Transcatheter Pulmonary Valve Implantation in Congenital Heart Diseases: Current Advances and Future Prospectives
Abstract
:1. Introduction
2. Indications for Pulmonary Valve Replacement
- -
- is recommended (class I, level C) in symptomatic patients with severe pulmonary regurgitation (CMR regurgitant fraction > 30–40%) and/or at least moderate right ventricle outflow tract (RVOT) obstruction (echo Vmax > 3 m/s);
- -
- should be considered (class IIa, level C) in asymptomatic patients with severe pulmonary regurgitation and/or RVOT obstruction when a decrease in objective exercise capacity and/or a significant right ventricle dilatation (RV end-systolic volume index ≥ 80 mL/m2, RV end-diastolic volume index ≥ 160 mL/m2) and/or a tricuspid valve regurgitation progression (at least moderate), and/or a progressive RV systolic dysfunction, and/or a very high right ventricle systolic pressure (>80 mmHg) are detected.
- -
- symptomatic patients with moderate or severe regurgitation (class I)
- -
- asymptomatic patient with moderate or severe pulmonary regurgitation and two or more of these elements: mild or moderate right or left ventricle systolic dysfunction; severe RV dilatation (RV end-diastolic volume index ≥ 160 mL/m2 or RV end-systolic volume index ≥ 80 mL/m2 or RV end-diastolic volume—left ventricle end-diastolic volume ratio ≥ 2); RV systolic pressure ≥ 2/3 of systemic arterial pressure due to RVOT obstruction; progressive reduction in objective exercise tolerance (class IIa)
- -
- asymptomatic patients with moderate or severe pulmonary regurgitation and sustained tachyarrhythmias (class IIb)
- -
- Asymptomatic patients with moderate or severe pulmonary regurgitation and residual lesions requiring surgical treatment (class IIb).
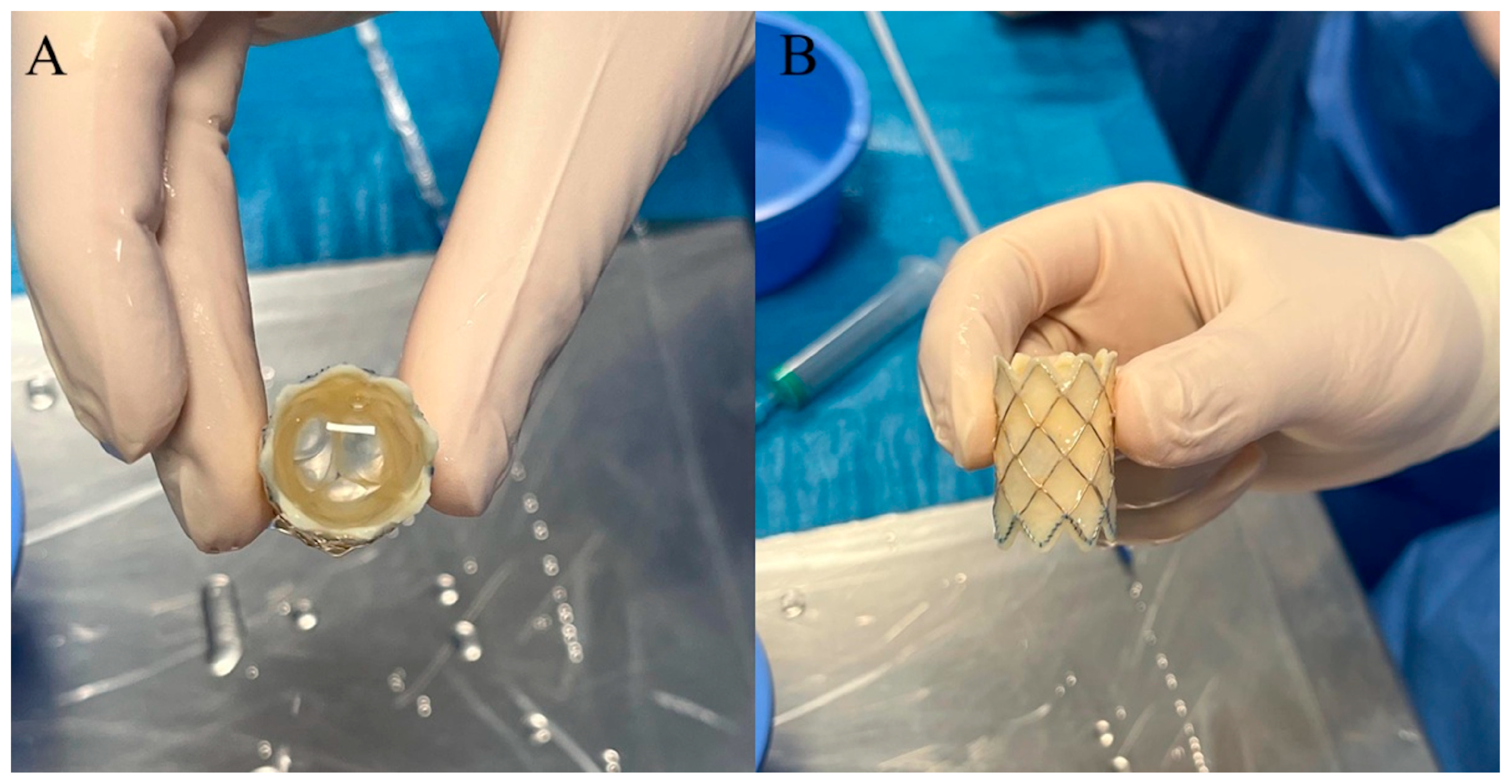
3. Melody Transcatheter Pulmonary Valve
4. Edwards Sapien Transcatheter Heart Valve
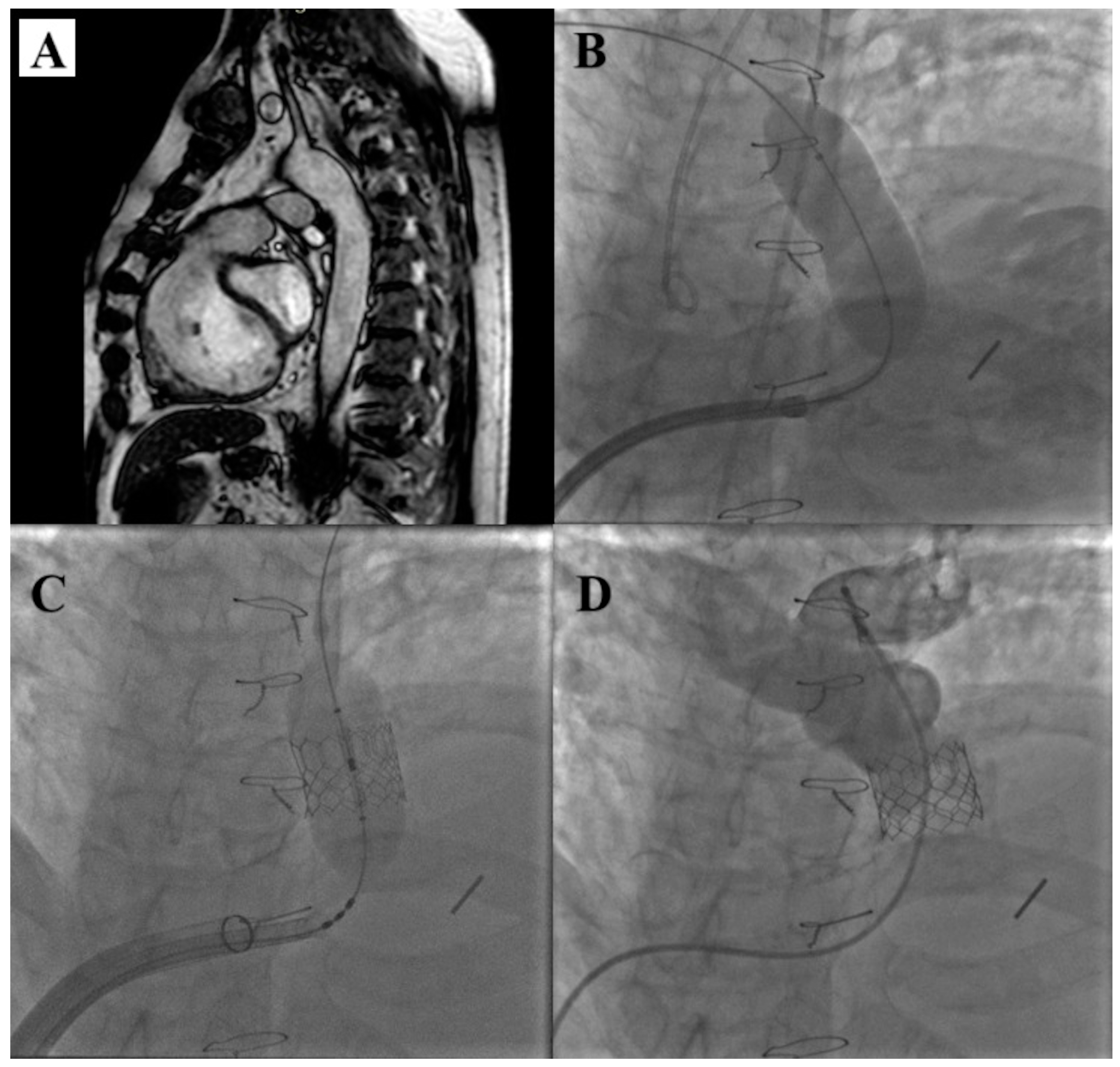
5. Venus P-Valve
6. Harmony Transcatheter Pulmonary Valve
- -
- at the distal (outflow) segment, Harmony TPV interference should be ≥15%.
- -
- at the proximal (inflow) segment, Harmony TPV interference should be ≥17%.
- -
- at the midsection, the Harmony TPV should be ideally uncompressed (0% of interference).
7. Alterra Adaptive Prestent
8. Adverse Events of Trans-Catheter Pulmonary Valve Replacement
9. Future Prospects
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larsen, S.H.; Dimopoulos, K.; Gatzoulis, M.A.; Uebing, A.; Shore, D.F.; Alonso-Gonzalez, R.; Kempny, A. Surgical and percutaneous pulmonary valve replacement in England over the past two decades. Heart 2019, 105, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; De Backer, J.; Babu-Narayan, S.V.; Budts, W.; Chessa, M.; Diller, G.P.; Lung, B.; Kluin, J.; Lang, I.M.; Meijboom, F.; et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur. Heart J. 2021, 42, 563–645. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e698–e800. [Google Scholar]
- Lee, C.; Kim, Y.M.; Lee, C.H.; Kwak, J.G.; Park, C.S.; Song, J.Y.; Shim, W.S.; Choi, E.Y.; Lee, S.Y.; Baek, J.S. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: Implications for optimal timing of pulmonary valve replacement. J. Am. Coll. Cardiol. 2012, 60, 1005–1014. [Google Scholar] [CrossRef]
- Ferraz Cavalcanti, P.E.; Sá, M.P.; Santos, C.A.; Esmeraldo, I.M.; de Escobar, R.R.; de Menezes, A.M.; de Azevedo, O.M., Jr.; de Vasconcelos Silva, F.P.; Lins, R.F.; Lima Rde, C. Pulmonary valve replacement after operative repair of tetralogy of Fallot: Meta-analysis and meta-regression of 3,118 patients from 48 studies. J. Am. Coll. Cardiol. 2013, 62, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Heng, E.L.; Gatzoulis, M.A.; Uebing, A.; Sethia, B.; Uemura, H.; Smith, G.C.; Diller, G.P.; McCarthy, K.P.; Ho, S.Y.; Li, W.; et al. Immediate and Midterm Cardiac Remodeling After Surgical Pulmonary Valve Replacement in Adults With Repaired Tetralogy of Fallot: A Prospective Cardiovascular Magnetic Resonance and Clinical Study. Circulation 2017, 136, 1703–1713. [Google Scholar] [CrossRef]
- Mongeon, F.P.; Ben Ali, W.; Khairy, P.; Bouhout, I.; Therrien, J.; Wald, R.M.; Dallaire, F.; Bernier, P.L.; Poirier, N.; Dore, A.; et al. Pulmonary Valve Replacement for Pulmonary Regurgitation in Adults With Tetralogy of Fallot: A Meta-analysis-A Report for the Writing Committee of the 2019 Update of the Canadian Cardiovascular Society Guidelines for the Management of Adults With Congenital Heart Disease. Can. J. Cardiol. 2019, 35, 1772–1783. [Google Scholar] [PubMed]
- Bokma, J.P.; Geva, T.; Sleeper, L.A.; Babu Narayan, S.V.; Wald, R.; Hickey, K.; Jansen, K.; Wassall, R.; Lu, M.; Gatzoulis, M.A.; et al. A propensity score-adjusted analysis of clinical outcomes after pulmonary valve replacement in tetralogy of Fallot. Heart 2018, 104, 738–744. [Google Scholar] [CrossRef]
- Bokma, J.P.; Geva, T.; Sleeper, L.A.; Lee, J.H.; Lu, M.; Sompolinsky, T.; Babu-Narayan, S.V.; Wald, R.M.; Mulder, B.J.M.; Valente, A.M. Improved Outcomes After Pulmonary Valve Replacement in Repaired Tetralogy of Fallot. J. Am. Coll. Cardiol. 2023, 81, 2075–2085. [Google Scholar] [CrossRef]
- Yamamura, K.; Yuen, D.; Hickey, E.J.; He, X.; Chaturvedi, R.R.; Friedberg, M.K.; Grosse-Wortmann, L.; Hanneman, K.; Billia, F.; Farkouh, M.E.; et al. Right ventricular fibrosis is associated with cardiac remodelling after pulmonary valve replacement. Heart 2019, 105, 855–863. [Google Scholar] [CrossRef]
- Ghonim, S.; Gatzoulis, M.A.; Ernst, S.; Li, W.; Moon, J.C.; Smith, G.C.; Heng, E.L.; Keegan, J.; Ho, S.Y.; McCarthy, K.P.; et al. Predicting Survival in Repaired Tetralogy of Fallot: A Lesion-Specific and Personalized Approach. JACC Cardiovasc. Imaging 2022, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Cools, B.; Nagaraju, C.K.; Vandendriessche, K.; van Puyvelde, J.; Youness, M.; Roderick, H.L.; Gewillig, M.; Sipido, K.; Claus, P.; Rega, F. Reversal of Right Ventricular Remodeling After Correction of Pulmonary Regurgitation in Tetralogy of Fallot. JACC Basic Transl. Sci. 2022, 8, 301–315. [Google Scholar] [CrossRef]
- Mayourian, J.; Sleeper, L.A.; Lee, J.H.; Lu, M.; Geva, A.; Mulder, B.; Babu-Narayan, S.V.; Wald, R.M.; Sompolinsky, T.; Valente, A.M.; et al. Development and Validation of a Mortality Risk Score for Repaired Tetralogy of Fallot. J. Am. Heart. Assoc. 2024, 13, e034871. [Google Scholar] [CrossRef] [PubMed]
- Geva, T.; Wald, R.M.; Bucholz, E.; Cnota, J.F.; McElhinney, D.B.; Mercer-Rosa, L.M.; Mery, C.M.; Miles, A.L.; Moore, J. Long-Term Management of Right Ventricular Outflow Tract Dysfunction in Repaired Tetralogy of Fallot: A Scientific Statement From the American Heart Association. Circulation 2024, 150, e689–e707. [Google Scholar] [CrossRef]
- Bonhoeffer, P.; Boudjemline, Y.; Saliba, Z.; Merckx, J.; Aggoun, Y.; Bonnet, D.; Acar, P.; Le Bidois, J.; Sidi, D.; Kachaner, J. Percutaneous replacement of pulmonary valve in a right ventricle to pulmonary artery prosthetic conduit with valve dysfunction. Lancet 2000, 21, 1403–1405. [Google Scholar] [CrossRef]
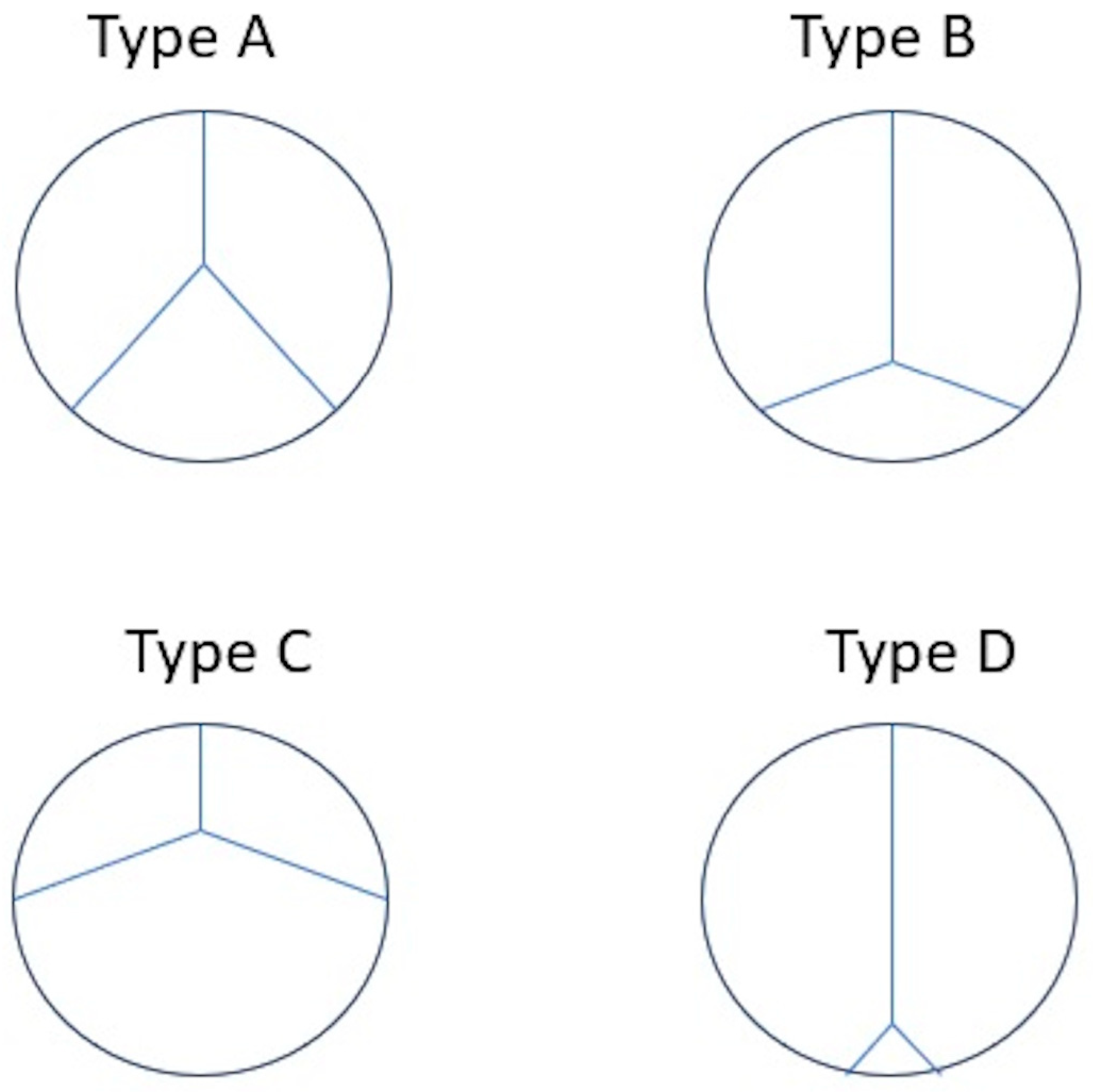
- Boe, B.A.; Cheatham, S.L.; Armstrong, A.K.; Berman, D.P.; Chisolm, J.L.; Cheatham, J.P. Leaflet morphology classification of the Melody Transcatheter Pulmonary Valve. Congenit. Heart Dis. 2019, 14, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Cheatham, S.L.; Holzer, R.J.; Chisolm, J.L.; Cheatham, J.P. The Medtronic melody(R) transcatheter pulmonary valve implanted at 24-mm diameter—It works. Catheter. Cardiovasc. Interv. 2013, 82, 816–823. [Google Scholar] [CrossRef]
- Malekzadeh-Milani, S.; Ladouceur, M.; Cohen, S.; Iserin, L.; Boudjem-line, Y. Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch. Cardiovasc. Dis. 2014, 107, 592–598. [Google Scholar] [CrossRef]
- Finch, W.; Levi, D.S.; Salem, M.; Hageman, A.; Aboulhosn, J. Transcatheter melody valve placement in large diameter bioprostheses and conduits: What is the optimal ”Landing zone”? Catheter. Cardiovasc. Interv. 2015, 86, E217–E223. [Google Scholar] [CrossRef]
- Ringewald, J.M.; Suh, E.J. Transcatheter pulmonary valve insertion, expanded use (beyond large conduits from the right ventricle to pulmonary artery), and future directions. Cardiol. Young 2014, 24, 1095–1100. [Google Scholar] [CrossRef]
- Hascoet, S.; Martins, J.D.; Baho, H.; Kadirova, S.; Pinto, F.; Paoli, F.; Bitar, F.; Haweleh, A.A.; Uebing, A.; Acar, P.; et al. Percutaneous pulmonary valve implantation in small conduits: A multicenter experience. Int. J. Card. 2018, 254, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Bensemlali, M.; Malekzadeh-Milani, S.; Mostefa-Kara, M.; Bonnet, D.; Boudjemline, Y. Percutaneous pulmonary Melody valve implantation in small conduits. Arch. Cardiovasc. Dis. 2017, 110, 517–524. [Google Scholar] [CrossRef]
- Berman, D.P.; McElhinney, D.B.; Vincent, J.A.; Hellenbrand, W.E.; Zahn, E.M. Feasibility and short- term outcomes of percutaneous transcatheter pulmonary valve replacement in small (<30 kg) children with dysfunctional right ventricular outflow tract conduits. Circ. Cardiovasc. Interv. 2014, 7, 142–148. [Google Scholar]
- Martin, M.H.; Shahanavaz, S.; Peng, L.F.; Asnes, J.D.; Riley, M.; Hellenbrand, W.E.; Balzer, D.T.; Gray, R.G.; McElhinney, D.B. Percutaneous transcatheter pulmonary valve replacement in children weighing less than 20kg. Cath. Cardiovasc. Interv. 2018, 91, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sumski, C.A.; Bartz, P.; Gudausky, T. Percutaneous melody valve implantation in a native tricuspid valve following failed surgical repair. Catheter. Cardiovasc. Interv. 2018, 92, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.W.; Cabalka, A.K.; Alli, O.O.; Pislaru, S.V.; Sorajja, P.; Nkomo, V.T.; Malouf, J.F.; Cetta, F.; Hagler, D.J.; Rihal, C.S. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: A case series in children and adults. JACC Cardiovasc. Interv. 2013, 6, 598–605. [Google Scholar] [CrossRef]
- Patel, M.; Iserin, L.; Bonnet, D.; Boudjemline, Y. Atypical malignant late infective endocarditis of Melody valve. J. Thorac. Cardiovasc. Surg. 2012, 143, e32–e35. [Google Scholar] [CrossRef]
- Boudjemline, Y.; Sarquella-Brugada, G.; Kamache, I.; Patel, M.; Ladouceur, M.; Bonnet, D.; Boughenou, F.M.; Fraisse, A.; Iserin, L. Impact of right ventricular outflow tract size and substrate on out- comes of percutaneous pulmonary valve implantation. Arch. Cardiovasc. Dis. 2013, 106, 19–26. [Google Scholar] [CrossRef]
- Cabalka, A.K.; Hellenbrand, W.E.; Eicken, A.; Kreutzer, J.; Gray, R.G.; Bergersen, L.; Berger, F.; Armstrong, A.K.; Cheatham, J.P.; Zahn, E.M.; et al. Relationships Among Conduit Type, Pre-Stenting, and Outcomes in Patients Undergoing Transcatheter Pulmonary Valve Replacement in the Prospective North American and European Melody Valve Trials. JACC Cardiovasc. Interv. 2017, 10, 1746–1759. [Google Scholar] [CrossRef]
- Boudjemline, Y. A new one-step procedure for pulmonary valve implantation of the melody valve: Simultaneous prestenting and valve implantation. Catheter. Cardiovasc. Interv. 2018, 91, 64–70. [Google Scholar] [CrossRef]
- Jones, T.K.; McElhinney, D.B.; Vincent, J.A.; Hellenbrand, W.E.; Cheatham, J.P.; Berman, D.P.; Zahn, E.M.; Khan, D.M.; Rhodes, J.F., Jr.; Weng, S.; et al. Long-Term Outcomes After Melody Transcatheter Pulmonary Valve Replacement in the US Investigational Device Exemption Trial. Circ. Cardiovasc. Interv. 2022, 15, e010852. [Google Scholar] [CrossRef] [PubMed]
- Shahanavaz, S.; Berger, F.; Jones, T.K.; Kreutzer, J.; Vincent, J.A.; Eicken, A.; Bergersen, L.; Rome, J.J.; Zahn, E.; Søndergaard, L.; et al. Outcomes After Transcatheter Reintervention for Dysfunction of a Previously Implanted Transcatheter Pulmonary Valve. JACC Cardiovasc. Interv. 2020, 13, 1529–1540. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Benson, L.N.; Eicken, A.; Kreutzer, J.; Padera, R.F.; Zahn, E.M. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: Combined results of 3 prospective North American and European studies. Circ. Cardiovasc. Interv. 2013, 6, 292–300. [Google Scholar] [CrossRef]
- Sadeghi, S.; Wadia, S.; Lluri, G.; Tarabay, J.; Fernando, A.; Salem, M.; Sinha, S.; Levi, D.S.; Aboulhosn, J. Risk factors for infective endocarditis following transcatheter pulmonary valve replacement in patients with congenital heart disease. Catheter. Cardiovasc. Interv. 2019, 94, 625–635. [Google Scholar] [CrossRef]
- Georgiev, S.; Ewert, P.; Eicken, A.; Hager, A.; Hörer, J.; Cleuziou, J.; Meierhofer, C.; Tanase, D. Munich comparative study: Prospective long-term outcome of the transcatheter melody valve versus surgical pulmonary bioprosthesis with up to 12 years of follow-up. Circ. Cardiovasc. Interv. 2020, 13, e008963. [Google Scholar] [CrossRef] [PubMed]
- Veloso, T.R.; Que, Y.A.; Chaouch, A.; Giddey, M.; Vouillamoz, J.; Rousson, V.; Moreillon, P.; Entenza, J.M. Prophylaxis of experimental endocarditis with antiplatelet and antithrombin agents: A role for long-term prevention of infective endocarditis in humans? J. Infect. Dis. 2015, 211, 72–79. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, D.B.; Sondergaard, L.; Armstrong, A.K.; Bergersen, L.; Padera, R.F.; Balzer, D.T.; Lung, T.H.; Berger, F.; Zahn, E.M.; Gray, R.G.; et al. Endocarditis After Transcatheter Pulmonary Valve Replacement. J. Am. Coll. Cardiol. 2018, 72, 2717–2728. [Google Scholar] [CrossRef]
- Sinha, S.; Aboulhosn, J.; Asnes, J.; Bocks, M.; Zahn, E.; Goldstein, B.H.; Zampi, J.; Hellenbrand, W.; Salem, M.; Levi, D. Initial results from the off-label use of the Sapien S3 valve for percutaneous transcatheter pulmonary valve replacement: A multi-institutional experience. Catheter. Cardiovasc. Interv. 2019, 93, 455–463. [Google Scholar] [CrossRef]
- Morgan, G.J.; Sadeghi, S.; Salem, M.M.; Wilson, N.; Kay, J.; Rothman, A.; Galindo, A.; Martin, M.H.; Gray, R.; Ross, M.; et al. SAPIEN valve for percutaneous transcatheter pulmonary valve replacement without “pre-stenting”: A multi-institutional experience. Catheter. Cardiovasc. Interv. 2019, 93, 324–329. [Google Scholar] [CrossRef]
- Shahanavaz, S.; Zahn, E.M.; Levi, D.S.; Aboulhousn, J.A.; Hascoet, S.; Qureshi, A.M.; Porras, D.; Morgan, G.J.; Bauser Heaton, H.; Martin, M.H.; et al. Transcatheter pulmonary valve replacement with the Sapien prosthesis. J. Am. Coll. Cardiol. 2020, 76, 2847–2858. [Google Scholar] [CrossRef]
- Le Ruz, R.; Plessis, J.; Houeijeh, A.; Baruteau, A.E.; Le Gloan, L.; Warin Fresse, K.; Karsenty, C.; Petit, J.; Godart, F.; Hascoët, S.; et al. Edwards SAPIEN XT transcatheter pulmonary valve implantation: 5-year follow-up in a French Registry. Catheter. Cardiovasc. Interv. 2021, 98, 990–999. [Google Scholar] [CrossRef]
- Shivaraju, A.; Kodali, S.; Thilo, C.; Ott, I.; Schunkert, H.; von Scheidt, W.; Leon, M.B.; Kastrati, A.; Kasel, A.M. Overexpansion of the SAPIEN 3 Transcatheter heart valve: A feasibility study. JACC Cardiovasc. Interv. 2015, 8, 2041–2043. [Google Scholar] [CrossRef]
- Kenny, D.; Morgan, G.J.; Murphy, M.; AlAlwi, K.; Giugno, L.; Zablah, J.; Carminati, M.; Walsh, K. Use of 65 cm large caliber Dryseal sheaths to facilitate delivery of the Edwards SAPIEN valve to dysfunctional right ventricular outflow tracts. Catheter. Cardiovasc. Interv. 2019, 94, 409–413. [Google Scholar] [CrossRef]
- Kenny, D.; Rhodes, J.F.; Fleming, G.A.; Kar, S.; Zahn, E.M.; Vincent, J.; Shirali, G.S.; Gorelick, J.; Fogel, M.A.; Fahey, J.T.; et al. 3-Year Outcomes of the Edwards SAPIEN Transcatheter Heart Valve for Conduit Failure in the Pulmonary Position From the COMPASSION Multicenter Clinical Trial. JACC Cardiovasc. Interv. 2018, 11, 1920–1929. [Google Scholar] [CrossRef]
- Hascoët, S.; Bentham, J.R.; Giugno, L.; Betrián-Blasco, P.; Kempny, A.; Houeijeh, A.; Baho, H.; Sharma, S.R.; Jones, M.I.; Biernacka, E.K.; et al. Outcomes of transcatheter pulmonary SAPIEN 3 valve implantation: An international registry. Eur. Heart J. 2024, 45, 198–210. [Google Scholar] [CrossRef]
- Boutsikou, M.; Tzifa, A. Noninvasive imaging prior to percutaneous pulmonary valve implantation. Hellenic. J. Cardiol. 2022, 67, 59–65. [Google Scholar] [CrossRef]
- Schievano, S.; Coats, L.; Migliavacca, F.; Norman, W.; Frigiola, A.; Deanfield, J.; Bonhoeffer, P.; Taylor, A.M. Variations in right ventricular outflow tract morphology following repair of congenital heart disease: Implications for percutaneous pulmonary valve implantation. J. Cardiovasc. Magn. Reson. 2007, 9, 687–695. [Google Scholar] [CrossRef]
- D’Aiello, A.F.; Schianchi, L.; Bevilacqua, F.; Ferrero, P.; Micheletti, A.; Negura, D.G.; Pasqualin, G.; Chessa, M. Holography-guided procedural planning for modifying Venus P-Valve implantation technique in patients with left pulmonary artery stents: A case-series. Front. Cardiovasc. Med. 2024, 11, 1378924. [Google Scholar] [CrossRef]
- Zablah, J.E.; Than, J.; Browne, L.P.; Rodriguez, S.; Morgan, G.J. Patient Screening for Self-Expanding Percutaneous Pulmonary Valves Using Virtual Reality. J. Am. Heart Assoc. 2024, 13, e033239. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Asch, F.M.; Manasse, E.; Ruiz, C.E.; Jelnin, V.; Kashif, M.; Kawamori, H.; Maeno, Y.; Kazuno, Y.; Takahashi, N.; et al. Systematic CT Methodology for the Evaluation of Subclinical Leaflet Thrombosis. JACC Cardiovasc. Imaging 2017, 10, 461–470. [Google Scholar] [CrossRef]
- Garay, F.; Pan, X.; Zhang, Y.J.; Wang, C.; Springmuller, D. Early experience with the Venus P-Valve for percutaneous pulmonary valve implantation in native outflow tract. Neth. Heart J. 2017, 25, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Prachasilchai, P.; Promphan, W.; Rosenthal, E.; Sivakumar, K.; Kappanayil, M.; Sakidjan, I.; Walsh, K.P.; Kenny, D.; Thomson, J.; et al. Medium-term results of percutaneous pulmonary valve implantation using the Venus P-Valve: International experience. EuroIntervention 2019, 14, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Long, Y.; Zhang, G.; Pan, X.; Chen, M.; Feng, Y.; Liu, J.; Yu, S.; Pan, W.; Zhou, D.; et al. Five-year follow-up after percutaneous pulmonary valve implantation using the Venus P-Valve system for patients with pulmonary regurgitation and an enlarged native right ventricular outflow tract. Catheter. Cardiovasc. Interv. 2024, 103, 359–366. [Google Scholar] [CrossRef]
- Gillespie, M.J.; Benson, L.N.; Bergersen, L.; Bacha, E.A.; Cheatham, S.L.; Crean, A.M.; Eicken, A.; Ewert, P.; Geva, T.; Hellenbrand, W.E.; et al. Patient Selection Process for the Harmony Transcatheter Pulmonary Valve Early Feasibility Study. Am. J. Cardiol. 2017, 120, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.J.; Bergersen, L.; Benson, L.N.; Weng, S.; Cheatham, J.P. 5-year outcomes from the harmony native outflow tract early feasibility study. JACC Cardiovasc. Interv. 2021, 14, 816–817. [Google Scholar] [CrossRef]
- Gillespie, M.; McElhinney, D.; Jones, T.; Levi, D.; Weng, S.; Cheatham, J. Midterm outcomes from the harmony transcatheter pulmonary valve pivotal trial and continued access study. Pediatr. Cardiol. 2021, 42, 1903–1904. [Google Scholar]
- Levi, D.S.; Gillespie, M.J.; McElhinney, D.B.; Jones, T.K.; Benson, L.N.; Justino, H.; Haugan, D.; Cheatham, J.P. One-year outcomes in an expanded cohort of harmony transcatheter pulmonary valve recipients. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100326–100327. [Google Scholar] [CrossRef]
- Zahn, E.M.; Chang, J.C.; Armer, D.; Garg, R. First human implant of the Alterra Adaptive Prestent TM: A new self-expanding device designed to remodel the right ventricular outflow tract. Catheter. Cardiovasc. Interv. 2018, 91, 1125–1129. [Google Scholar] [CrossRef]
- Dimas, V.V.; Babaliaros, V.; Kim, D.; Lim, D.S.; Morgan, G.; Jones, T.K.; Armstrong, A.K.; Berman, D.; Aboulhosn, J.; Mahadevan, V.S.; et al. Multicenter Pivotal Study of the Alterra Adaptive Prestent for the Treatment of Pulmonary Regurgitation. JACC Cardiovasc. Interv. 2024, 17, 2287–2297. [Google Scholar] [CrossRef]
- Fraisse, A.; Assaidi, A.; Mauri, L.; Malekzadeh-Milani, S.; Thambo, J.B.; Bonnet, D.; Iserin, L.; Mancini, J.; Boudjemline, Y. Coronary artery compression during intention to treat right ventricle outflow with percutaneous pulmonary valve implantation: Incidence, diagnosis, and out-come. Catheter. Cardiovasc. Interv. 2014, 83, E260–E268. [Google Scholar] [CrossRef]
- Boudjemline, Y.; Malekzadeh-Milani, S.; Patel, M.; Thambo, J.B.; Bonnet, D.; Iserin, L.; Fraisse, A. Predictors and outcomes of right ventricular outflow tract conduit rupture during percutaneous pulmonary valve implantation: A multicentre study. EuroIntervention 2016, 11, 1053–1062. [Google Scholar] [CrossRef]
- Alsulami, G.; Patel, M.; Malekzadeh-Milani, S.; Bonnet, D.; Boudjemline, Y. Hyperacute flash pulmonary oedema after transcatheter pulmonary valve implantation: The Melody® of an over whelmed left ventricle. Arch. Cardiovasc. Dis. 2014, 107, 219–224. [Google Scholar] [CrossRef]
- Faccini, A.; Butera, G. Tricuspid regurgitation as a complication of Edwards Sapien XT valve implantation in pulmonary position a problem to deal with. Catheter. Cardiovasc. Interv. 2018, 91, 927–931. [Google Scholar] [CrossRef]
- Butera, G.; Hansen, J.H.; Jones, M.I. Tricuspid regurgitation complicating SAPIEN 3 valve implantation in pulmonary position. Catheter. Cardiovasc. Interv. 2019, 94, 894. [Google Scholar] [CrossRef]
- Nordmeyer, J.; Khambadkone, S.; Coats, L.; Schievano, S.; Lurz, P.; Parenzan, G.; Taylor, A.M.; Lock, J.E.; Bonhoeffer, P. Risk stratification, systematic classification, and anticipatory management strategies for stent fracture after percutaneous pulmonary valve implantation. Circulation 2007, 115, 1392–1397. [Google Scholar] [CrossRef]
- Hascoet, S.; Mauri, L.; Claude, C.; Fournier, E.; Lourtet, J.; Riou, J.Y.; Brenot, P.; Petit, J. Infective Endocarditis Risk After Percutaneous Pulmonary Valve Implantation With the Melody and Sapien Valves. JACC Cardiovasc. Interv. 2017, 10, 510–517. [Google Scholar] [CrossRef]
- Patel, N.D.; Levi, D.S.; Cheatham, J.P.; Qureshi, S.A.; Shahanavaz, S.; Zahn, E.M. Transcatheter Pulmonary Valve Replacement: A Review of Current Valve Technologies. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100452. [Google Scholar] [CrossRef]
- Malekzadeh-Milani, S.; Ladouceur, M.; Patel, M.; Boughenou, F.M.; Iserin, L.; Bonnet, D.; Boudjemline, Y. Incidence and predictors of Melody® valve endocarditis: A prospective study. Arch. Cardiovasc. Dis. 2015, 108, 97–106. [Google Scholar] [CrossRef]
- Shang, X.; Dong, N.; Zhang, C.; Wang, Y. The clinical trial outcomes of Med-zenith PT-Valve in the treatment of patients with severe pulmonary regurgitation. Front. Cardiovasc. Med. 2022, 9, 887886. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, G.B.; Kim, S.H.; Jang, S.I.; Choi, J.Y.; Kang, I.S.; Kim, Y.H. Mid-term outcomes of the Pulsta transcatheter pulmonary valve for the native right ventricular outflow tract. Catheter. Cardiovasc. Interv. 2021, 98, E724–E732. [Google Scholar] [CrossRef]
- Odemis, E.; Yenidogan, I. First experiences with Myval Transcatheter Heart Valve System in the treatment of severe pulmonary regurgitation in native right ventricular outflow tract and conduit dysfunction. Cardiol. Young 2022, 32, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Turner, M.; Caputo, M.; Stoica, S.; Marianeschi, S.; Parry, A. Pulmonary valve implantation using self-expanding tissue valve without cardiopulmonary bypass reduces operation time and blood product use. J. Thorac. Cardiovasc. Surg. 2013, 145, 1040–1045. [Google Scholar] [CrossRef] [PubMed]



| Proactive Approach | Conservative Approach | |
|---|---|---|
| RVEDVi | >160 mL/m2 | >180 mL/m2 |
| RVESVi | >80 mL/m2 | >95 mL/m2 |
| RVEF | <47% | <40% |
| LVEF | <55% | <45% |
| QRS width | >160 ms | >180 ms |
| Model | Bovine Jugular Vein | NuMed Platinum Iridium Stent | Length (Out of the Jar) | Landing Zone |
|---|---|---|---|---|
| Melody TPV 20 | 16 mm | CP-34 (8 crown-6 zig) | 30 mm | 16–20 mm |
| Melody TPV 22 | 18 mm | CP-34 (8 crown-6 zig) | 28 mm | 18–22 mm |
| Model | Diameter | Length | Landing Zone |
|---|---|---|---|
| Edwards Sapien THV 20 | 20 mm | 13.5 mm | 16–19 mm |
| Edwards Sapien THV 23 | 23 mm | 14.3 mm | 18–22 mm |
| Edwards Sapien THV 26 | 26 mm | 17.2 mm | 21–25 mm |
| Edwards Sapien THV 29 | 29 mm | 19.1 mm | 24–27 mm |
 | Model | Specification | Diameter (mm) | |||
| A | B | C | D | |||
| L28P | P28-25 | 38 | 28 | 38 | 25 | |
| P28-30 | 30 | |||||
| L30P | P30-25 | 40 | 30 | 40 | 25 | |
| P30-30 | 30 | |||||
| L32P | P32-25 | 42 | 32 | 42 | 25 | |
| P32-30 | 30 | |||||
| L34P | P34-25 | 44 | 34 | 44 | 25 | |
| P34-30 | 30 | |||||
| L36P | P36-25 | 46 | 36 | 46 | 25 | |
| P36-30 | 30 | |||||
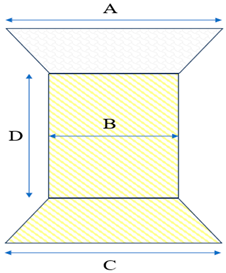 | Model | Diameter (mm) | |||
| A | B | C | D | ||
| Harmony TPV 22 | 32 | 22 | 41 | 55 | |
| Harmony TPV 25 | 43 | 25 | 54 | 51 | |
| Model | Conduit RV-PA | Bioprosthetic PV | Native RVOT | Landing Zone |
|---|---|---|---|---|
| Melody TPV (balloon-expandable) | X | X | 16–22 mm | |
| Edwards Sapien THV (balloon-expandable) | X | X | X | 16–27 mm |
| Venus P-Valve (self-expandable) | X | 26–34 mm | ||
| Harmony TPV (self-expandable) | X | CT analysis | ||
| Alterra Adaptive Prestent (self-expandable) | X | 27–38 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, M.; Marzullo, R.; Gaio, G.; Cappelli Bigazzi, M.; Ciriello, G.D.; Palladino, M.T.; Sarubbi, B.; Russo, M.G. Transcatheter Pulmonary Valve Implantation in Congenital Heart Diseases: Current Advances and Future Prospectives. Children 2025, 12, 547. https://doi.org/10.3390/children12050547
Giordano M, Marzullo R, Gaio G, Cappelli Bigazzi M, Ciriello GD, Palladino MT, Sarubbi B, Russo MG. Transcatheter Pulmonary Valve Implantation in Congenital Heart Diseases: Current Advances and Future Prospectives. Children. 2025; 12(5):547. https://doi.org/10.3390/children12050547
Chicago/Turabian StyleGiordano, Mario, Raffaella Marzullo, Gianpiero Gaio, Maurizio Cappelli Bigazzi, Giovanni Domenico Ciriello, Maria Teresa Palladino, Berardo Sarubbi, and Maria Giovanna Russo. 2025. "Transcatheter Pulmonary Valve Implantation in Congenital Heart Diseases: Current Advances and Future Prospectives" Children 12, no. 5: 547. https://doi.org/10.3390/children12050547
APA StyleGiordano, M., Marzullo, R., Gaio, G., Cappelli Bigazzi, M., Ciriello, G. D., Palladino, M. T., Sarubbi, B., & Russo, M. G. (2025). Transcatheter Pulmonary Valve Implantation in Congenital Heart Diseases: Current Advances and Future Prospectives. Children, 12(5), 547. https://doi.org/10.3390/children12050547







