.00 [75.00, 95.00] | 86.00 [77.50, 94.00] | 0.751 |
| SES at age 6 (%) | 0.282 | ||
| D− (lowest) | 1 (0.4) | 1 (0.4) | |
| D | 47 (18.1) | 56 (22.0) | |
| D+ | 93 (35.8) | 67 (26.3) | |
| C− | 58 (22.3) | 67 (26.3) | |
| C | 36 (13.8) | 45 (17.6) | |
| C+ | 20 (7.7) | 15 (5.9) | |
| A/B (highest) | 5 (1.9) | 4 (1.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.H.; Bellinger, D.; Dams-O’Connor, K.; Teresi, J.A.; Pantic, I.; Martínez-Medina, S.; Chelonis, J.; Téllez-Rojo, M.M.; Wright, R.O. Associations of Prenatal Socioeconomic Status and Childhood Working Memory: A Structural Equation Modeling Approach. Children 2025, 12, 537. https://doi.org/10.3390/children12050537
Liu SH, Bellinger D, Dams-O’Connor K, Teresi JA, Pantic I, Martínez-Medina S, Chelonis J, Téllez-Rojo MM, Wright RO. Associations of Prenatal Socioeconomic Status and Childhood Working Memory: A Structural Equation Modeling Approach. Children. 2025; 12(5):537. https://doi.org/10.3390/children12050537
Chicago/Turabian StyleLiu, Shelley H., David Bellinger, Kristen Dams-O’Connor, Jeanne A. Teresi, Ivan Pantic, Sandra Martínez-Medina, John Chelonis, Martha M. Téllez-Rojo, and Robert O. Wright. 2025. "Associations of Prenatal Socioeconomic Status and Childhood Working Memory: A Structural Equation Modeling Approach" Children 12, no. 5: 537. https://doi.org/10.3390/children12050537
APA StyleLiu, S. H., Bellinger, D., Dams-O’Connor, K., Teresi, J. A., Pantic, I., Martínez-Medina, S., Chelonis, J., Téllez-Rojo, M. M., & Wright, R. O. (2025). Associations of Prenatal Socioeconomic Status and Childhood Working Memory: A Structural Equation Modeling Approach. Children, 12(5), 537. https://doi.org/10.3390/children12050537







