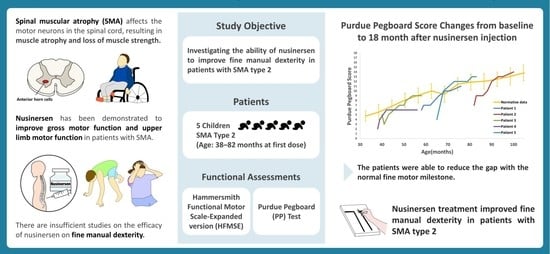
Improvement in Fine Manual Dexterity in Children with Spinal Muscular Atrophy Type 2 after Nusinersen Injection: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Functional Assessments
3. Results
3.1. Baseline Characteristics
3.2. Efficacy Results
3.2.1. Hammersmith Functional Motor Scale (Expanded Version)
3.2.2. Purdue Pegboard Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Prasad, A.N.; Prasad, C.J.B. The floppy infant: Contribution of genetic and metabolic disorders. Brain Dev. 2003, 25, 457–476. [Google Scholar] [CrossRef]
- Lefebvre, S.; Burglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Iyer, C.C.; McGovern, V.L.; Murray, J.D.; Gombash, S.E.; Zaworski, P.G.; Foust, K.D.; Janssen, P.M.L.; Burghes, A.H.M. Low levels of Survival Motor Neuron protein are sufficient for normal muscle function in the SMNDelta7 mouse model of SMA. Hum. Mol. Genet. 2015, 24, 6160–6173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercuri, E.; Darras, B.T.; Chiriboga, C.A.; Day, J.W.; Campbell, C.; Connolly, A.M.; Iannaccone, S.T.; Kirschner, J.; Kuntz, N.; Saito, K.; et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N. Engl. J. Med. 2018, 378, 625–635. [Google Scholar] [CrossRef] [PubMed]
- De Wel, B.; Goosens, V.; Sobota, A.; Camp, E.V.; Geukens, E.; Van Kerschaver, G.; Jagut, M.; Claes, K.; Claeys, K.G. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J. Neurol. 2021, 268, 923–935. [Google Scholar] [CrossRef]
- O’Hagen, J.M.; Glanzman, A.M.; McDermott, M.P.; Ryan, P.A.; Flickinger, J.; Quigely, J.; Riley, S.; Saborn, E.; Irvine, C.; Martens, W.B.; et al. An expanded version of the Hammersmith Functional Motor Scale for SMA II and III patients. Neuromusc. Disord. 2007, 17, 693–697. [Google Scholar] [CrossRef]
- Glanzman, A.M.; O’Hagen, J.M.; McDermott, M.P.; Martens, W.B.; Flickinger, J.; Riley, S.; Quigely, J.; Montes, J.; Dunaway, S.; Deng, L.; et al. Validation of the Expanded Hammersmith Functional Motor Scale in spinal muscular atrophy type II and III. J. Child Neurol. 2011, 26, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.C.; Wilson, J.J.; Iacoviello, J.M.; Risucci, D. Purdue Pegboard performance of normal preschool children. J. Clin. Neuropsychol. 1982, 4, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Pin, T.W.; Butler, P.B.; Cheung, H.-M.; Shum, S.L.F. Relationship between segmental trunk control and gross motor development in typically developing infants aged from 4 to 12 months: A pilot study. BMC Pediatr. 2019, 19, 425. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, S.; Josman, N.J.P. The relationship between postural control and fine manual dexterity. Phys. Occup. Ther. Pediatr. 2003, 23, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-N.; Howe, T.-H.; Hinojosa, J.; Weinberg, S.L. Relationship between postural control and fine motor skills in preterm infants at 6 and 12 months adjusted age. Am. J. Occup. Ther. 2011, 65, 695–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, Y.; Kobayashi, R.; Kelepecz, D.; Nakajima, M. Core exercises elevate trunk stability to facilitate skilled motor behavior of the upper extremities. J. Bodyw. Mov. Ther. 2013, 17, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.A.; Broman, M. The Purdue Pegboard: Normative data on 1334 school children. J. Clin. Child Psychol. 1979, 8, 156–162. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul. Disord. 2019, 29, 842–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Dunaway Young, S.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve. 2017, 55, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Chiriboga, C.A.; Iannaccone, S.T.; Swoboda, K.J.; Montes, J.; Mignon, L.; Xia, S.; Bennett, C.F.; Bishop, K.M.; Shefner, J.M.; et al. Nusinersen in later-onset spinal muscular atrophy: Long-term results from the phase 1/2 studies. Neurology. 2019, 92, e2492–e2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Patient Number | Sex | SMA Type | SMN2 Copy Number | Age at Symptom Onset (Month) | Age at Diagnosis (Month) | Age at First Dose (Month) |
|---|---|---|---|---|---|---|
| 1 | Female | 2 | 3 | 12 | 2 | 58 |
| 2 | Female | 2 | 3 | 13 | 24 | 82 |
| 3 | Female | 2 | 3 | 14 | 23 | 40 |
| 4 | Female | 2 | 3 | 12 | 21 | 38 |
| 5 | Female | 2 | 3 | 13 | 21 | 65 |
| Patient Number | Baseline HFMSE Score | ∆ Lying and Rolling | ∆ Sitting | ∆ Crawling and Kneeling | ∆ Standing | ∆ Walking, Running, and Jumping | HFMSE Score after 7th Dose | ∆ HFMSE Score (Δ%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | +2 | +3 | 0 | 0 | 0 | 15 | +5 (+50%) |
| 2 | 19 | +5 | +1 | +4 | 0 | 0 | 29 | +10 (+53%) |
| 3 | 40 | +3 | 0 | +1 | +1 | +1 | 46 | +6 (+15%) |
| 4 | 13 | 0 | +3 | 0 | 0 | 0 | 16 | +3 (+23%) |
| 5 | 30 | +3 | 0 | +1 | 0 | 0 | 34 | +4 (+13%) |
| Patient Number | Δ HFMSE Score (Δ%) | Preferred Hand PP Score | Non-Preferred Hand PP Score | ||
|---|---|---|---|---|---|
| Baseline | After 7th Dose | Baseline | After 7th Dose | ||
| 1 | +5 (+50%) | 6 | 11 | 4 | 8 |
| Δ PP score (Δ%) | +5 (+83%) | +4 (+100%) | |||
| 2 | +10 (+53%) | 7 | 14 | 6 | 13 |
| Δ PP score (Δ%) | +7 (+100%) | +7 (+117%) | |||
| 3 | +6 (+15%) | 5 | 9 | 5 | 8 |
| Δ PP score (Δ%) | +4 (+80%) | +3 (+60%) | |||
| 4 | +3 (+23%) | 2 | 6 | 1 | 6 |
| Δ PP score (Δ%) | +4 (+200%) | +5 (+500%) | |||
| 5 | +4 (+13%) | 4 | 13 | 5 | 11 |
| Δ PP score (Δ%) | +9 (+225%) | +6 (+120%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, M.; Kong, H.-H. Improvement in Fine Manual Dexterity in Children with Spinal Muscular Atrophy Type 2 after Nusinersen Injection: A Case Series. Children 2021, 8, 1039. https://doi.org/10.3390/children8111039
Gu M, Kong H-H. Improvement in Fine Manual Dexterity in Children with Spinal Muscular Atrophy Type 2 after Nusinersen Injection: A Case Series. Children. 2021; 8(11):1039. https://doi.org/10.3390/children8111039
Chicago/Turabian StyleGu, Minsu, and Hyun-Ho Kong. 2021. "Improvement in Fine Manual Dexterity in Children with Spinal Muscular Atrophy Type 2 after Nusinersen Injection: A Case Series" Children 8, no. 11: 1039. https://doi.org/10.3390/children8111039
APA StyleGu, M., & Kong, H.-H. (2021). Improvement in Fine Manual Dexterity in Children with Spinal Muscular Atrophy Type 2 after Nusinersen Injection: A Case Series. Children, 8(11), 1039. https://doi.org/10.3390/children8111039