Cytomegalovirus Infection in Infancy May Increase the Risk of Subsequent Epilepsy and Autism Spectrum Disorder in Childhood
Abstract
:1. Introduction
2. Materials and Methods
3. Statistical Analysis
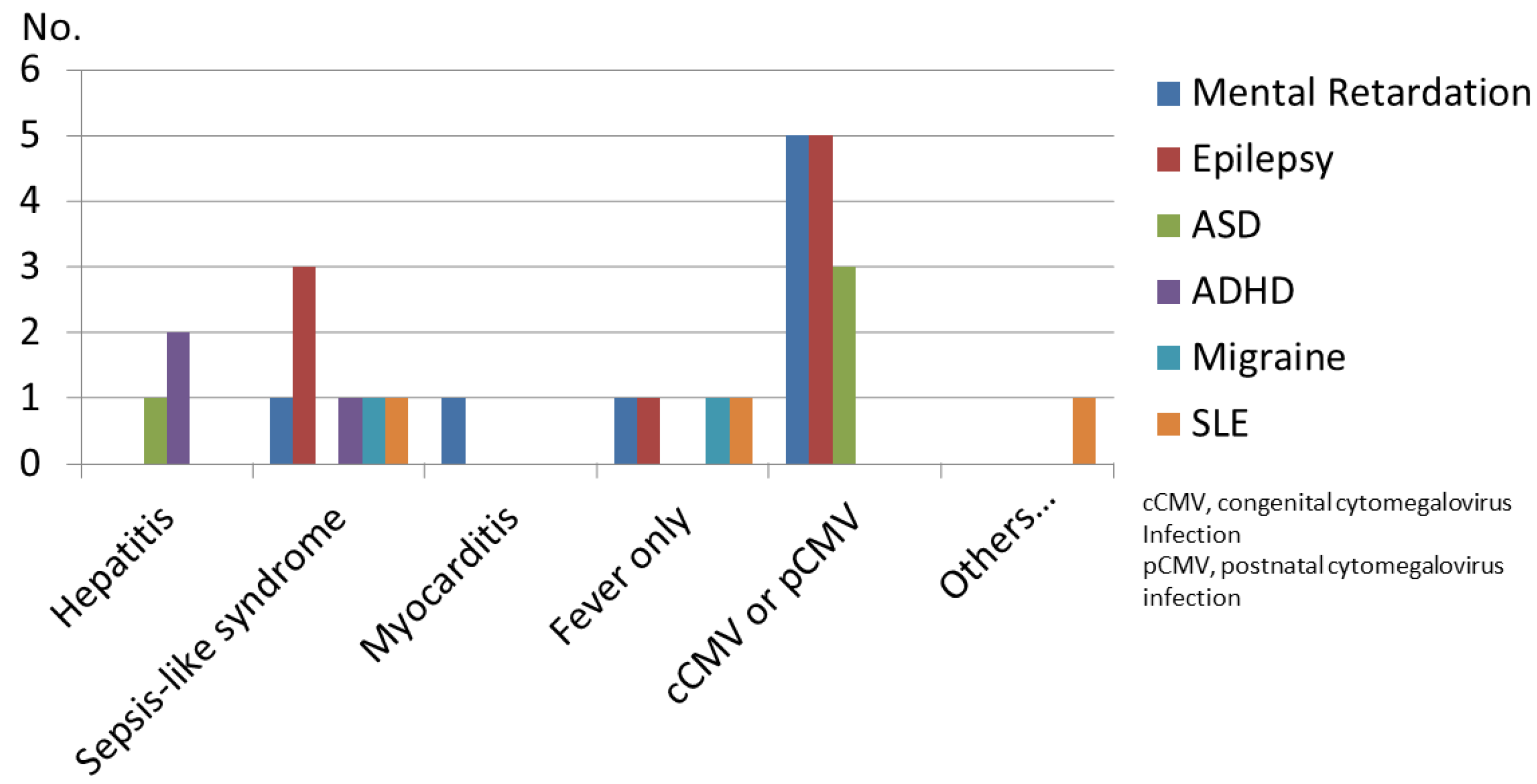
4. Results
4.1. Data Analysis
| Demographic Data | CMV Infection, n = 69 (%) | Controls, n = 292 (%) | p-Value |
|---|---|---|---|
| Mean age of infection (years) (SD) | 3.78 (4.66) | 3.62 (4.50) | 0.90 |
| Gender | 0.27 | ||
| Male | 38 (55.1) | 182 (62.3) | |
| Female | 31 (44.9) | 110 (37.7) | |
| Labor | 0.68 | ||
| Preterm | 7 (10.1) | 25 (8.6) | |
| Term | 62 (89.9) | 267 (91.4) | |
| Stratified by age (years) | 0.18 | ||
| 0–2 | 33 (47.8) | 122 (41.8) | |
| 2–5 | 19 (27.5) | 89 (30.5) | |
| 6–10 | 9 (13.0) | 63 (21.6) | |
| >10 | 8(11.6) | 18 (6.2) | |
| Associated diseases or symptoms | |||
| SGA | 5 (7.2) | - | |
| Thrombocytopenia | 5 (7.2) | - | |
| Mononucleosis-like syndrome | 11 (15.9) | - | |
| Prolonged fever | 9 (13.0) | - | |
| Neurological manifestations | 9 (13.0) | - | |
| Hepatitis and/or HSM | 28 (40.6) | - | |
| Myocarditis and sepsis-like syndrome | 6 (8.7) | - | |
| Skin manifestations | 2 (2.9) | - |
4.2. Neurodevelopmental Outcomes
4.3. Neurodevelopmental Disabilities in Children with CMV Infection
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention Deficit Hyperactivity Disorder |
| ASD | Autism Spectrum Disorder |
| CI | Confidence Interval |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| FS | Febrile Seizure |
| MR | Mental Retardation |
| NDDs | Neurodevelopmental Disorders |
| OR | Odds Ratio |
| TS | Tourette Syndrome |
References
- Harrison, G.J. Cytomegalovirus. In Feigin and Cherry’s Textbook of Pediatric Infectious Diseases, 7th ed.; Cherry, J.D., Harrison, G.J., Kaplan, S.L., Steinbach, W.J., Hotez, P.J., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; p. 1969. [Google Scholar]
- American Academy of Pediatrics. Cytomegalovirus infection. In Red Book: 2015 Report of the Committee on Infectious Diseases, 30th ed.; Kimberlin, D.W., Ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2015; p. 317. [Google Scholar]
- Boppana, S.B.; Fowler, K.B.; Vaid, Y.; Hedlund, G.; Stagno, S.; Britt, W.J.; Pass, R. Neuroradiographic Findings in the Newborn Period and Long-term Outcome in Children With Symptomatic Congenital Cytomegalovirus Infection. Pediatrics 1997, 99, 409–414. [Google Scholar] [CrossRef]
- Stagno, S.; Pass, R.F.; Cloud, G.; Britt, W.J.; Henderson, R.E.; Walton, P.D.; Veren, D.A.; Page, F.; Alford, C.A. Primary Cytomegalovirus Infection in Pregnancy. JAMA 1986, 256, 1904–1908. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Permar, S.R. Preventing Postnatal Cytomegalovirus Infection in the Preterm Infant: Should It Be Done, Can It Be Done, and at What Cost? J. Pediatr. 2015, 166, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Gunkel, J.; De Vries, L.S.; Jongmans, M.; Koopman-Esseboom, C.; Van Haastert, I.C.; Eijsermans, M.C.J.; Van Stam, C.; Van Zanten, B.G.A.; Wolfs, T.F.W.; Nijman, J. Outcome of Preterm Infants With Postnatal Cytomegalovirus Infection. Pediatrics 2018, 141, e20170635. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Kumari, N.; Trehan, A.; Khadwal, A.; Dogra, M.R.; Gupta, V.; Sharma, A.; Gupta, A.; Singh, R. Outcome of cytomegalovirus retinitis in immunocompromised patients without Human Immunodeficiency Virus treated with intravitreal ganciclovir injection. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Hartley, S.L.; Sikora, D.M. Which DSM-IV-TR criteria best differentiate high-functioning autism spectrum disorder from ADHD and anxiety disorders in older children? Autism 2009, 13, 485–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doernberg, E.; Hollander, E. Neurodevelopmental Disorders (ASD and ADHD): DSM-5, ICD-10, and ICD-11. CNS Spectr. 2016, 21, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Uematsu, M.; Haginoya, K.; Kikuchi, A.; Hino-Fukuyo, N.; Ishii, K.; Shiihara, T.; Kato, M.; Kamei, A.; Kure, S. Asymptomatic congenital cytomegalovirus infection with neurological sequelae: A retrospective study using umbilical cord. Brain Dev. 2016, 38, 819–826. [Google Scholar] [CrossRef]
- Smithers-Sheedy, H.; Raynes-Greenow, C.; Badawi, N.; McIntyre, S.; Jones, C.A.; Australian Cerebral Palsy Register Group. Congenital cytomegalovirus is associated with severe forms of cerebral palsy and female sex in a retrospective population-based study. Dev. Med. Child Neurol. 2014, 56, 846–852. [Google Scholar] [CrossRef]
- Muhle, R.A.; Reed, H.E.; Stratigos, K.A.; Veenstra-Vander Weele, J. The Emerging Clinical Neuroscience of Autism Spectrum Disorder: A Review. JAMA Psychiatry 2018, 75, 514–523. [Google Scholar] [CrossRef]
- Mazina, V.; Gerdts, J.; Trinh, S.; Ankenman, K.; Ward, T.; Dennis, M.Y.; Girirajan, S.; Eichler, E.E.; Bernier, R. Epigenetics of autism-related impairment: Copy number variation and maternal infection. J. Dev. Behav. Pediatr. 2015, 36, 61–67. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, M.; Kortekaas, J.; Herden, C.; Hoffmann, B.; Tappe, D.; Trebst, C.; Griffin, D.E.; Brindle, H.; Solomon, T.; Brown, A.S.; et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2015, 131, 159–184. [Google Scholar] [CrossRef] [Green Version]
- Cordeiro, C.N.; Tsimis, M.; Burd, I. Infections and Brain Development. Obstet. Gynecol. Surv. 2015, 70, 644–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, Y.; Toribe, Y.; Mogami, Y.; Yanagihara, K.; Nishikawa, M. Epilepsy in patients with congenital cytomegalovirus infection. Brain Dev. 2008, 30, 420–424. [Google Scholar] [CrossRef]
- Hayashi, M.; Nishiyama, I.; Moriuchi, M.; Moriuchi, H. A Twenty-Year Retrospective Diagnosis of Congenital Cytomegalovirus Infection. Pediatr. Neurol. 2018, 86, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Alarcon, A.; Martinez-Biarge, M.; Cabañas, F.; Quero, J.; García-Alix, A. A Prognostic Neonatal Neuroimaging Scale for Sympto-matic Congenital Cytomegalovirus Infection. Neonatology 2016, 110, 277–285. [Google Scholar] [CrossRef]
- Hrvoj-Mihic, B.; Bienvenu, T.; Stefanacci, L.; Muotri, A.R.; Semendeferi, K. Evolution, development, and plasticity of the human brain: From molecules to bones. Front. Hum. Neurosci. 2013, 7, 707. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, B.; Seibold-Weiger, K.; Schmitz-Salue, C.; Hamprecht, K.; Goelz, R.; Krageloh-Mann, I.; Speer, C.P. Postnatally acquired cy-tomegalovirus infection via breast milk: Effects on hearing and development in preterm infants. Pediatr Infect Dis. J. 2004, 23, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Goelz, R.; Meisner, C.; Bevot, A.; Hamprecht, K.; Kraegeloh-Mann, I.; Poets, C.F. Long-term cognitive and neurological outcome of preterm infants with postnatally acquired CMV infection through breast milk. Arch. Dis. Child.-Fetal Neonatal Ed. 2013, 98, F430–F433. [Google Scholar] [CrossRef] [PubMed]
- Bevot, A.; Hamprecht, K.; Krägeloh-Mann, I.; Brosch, S.; Goelz, R.; Vollmer, B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2011, 101, e167–e172. [Google Scholar] [CrossRef]
- Smithers-Sheedy, H.; Raynes-Greenow, C.; Badawi, N.; Fernandez, M.A.; Kesson, A.; McIntyre, S.; Leung, K.-C.; Jones, C.A. Congenital Cytomegalovirus among Children with Cerebral Palsy. J. Pediatr. 2016, 181, 267–271.e1. [Google Scholar] [CrossRef]
- Valayi, S.; Eftekharian, M.M.; Taheri, M.; Alikhani, M.Y. Evaluation of antibodies to cytomegalovirus and Epstein-Barr virus in patients with autism spectrum disorder. Hum Antibodies. 2017, 26, 165–169. [Google Scholar] [CrossRef]
- Maeyama, K.; Tomioka, K.; Nagase, H.; Yoshioka, M.; Takagi, Y.; Kato, T.; Mizobuchi, M.; Kitayama, S.; Takada, S.; Nagai, M.; et al. Congenital Cytomegalovirus Infection in Children with Autism Spectrum Disorder: Systematic Review and Meta-Analysis. J. Autism Dev. Disord. 2017, 48, 1483–1491. [Google Scholar] [CrossRef]
- Slawinski, B.L.; Talge, N.; Ingersoll, B.; Smith, A.; Glazier, A.; Kerver, J.; Paneth, N.; Racicot, K. Maternal cytomegalovirus sero-positivity and autism symptoms in children. Am. J. Reprod. Immunol. 2018, 79, e12840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Fang, F. Congenital human cytomegalovirus infection and neurologic diseases in newborns. Chin. Med. J. 2019, 132, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.; Petri, W.A.J. Microglia: Immune Regulators of Neurodevelopment. Front. Immunol. 2018, 9, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Group | Epilepsy | ASD | ADHD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event (No.) | IR(%) | OR (95% CI) | p | Event (No.) | IR(%) | OR (95% CI) | p | Event (No.) | IR(%) | OR (95% CI) | p | |
| Controls (n = 292) | 2 | 0.68 | reference | 1 | 0.34 | reference | 8 | 2.73 | reference | |||
| CMV group (n = 69) | 7 | 10.1 | 16.4 (3.32–80.7) | 0.001 | 4 | 5.8 | 17.9 (1.96–162.8) | 0.01 | 2 | 2.89 | 1.06 (0.22–5.10) | 0.94 |
| CMV infection at age 0 to 2 years (n = 33) | 7 | 21.2 | 32.6 (3.84–276.2) | 0.001 | 3 | 9.1 | 12.1 (1.21–120.5) | 0.03 | 0 | 0 | N/A | >0.99 |
| Patient No. | Sex/Labor | Age of CMV INF | Type of Infection | Antiviral Treatment | MR | Epilepsy/ Age of Dx | ASD/ Age of Dx | Last EEG Patterns/Age | Number of AED Uses | Seizure Control/Freq | Brain MRI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMV01 | F/P | 0 | Congenital | GCV | S | +/1 y | - | H yps/2 y | 4 | Unfavorable | WM |
| CMV02 | M/T | 0 | Congenital | GCV | M to S | +/1 y | +/3 y | Multi/5 y | 1 | Controlled | VM |
| CMV03 | M/T | 0 | Congenital | GCV | M | +/1 y | +/2 y | Multi/2 y | 1 | Controlled | HCP |
| CMV04 | F/T | 0 | Congenital | GCV | S | +/1 y | - | Multi/3 y | 1 | Controlled | HCP |
| CMV05 | M/T | 0 | E/P | - | - | +/2 y | - | Normal/9 y | 1 | Controlled | Normal |
| CMV06 | M/T | 1.5 y | Immunocompetent | - | - | +/2 y | - | Normal/8 y | 0 | Yearly | Normal |
| CMV07 | F/T | 1 y | Immunocompetent | - | M to S | +/1 y | - | Multi/2 y | 2 | Monthly | WM |
| CMV08 | F/P | 0 | Congenital | GCV | Mild | - | +/3 y | N/A | N/A | N/A | Normal |
| CMV09 | F/T | 2.1 y | Immunocompetent | - | - | - | +/3 y | N/A | N/A | N/A | Normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chou, I.-C.; Lee, I.-C.; Hong, S.-Y. Cytomegalovirus Infection in Infancy May Increase the Risk of Subsequent Epilepsy and Autism Spectrum Disorder in Childhood. Children 2021, 8, 1040. https://doi.org/10.3390/children8111040
Lin C-H, Chou I-C, Lee I-C, Hong S-Y. Cytomegalovirus Infection in Infancy May Increase the Risk of Subsequent Epilepsy and Autism Spectrum Disorder in Childhood. Children. 2021; 8(11):1040. https://doi.org/10.3390/children8111040
Chicago/Turabian StyleLin, Chien-Heng, I.-Ching Chou, Inn-Chi Lee, and Syuan-Yu Hong. 2021. "Cytomegalovirus Infection in Infancy May Increase the Risk of Subsequent Epilepsy and Autism Spectrum Disorder in Childhood" Children 8, no. 11: 1040. https://doi.org/10.3390/children8111040





