Altered Brain Functional Connectivity and Topological Structural in Girls with Idiopathic Central Precocious Puberty: A Graph Theory Analysis Based on Resting-State fMRI
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Clinical Data
2.3. MRI Data Acquisition
2.4. Data Preprocessing
2.5. Network Construction
2.6. Network Analysis
2.7. Statistical Analysis
3. Results
3.1. Demographics and Characteristics
3.2. Comparison of RS-FC Between Two Groups
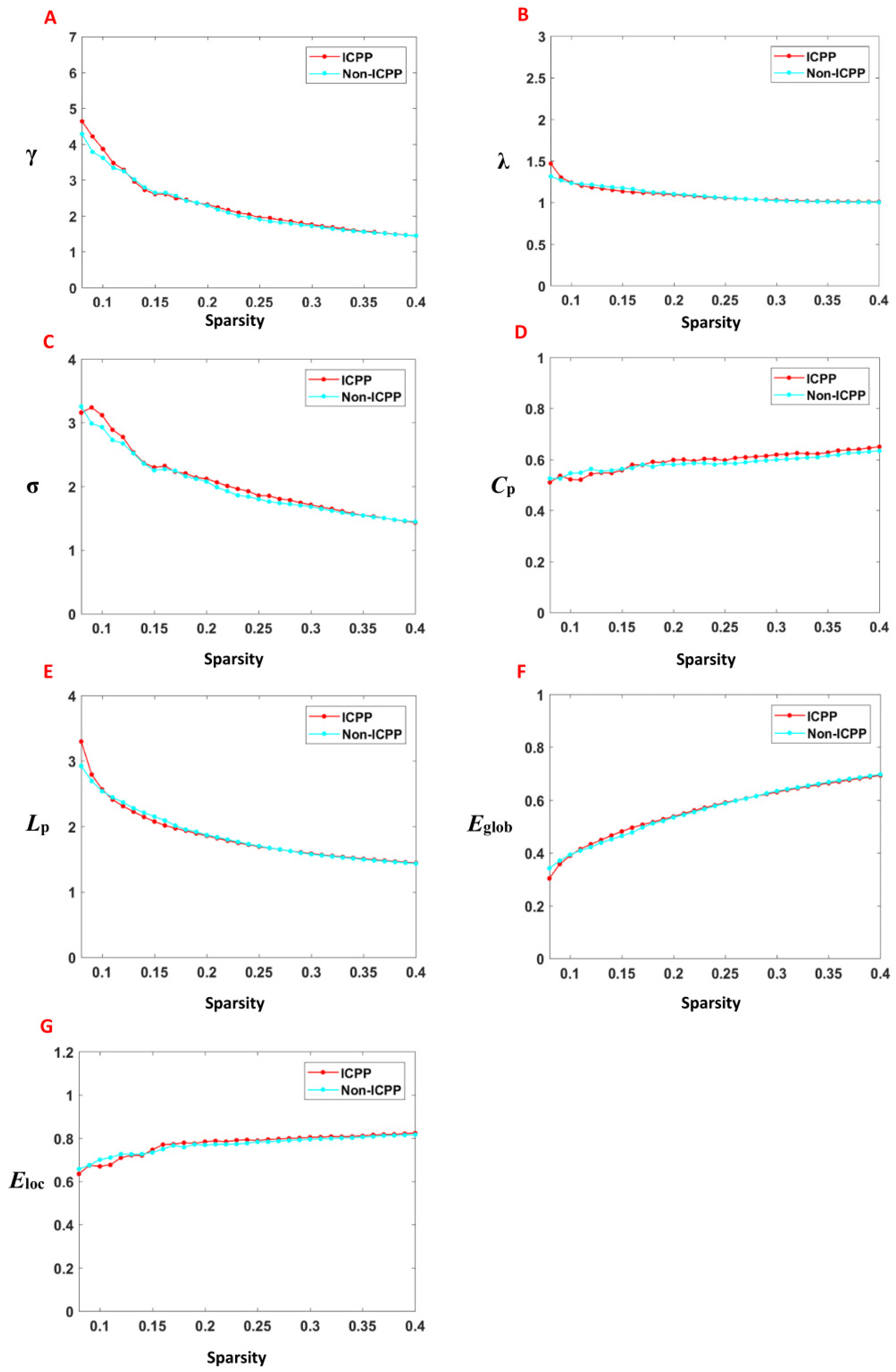
3.3. Comparison of Global Topological Properties Between Two Groups
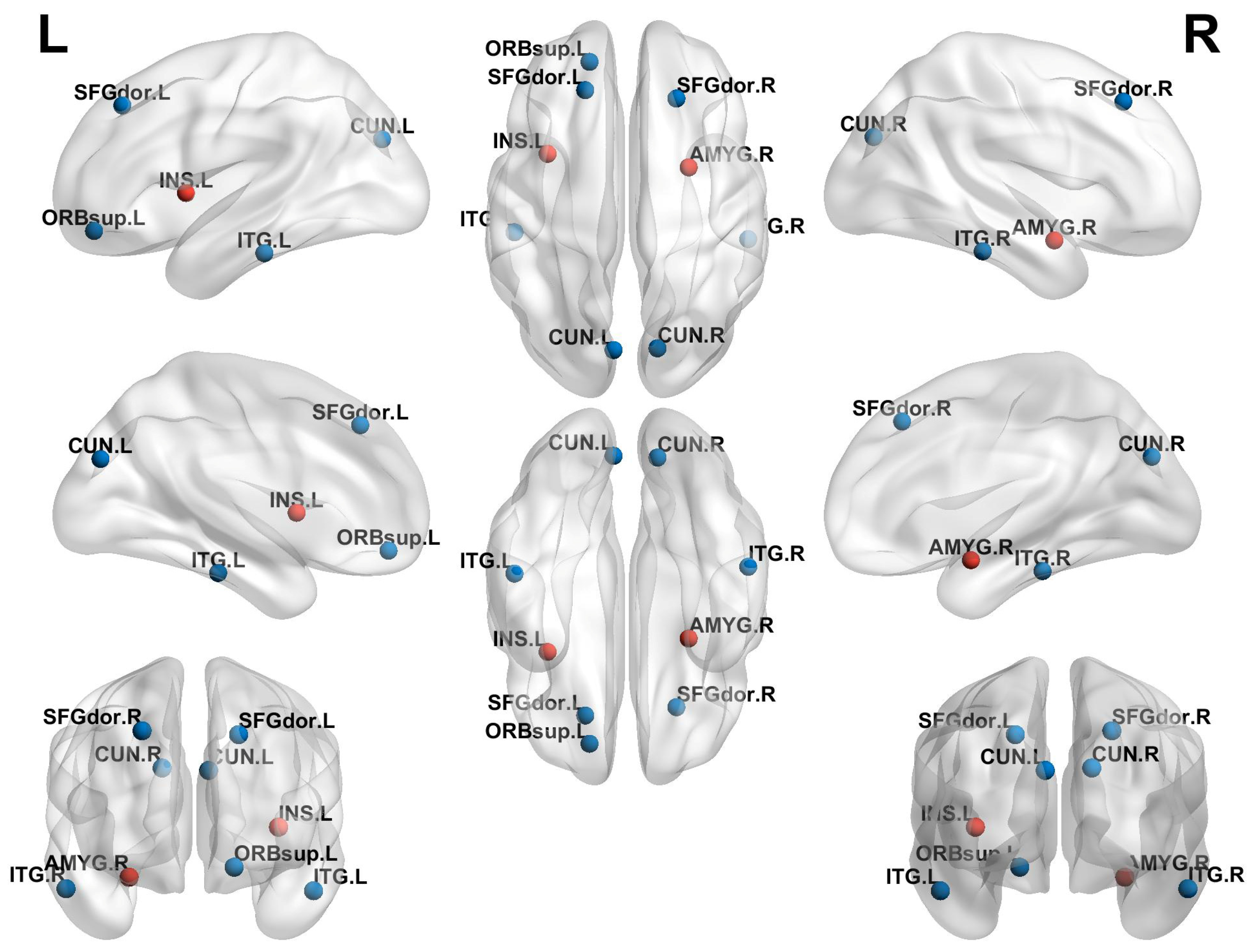
3.4. Comparison of Local Topological Properties Between Two Groups
3.5. Relationships Between Sex Hormone and Topological Properties
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Feng, G.; Cao, B.; Zheng, Y.; Gong, C.X. Development of a disease diagnostic model to predict the occurrence of central precocious puberty of female. J. Pediatr. Endocrinol. Metab. 2025, 38, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Corripio, R.; Soriano-Guillen, L.; Herrero, F.J.; Castro-Feijoo, L.; Escribano, A.; Sol-Ventura, P.; Espino, R.; Vela, A.; Labarta, J.I.; Spanish, P.G.; et al. Adult height in girls with idiopathic central precocious puberty treated with triptorelin. Front. Endocrinol. 2024, 15, 1498726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arcari, A.J.; Freire, A.V.; Ballerini, M.G.; Escobar, M.E.; Diaz Marsiglia, Y.M.; Bergada, I.; Ropelato, M.G.; Gryngarten, M.G. Prevalence of Polycystic Ovarian Syndrome in Girls with a History of Idiopathic Central Precocious Puberty. Horm. Res. Paediatr. 2024, 97, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Canton, A.P.M.; Macedo, D.B.; Abreu, A.P.; Latronico, A.C. Genetics and Epigenetics of Human Pubertal Timing: The Contribution of Genes Associated with Central Precocious Puberty. J. Endocr. Soc. 2025, 9, bvae228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knific, T.; Lazarevic, M.; Zibert, J.; Obolnar, N.; Aleksovska, N.; Suput Omladic, J.; Battelino, T.; Avbelj Stefanija, M. Final adult height in children with central precocious puberty—A retrospective study. Front. Endocrinol. 2022, 13, 1008474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soriano-Guillen, L.; Argente, J. Central precocious puberty, functional and tumor-related. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101262. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.L.; Yang, Y.; Xiong, X.Y.; Yang, L.; Wu, X.; Zhang, D.G. A Clinical Study of Girls With Idiopathic Central Precocious Puberty and Psychological Behavior Problems. Clin. Pediatr. 2023, 62, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Uthayo, W.; Chunin, H.; Sudnawa, K.K.; Arunyanart, W.; Phatarakijnirund, V. Psychological and behavioral assessments in girls with idiopathic central precocious puberty. J. Pediatr. Endocrinol. Metab. 2024, 38, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Zevin, E.L.; Eugster, E.A. Central precocious puberty: A review of diagnosis, treatment, and outcomes. Lancet Child. Adolesc. Health 2023, 7, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.R.; Kim, Y.J.; Oh, K.E.; Park, G.H.; Kang, E.; Nam, H.K.; Rhie, Y.J.; Oh, S.; Lee, K.H. Brain magnetic resonance imaging (MRI) findings in central precocious puberty patients: Is routine MRI necessary for newly diagnosed patients? Ann. Pediatr. Endocrinol. Metab. 2023, 28, 200–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eby, A.L.; Remedios, L.W.; Kim, M.E.; Li, M.; Gao, Y.; Gore, J.C.; Schilling, K.G.; Landman, B.A. Identification of functional white matter networks in BOLD fMRI. Proc. SPIE Int. Soc. Opt. Eng. 2024, 12926, 179–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van den Boomen, M.; Manhard, M.K.; Snel, G.J.H.; Han, S.; Emblem, K.E.; Slart, R.; Sosnovik, D.E.; Catana, C.; Rosen, B.R.; Prakken, N.H.J.; et al. Blood Oxygen Level-Dependent MRI of the Myocardium with Multiecho Gradient-Echo Spin-Echo Imaging. Radiology 2020, 294, 538–545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, W.; Lu, Y.; Chen, T.; Xia, Y.; Tang, J.; Hussein, N.M.; Meng, S.; Liu, X.; Liu, P.; Yan, Z. Frequency-dependent alterations in regional homogeneity associated with puberty hormones in girls with central precocious puberty: A resting-state fMRI study. J. Affect. Disord. 2023, 332, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Lu, Y.; Wang, Y.; Guo, A.; Xie, X.; Fu, Y.; Shen, B.; Lin, W.; Yang, D.; Zhou, L.; et al. Altered Brain Structure and Functional Connectivity Associated with Pubertal Hormones in Girls with Precocious Puberty. Neural Plast. 2019, 2019, 1465632. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajagopalan, V.; Pioro, E.P. Graph theory network analysis reveals widespread white matter damage in brains of patients with classic ALS. Amyotroph. Lateral Scler. Front. Degener. 2025, 26, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xin, H.; Wang, L.; Qi, Q.; Wang, Y.; Jia, Y.; Zheng, W.; Sun, C.; Chen, X.; Du, J.; et al. Distinct brain network patterns in complete and incomplete spinal cord injury patients based on graph theory analysis. CNS Neurosci. Ther. 2024, 30, e14910. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mamat, M.; Wang, Z.; Jin, L.; He, K.; Li, L.; Chen, Y. Beyond nodes and edges: A bibliometric analysis on graph theory and neuroimaging modalities. Front. Neurosci. 2024, 18, 1373264. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, R.B.; Li, X.T.; Huang, X. Topological Organization of the Brain Network in Patients with Primary Angle-closure Glaucoma Through Graph Theory Analysis. Brain Topogr. 2024, 37, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhan, P.; Wang, G.; Yu, X.; Liu, H.; Wang, W. Changes of brain functional network in Alzheimer’s disease and frontotemporal dementia: A graph-theoretic analysis. BMC Neurosci. 2024, 25, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Xia, J.; Hu, J.; Chen, Q.; Li, Y.; Yin, M.; Zou, H.; Zhou, W.; Zhang, P. Functional Brain Network Alterations in Patients With Systemic Lupus Erythematosus With Different Cognitive Function States: A Graph Theory Analysis Study. J. Comput. Assist. Tomogr. 2024, 48, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Carel, J.C.; Eugster, E.A.; Rogol, A.; Ghizzoni, L.; Palmert, M.R.; Group, E.-L.G.A.C.C.; Antoniazzi, F.; Berenbaum, S.; Bourguignon, J.P.; Chrousos, G.P.; et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009, 123, e752–e762. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.B.; Demirbilek, H. Diagnostic utility of the average peak LH levels measured during GnRH stimulation test. J. Pediatr. Endocrinol. Metab. 2024, 37, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, L.A.; Trout, A.T.; Schuster, J.G.; Schwartz, B.I.; Breech, L.L.; Zhang, B.; Towbin, A.J. Normative values for ultrasound measurements of the female pelvic organs throughout childhood and adolescence. Pediatr. Radiol. 2019, 49, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Sousa, E.S.P.; Coelho, E.S.M.J.; Seabra, A.; Costa, D.C.; Martinho, D.V.; Duarte, J.P.; Oliveira, T.; Goncalves-Santos, J.; Rodrigues, I.; Ribeiro, L.P.; et al. Skeletal age assessed by TW2 using 20-bone, carpal and RUS score systems: Intra-observer and inter-observer agreement among male pubertal soccer players. PLoS ONE 2022, 17, e0271386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Y.T.; Yuan, Y.S.; Wang, M.; Zhi, Y.; Wang, J.W.; Wang, L.N.; Ma, K.W.; Si, Q.Q.; Zhang, K.Z. Dysfunction in superior frontal gyrus associated with diphasic dyskinesia in Parkinson’s disease. NPJ Park. Dis. 2020, 6, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfurtscheller, G.; Rassler, B.; Porta, A.; Schwarz, G.; Kaminski, M.; Pfurtscheller, K.; Klimesch, W. Modulation of amygdala and hippocampus during anxiety by heart and middle frontal gyrus. Cardiovasc. Res. 2025, cvaf007. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, W.M.; Aljundi, Z.E.; Sulaiman, A.A.; Bagadood, R.M. Acute spasticity secondary to ischemic stroke involving superior frontal gyrus and anterior cingulate gyrus. J. Neurosci. Rural. Pract. 2023, 14, 741–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, Z.; Qu, H.; Zou, W.; Du, X.; Li, Y.; Wang, W. Altered degree centrality and functional connectivity in girls with central precocious puberty. Brain Imaging Behav. 2024, 19, 138–147. [Google Scholar] [CrossRef]
- Shackman, A.J.; Grogans, S.E.; Fox, A.S. Fear, anxiety and the functional architecture of the human central extended amygdala. Nat. Rev. Neurosci. 2024, 25, 587–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.; Huang, J.; Hong, B.; Wang, X. Neural correlates of perceived emotions in human insula and amygdala for auditory emotion recognition. Neuroimage 2022, 260, 119502. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, Z.; Wu, S.; Hong, J.; Wen, G. Small-world network of patients with primary insomnia: A resting-state functional magnetic resonance imaging study. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 424–429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Y.; Jin, X.; Xue, Y.; Li, X.; Chen, Y.; Kang, N.; Yan, W.; Li, P.; Guo, X.; Luo, B.; et al. Right superior frontal gyrus: A potential neuroimaging biomarker for predicting short-term efficacy in schizophrenia. Neuroimage Clin. 2024, 42, 103603. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, J.; Li, Y.; Chen, K.; Cao, Y.; Liu, K.; Zhang, X.; Wu, X.; Wang, Z.; Liu, X.; Li, L. Aberrant functional connectivity in anterior cingulate gyrus subregions in migraine without aura patients. Front. Neurol. 2024, 15, 1412117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeager, B.E.; Bruss, J.; Duffau, H.; Herbet, G.; Hwang, K.; Tranel, D.; Boes, A.D. Central precuneus lesions are associated with impaired executive function. Brain Struct. Funct. 2022, 227, 3099–3108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, C.; Zheng, Y.; Li, J.; Zhang, B.; Li, R.; Wu, H.; She, S.; Liu, S.; Peng, H.; Ning, Y.; et al. Activation and Functional Connectivity of the Left Inferior Temporal Gyrus during Visual Speech Priming in Healthy Listeners and Listeners with Schizophrenia. Front. Neurosci. 2017, 11, 107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]




| Parameters | ICPP Group (n = 53) | Non-ICPP Group (n = 51) | Statistic | p |
|---|---|---|---|---|
| General parameters | ||||
| CA (year) | 6.86 ± 1.01 | 6.54 ± 1.31 | 1.549 a | 0.121 |
| Height (cm) | 128.78 ± 6.90 | 119.11 ± 6.11 | 3.558 a | <0.001 |
| Weight (kg) | 29.01 ± 6.08 | 24.81 ± 4.32 | 3.924 a | <0.001 |
| BMI (kg/m2) | 17.79 (16.14, 19.07) | 16.76 (15.06, 18.5) | 1.468 c | 0.143 |
| Clinical manifestation | ||||
| Breast development | 45 (85%) | 42 (82%) | 1.814 b | 0.070 |
| Vaginal bleeding | 21 (39%) | 18 (35%) | 1.549 b | 0.080 |
| Biochemical indicators | ||||
| Basal LH (mIU/mL) | 0.24 (0.14, 0.43) | 0.10 (0.10, 0.12) | 2.033 c | <0.001 |
| Basal FSH (mIU/m) | 2.61 (1.69, 3.52) | 1.85 (1.24, 2.44) | 3.110 c | 0.002 |
| Peak LH (mIU/mL) | 10.80 (8.22, 12.69) | 3.38 (2.17, 4.84) | 8.043 c | <0.001 |
| Peak FSH (mIU/mL) | 11.48 (9.56, 12.60) | 11.38 (9.43, 13.28) | 1.278 c | 0.095 |
| Peak LH/Peak FSH | 0.94 (0.85, 1.05) | 0.29 (0.23, 0.36) | 9.241 c | <0.001 |
| E2 (pmol/L) | 78.50 (73.40, 82.00) | 73.40 (73.40, 82.01) | 2.238 c | 0.055 |
| Imaging parameters | ||||
| BA (years) | 9.2 (8.40, 9.60) | 8.30 (7.40, 9.30) | 3.186 c | 0.001 |
| Left ovarian volume (cm3) | 1.34 (1.01, 1.96) | 0.86 (0.50, 1.61) | 3.161 c | <0.001 |
| Right ovarian volume (cm3) | 1.31 (0.98, 1.87) | 0.88 (0.58, 1.66) | 3.124 a | <0.001 |
| Uterine volume (cm3) | 2.63 (2.26, 3.12) | 2.24 (1.63, 2.86) | 2.245 c | 0.003 |
| Scale | ||||
| WISC-CR (IQ) score | 104.12 ± 10.28 | 102.25 ± 13.96 | 1.428 a | 0.820 |
| CBCL score | 7.90 ± 6.40 | 8.10 ± 5.87 | 1.338 a | 0.736 |
| HAMA score | 5.23 ± 1.21 | 5.42 ± 1.00 | 1.268 a | 0.870 |
| Group | n | γ | λ | σ | Cp | Lp | Eglob | Eloc |
|---|---|---|---|---|---|---|---|---|
| ICPP | 53 | 0.60 ± 0.12 | 0.35 ± 0.02 | 0.54 ± 0.11 | 0.19 ± 0.01 | 0.60 ± 0.04 | 0.178 ± 0.08 | 0.24 ± 0.01 |
| Non-ICPP | 51 | 0.60 ± 0.12 | 0.36 ± 0.02 | 0.53 ± 0.12 | 0.20 ± 0.02 | 0.62 ± 0.05 | 0.17 ± 0.01 | 0.25 ± 0.01 |
| T | 0.10 | −1.14 | 0.34 | −1.78 | −1.20 | 1.43 | −1.65 | |
| p | 0.92 | 0.27 | 0.74 | 0.09 | 0.24 | 0.16 | 0.12 |
| AAL No. | Region | MNI Coordinates | T | p | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 3 | SFGdor.L | −22 | 18 | 72 | −3.4474 | 0.0010 |
| 4 | SFGdor.R | 24 | 20 | 70 | −2.1161 | 0.0437 |
| 31 | ACG.L | −6 | 40 | 2 | −2.1415 | 0.0414 |
| 32 | ACG.R | 8 | 42 | 4 | −2.0243 | 0.0410 |
| 42 | AMYG.R | 24 | −2 | −14 | 2.2437 | 0.0333 |
| 45 | CUN.L | −8 | −76 | 20 | −2.2693 | 0.0315 |
| 89 | ITG.L | −50 | −40 | −20 | −2.2247 | 0.0347 |
| 90 | ITG.R | 52 | −38 | −20 | −2.7442 | 0.0107 |
| AAL No. | Region | MNI Coordinates | T | p | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| 3 | SFGdor.L | −22 | 18 | 72 | −2.9722 | 0.004 |
| 4 | SFGdor.R | 24 | 20 | 70 | −2.8655 | 0.006 |
| 5 | ORBsup.L | −30 | 50 | −14 | −2.4309 | 0.008 |
| 29 | INS.L | −40 | 0 | −10 | 2.2595 | 0.040 |
| 42 | AMYG.R | 24 | −2 | −14 | 3.1447 | 0.010 |
| 45 | CUN.L | −8 | −76 | 20 | −2.0810 | 0.009 |
| 46 | CUN.R | 10 | −74 | −20 | −2.4647 | 0.048 |
| 89 | ITG.L | −50 | −40 | −20 | −2.3539 | 0.042 |
| 90 | ITG.R | 52 | −38 | −20 | −2.7402 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Zeng, Y.; Zheng, H.; Cai, J. Altered Brain Functional Connectivity and Topological Structural in Girls with Idiopathic Central Precocious Puberty: A Graph Theory Analysis Based on Resting-State fMRI. Children 2025, 12, 565. https://doi.org/10.3390/children12050565
Tian L, Zeng Y, Zheng H, Cai J. Altered Brain Functional Connectivity and Topological Structural in Girls with Idiopathic Central Precocious Puberty: A Graph Theory Analysis Based on Resting-State fMRI. Children. 2025; 12(5):565. https://doi.org/10.3390/children12050565
Chicago/Turabian StyleTian, Lu, Yan Zeng, Helin Zheng, and Jinhua Cai. 2025. "Altered Brain Functional Connectivity and Topological Structural in Girls with Idiopathic Central Precocious Puberty: A Graph Theory Analysis Based on Resting-State fMRI" Children 12, no. 5: 565. https://doi.org/10.3390/children12050565
APA StyleTian, L., Zeng, Y., Zheng, H., & Cai, J. (2025). Altered Brain Functional Connectivity and Topological Structural in Girls with Idiopathic Central Precocious Puberty: A Graph Theory Analysis Based on Resting-State fMRI. Children, 12(5), 565. https://doi.org/10.3390/children12050565






