Adverse Events of Mind-Body Interventions in Children: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Selection
2.3. Data Extraction
2.4. Data Synthesis
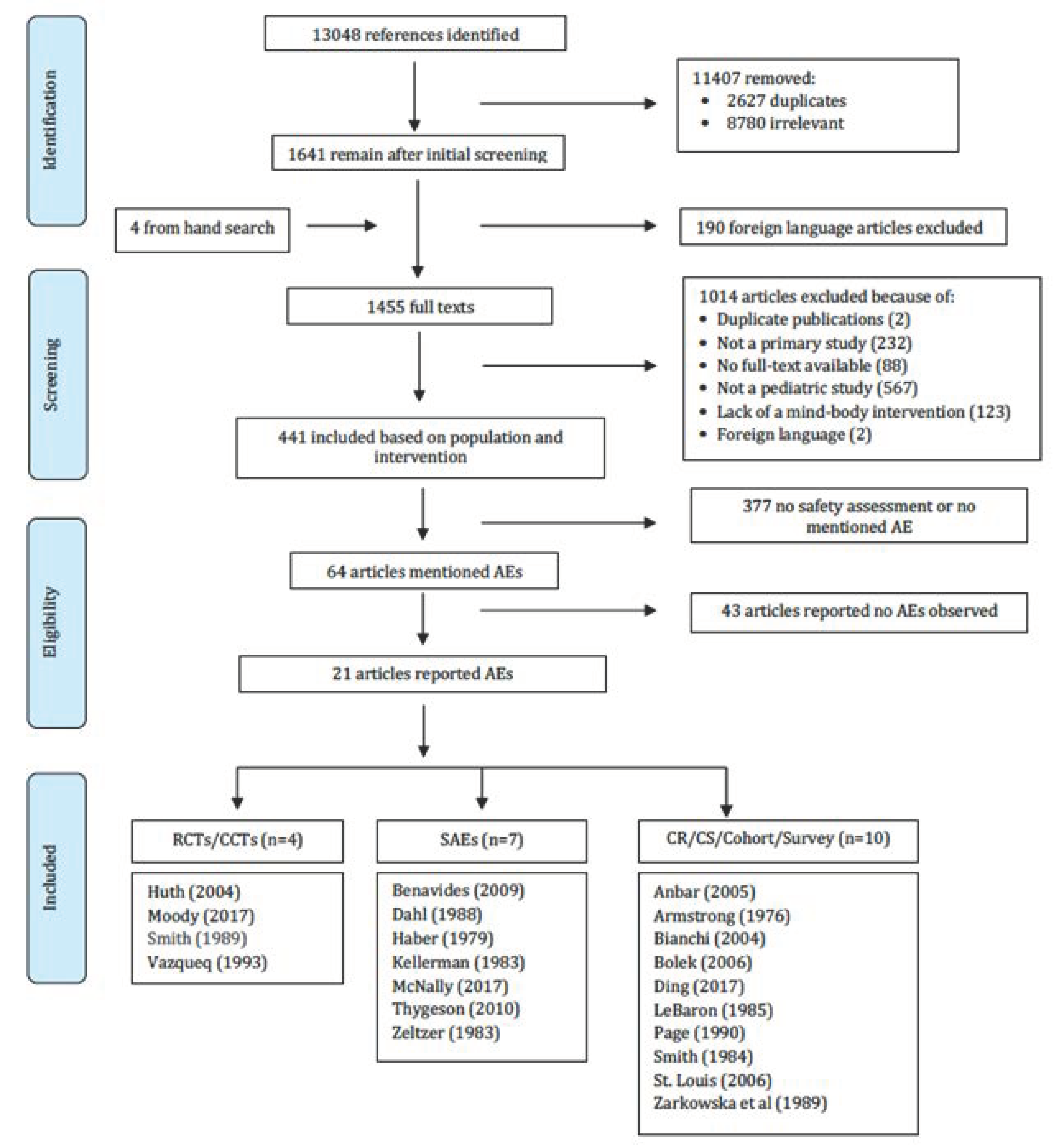
3. Results
3.1. Adverse Events
3.2. Adverse Events of Pediatric Mind-Body Interventions by Severity
3.2.1. Grade 3
3.2.2. Grade 2
3.2.3. Grade 1
3.3. Unclear Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| 1. Safety/or patient safety/ |
| 2. Drug Toxicity/ |
| 3. Adverse Effects.mp. |
| 4. Adverse Event.mp. |
| 5. Medication Side Effects.mp. |
| 6. Risk benefit analysis.mp. |
| 7. Risk/ |
| 8. Causality/ |
| 9. Safe$.mp. |
| 10. Adverse.m_titl. |
| 11. (Advers* effect or advers* affect).mp. |
| 12. ((Side or Advers$) adj3 (effect$ or affect$ or reaction$ or events$)).tw. |
| 13. $etiolog$.mp. |
| 14. Harm$.m_titl. |
| 15. Risks$.m_titl. |
| 16. Significant event.tw. |
| 17. Toxicity.ti. |
| 18. Consequence$.tw. |
| 19. Complication$.tw. |
| 20. Injury.tw. |
| 21. Incident$.tw. |
| 22. Therapeutic safet$.tw. |
| 23. Symptom$.tw. |
| 24. (ae or to or co).fs |
| 25. Or/1-24 |
| 26. Meditation/ |
| 27. Relaxation Therapy/ |
| 28. Biofeedback, Psychology/ |
| 29. Yoga/ |
| 30. Breathing Exercises/ |
| 31. “Imagery (Psychotherapy)”/ |
| 32. Hypnosis/ |
| 33. Tai Ji/ |
| 34. Qi gong.mp. |
| 35. Biofeedback.ti,ab. |
| 36. Creative arts therapies.mp. |
| 37. Deep breathing exercises.mp. |
| 38. Guided imagery.mp. |
| 39. Hypnotherapy.mp. |
| 40. Mantra meditation.mp. |
| 41. MBSR.mp. |
| 42. Mindfullness-based stress reduction.mp. |
| 43. Mediation.ti,ab. |
| 44. Relaxation techniques.mp. |
| 45. Tai Chi.mp. |
| 46. Tai Ji.mp. |
| 47. Yoga.ti,ab. |
| 48. Or/26-47 |
| 49. 25 and 48 |
Appendix B
| Intervention | Number of Studies |
|---|---|
| Biofeedback | 180 |
| Breathing Exercise | 10 |
| Healing Touch | 2 |
| Hypnosis | 82 |
| Imagery | 13 |
| Massage | 1 |
| Meditation | 11 |
| Mindfulness/MBSR | 14 |
| Music Therapy | 2 |
| Relaxation | 36 |
| Qi Gong | 2 |
| Tai Chi | 2 |
| Yoga | 27 |
| Multiple Interventions | 59 |
| Total | 441 |
Appendix C
| Biofeedback | A technique that uses simple electronic devices to teach clients how to consciously regulate bodily functions such as breathing, heart rate, and blood pressure, to improve overall health. |
| Breathing Exercises | An active process that involves conscious control over breathing in and out. This may involve controlling the way in which air is drawn in, the rate, the depth, and the control of other body parts. |
| Hypnosis | An altered state of consciousness characterized by increased responsiveness to suggestion. This hypnotic state is attained by first relaxing the body, then shifting attention toward a narrow range of suggested objects or ideas. |
| Imagery | Used for healing or health maintenance and involves a series of relaxation techniques followed by the visualization of detailed images, usually calm and peaceful in nature. |
| Massage | Therapists manipulate muscle and connective tissue to enhance function of those tissues and promote relaxation and wellbeing. |
| Meditation | A group of techniques, most of which started in Eastern religious or spiritual traditions. In meditation, individuals learn to focus their attention and suspend the stream of thoughts that normally occupy the mind. |
| Relaxation | A technique used to relieve tension and stress by systematically tensing and relaxing successive muscle groups. |
| Qi Gong | An ancient Chinese discipline combining the use of gentle physical movements, mental focus, and deep breathing directed toward specific parts of the body. |
| Tai Chi | A mind-body practice that originated in China as a martial art. Individuals doing tai chi move their bodies slowly and gently, while breathing deeply and meditating |
| Yoga | A combination of breathing exercises, physical postures, and meditation to calm the nervous system and balance the body, mind, and spirit. |
References
- National Institutes of Health. The Science of Mind and Body Therapies. 2016. Available online: https://nccih.nih.gov/video/series/mindbody (accessed on 28 December 2016).
- Data Resource Center for Child & Adolescents Health. Prevalence of Complementary Alternative Medicine Use. 2016. Available online: http://childhealthdata.org/browse./survey/results?q=2856&r=1# (accessed on 14 October 2016).
- Section on Integrative Medicine: Mind-Body Therapies in Children and Youth. Pediatrics 2016, 138, e20161896. [CrossRef] [Green Version]
- Feeney, K.; Moser, C.S. Yoga in Pediatrics. J. Occup. Ther. Sch. Early Interv. 2014, 7, 161–171. [Google Scholar] [CrossRef]
- Kanitz, J.L.; Camus, M.E.; Seifert, G. Keeping the balance—an overview of mind-body therapies in pediatric oncolo-gy. Complem. Ther. Med. 2013, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Black, L.I.; Clarke, T.C.; Barnes, P.M.; Stussman, B.J.; Nahin, R.L. Use of complementary health approaches among children aged 4–17 years in the United States: National Health Interview Survey, 2007. Natl. Health Stat. Rep. 2015, 78, 1–19. [Google Scholar]
- McClafferty, H. Mind-Body Medicine in Pediatrics. Children 2017, 4, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, R.; Aronson, N.; Atkins, D.; Ismaila, A.S.; Santaguida, P.; Smith, D.H.; Whitlock, E.; Wilt, T.J.; Moher, D. AHRQ Series Paper 4: Assessing harms when comparing medical interventions: AHRQ and the Effective Health-Care Program. J. Clin. Epidemiol. 2010, 63, 502–512. [Google Scholar] [CrossRef]
- CTCAE. Common Terminology Criteria for Adverse Events; Version 4. 2010. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_ (accessed on 14 October 2016).
- Singh, S.; Loke, Y.K. Drug safety assessment in clinical trials: Methodological challenges and opportunities. Trials 2012, 13, 138. [Google Scholar] [CrossRef] [Green Version]
- Ioannidis, J.P. Adverse events in randomized trials: Neglected, restricted, distorted, and silenced. Arch. Intern. Med. 2009, 169, 1737–1739. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, P.M. External validity of randomised controlled trials: “to whom do the results of this trial apply? Lancet 2005, 365, 82–93. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Evans, S.J.; Gøtzsche, P.C.; O’Neill, R.T.; Altman, D.G.; Schulz, K.; Moher, D. Better Reporting of Harms in Randomized Trials: An Extension of the CONSORT Statement. Ann. Intern. Med. 2004, 141, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P. Benefits and harms of drug treatments. BMJ 2004, 329, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Database of Systematic Reviews; John Wiley & Sons: Oxford, UK, 2019; Volume 10, p. ED000142. [Google Scholar]
- Zorzela, L.; Golder, S.; Liu, Y.; Pilkington, K.; Hartling, L.; Joffe, A.; Loke, Y.; Vohra, S. Quality of reporting in systematic reviews of adverse events: Systematic review. BMJ 2013, 348, f7668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Bolek, J.E. Use of Multiple-Site Performance-Contingent SEMG Reward Programming in Pediatric Rehabilitation: A Retrospective Review. Appl. Psychophysiol. Biofeedback 2006, 31, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Melin, L.; Leissner, P. Effects of a Behavioral Intervention on Epileptic Seizure Behavior and Paroxysmal Activity: A Systematic Replication of Three Cases of Children with Intractable Epilepsy. Epilepsia 1988, 29, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Anbar, R.D.; Savedoff, A.D. Hypnosis-associated blue-tinted vision: A case report. BMC Ophthalmol. 2005, 5, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haber, C.H.; Nitkin, R.; Shenker, I.R. Adverse reactions to hypnotherapy in obese adolescents: A developmental viewpoint. Psychiatr. Q. 1979, 51, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, J.; Zeltzer, L.; Ellenberg, L.; Dash, J. Adolescents with cancer. J. Adolesc. Health Care 1983, 4, 85–90. [Google Scholar] [CrossRef]
- LeBaron, S.; Zeltzer, L. Hypnosis for hemophiliacs: Methodologic problems and risks. Am. J. Pediatr. Hematol. Oncol. 1985, 7, 316–319. [Google Scholar]
- Page, R.A.; Handley, G.W. Psychogenic and Physiological Sequelae to Hypnosis: Two Case Reports. Am. J. Clin. Hypn. 1990, 32, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; Kamitsuka, M. Self-Hypnosis Misinterpreted as CNS Deterioration in an Adolescent with Leukemia and Vincristine Toxicity. Am. J. Clin. Hypn. 1984, 26, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Zeltzer, L.; Kellerman, J.; Ellenberg, L.; Dash, J. Hypnosis for reduction of vomiting associated with chemotherapy and disease in adolescents with cancer. J. Adolesc. Health Care 1983, 4, 77–84. [Google Scholar] [CrossRef]
- Huth, M.M.; Broome, M.E.; Good, M. Imagery reduces children’s post-operative pain. Pain 2004, 110, 439–448. [Google Scholar] [CrossRef] [PubMed]
- St Louis, E.K.; Lansky, E.P. Meditation and epilepsy: A still hung jury. Med. Hypothes. 2006, 67, 247–250. [Google Scholar] [CrossRef]
- McNally, K.A.; Patrick, K.E.; LaFleur, J.E.; Dykstra, J.B.; Monahan, K.; Hoskinson, K.R. Brief cognitive behavioral intervention for children and adolescents with persistent post-concussive symptoms: A pilot study. Child. Neuropsychol. 2017, 24, 396–412. [Google Scholar] [CrossRef]
- Zarkowska, E.; Crawley, B.; Locke, J. A behavioural intervention for Gilles de la Tourette syndrome in a severely mentally handicapped girl. J. Intellect. Disabil. Res. 2008, 33, 245–253. [Google Scholar] [CrossRef]
- Benavides, S.; Caballero, J. Ashtanga yoga for children and adolescents for weight management and psychological well being: An uncontrolled open pilot study. Complement. Ther. Clin. Pr. 2009, 15, 110–114. [Google Scholar] [CrossRef]
- Bianchi, G.; Cavenago, C.; Marchese, M. Can the practice of yoga be dangerous? Considerations over a case of epiphyseal separation of the distal tibia in a teenager. J. Orthop. Traumatol. 2004, 5, 188–190. [Google Scholar] [CrossRef]
- Moody, K.; Abrahams, B.; Baker, R.; Santizo, R.; Manwani, D.; Carullo, V.; Eugenio, D.; Carroll, A. A Randomized Trial of Yoga for Children Hospitalized with Sickle Cell Vaso-Occlusive Crisis. J. Pain Symptom Manag. 2017, 53, 1026–1034. [Google Scholar] [CrossRef] [Green Version]
- Thygeson, M.V.; Hooke, M.C.; Clapsaddle, J.; Robbins, A.; Moquist, K. Peaceful Play Yoga: Serenity and Balance for Children With Cancer and Their Parents. J. Pediatr. Oncol. Nurs. 2010, 27, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.H.; Hendrix, M.; Hendrix, E. Character Defenses and Biofeedback. Psychother. Psychosom. 1976, 27, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.S.; Doroshow, C.; Womack, W.M.; Tenckhoff, L.; Stamm, S.; Pertik, M. Symptomatic mitral valve prolapse in children and adolescents: Catecholamines, anxiety, and biofeedback. Pediatrics 1989, 84, 290. [Google Scholar] [PubMed]
- Vazquez, M.I.; Buceta, J.M. Effectiveness of self-management programmes and relaxation training in the treat-ment of bronchial asthma: Relationships with trait anxiety and emotional attack triggers. J. Psychosom. Res. 1993, 37, 71–81. [Google Scholar] [CrossRef]
- Ding, J.-L.; Taylor, D.M.; Lee, M.; Johnson, O.G.; Ashok, A.; Griffiths, M.; Simma, L.; Craig, S.S.; Cheek, J.A.; Babl, F.E. Observational study of alternative therapies among paediatric emergency department patients. Emerg. Med. Australas. 2017, 29, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Ling, M.; Hill, B.; Rinehart, N.; Austin, D.; Sciberras, E. Systematic review of meditation-based interventions for children with ADHD. Eur. Child. Adolesc. Psychiatry 2017, 27, 9–27. [Google Scholar] [CrossRef]
- Novak, I.; McIntyre, S.; Morgan, C.; Campbell, L.; Dark, L.; Morton, N.; Stumbles, E.; Wilson, S.-A.; Goldsmith, S. A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev. Med. Child. Neurol. 2013, 55, 885–910. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Mu, P.-F.; Jou, S.-T.; Wong, T.-T.; Chen, Y.-C. Systematic Review and Meta-Analysis of Nonpharmacological Interventions for Fatigue in Children and Adolescents with Cancer. Worldviews Evid. Based Nurs. 2013, 10, 208–217. [Google Scholar] [CrossRef]
- Posadzki, P.; Lewandowski, W.; Terry, R.; Ernst, E.; Stearns, A. Guided Imagery for Non-Musculoskeletal Pain: A Systematic Review of Randomized Clinical Trials. J. Pain Symptom Manag. 2012, 44, 95–104. [Google Scholar] [CrossRef]
- Birdee, G.S.; Yeh, G.Y.; Wayne, P.M.; Phillips, R.S.; Davis, R.B.; Gardiner, P. Clinical Applications of Yoga for the Pediatric Population: A Systematic Review. Acad. Pediatr. 2009, 9, 212–220.e9. [Google Scholar] [CrossRef] [Green Version]
- Chambers, C.T.; Taddio, A.; Uman, L.S.; McMurtry, C. Psychological interventions for reducing pain and distress during routine childhood immunizations: A systematic review. Clin. Ther. 2009, 31, S77–S103. [Google Scholar] [CrossRef] [PubMed]
- Galantino, M.L.; Galbavy, R.; Quinn, L. Therapeutic Effects of Yoga for Children: A Systematic Review of the Literature. Pediatr. Phys. Ther. 2008, 20, 66–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, J.; Smith, J.E.; McCall, G.; Pilkington, K. Hypnosis for Procedure-Related Pain and Distress in Pediatric Cancer Patients: A Systematic Review of Effectiveness and Methodology Related to Hypnosis Interventions. J. Pain Symptom Manag. 2006, 31, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Brazzelli, M.; Griffiths, P.V.; Cody, J.D.; Tappin, D. Behavioural and cognitive interventions with or without other treatments for the management of faecal incontinence in children. Cochrane Database Syst. Rev. 2011, 2011, CD002240. [Google Scholar] [CrossRef]
- Fisher, E.; Law, E.; Dudeney, J.; Palermo, T.M.; Stewart, G.; Eccleston, C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst. Rev. 2018, 9, CD003968. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.Z.; Brignall, M.; Hamilton, M.; Beardsley, J.; Batson, R.D.; Hawrelak, J.; Lichtenstein, B.; Johnston, B.C. Biofeedback for treatment of irritable bowel syndrome. Cochrane Database Syst. Rev. 2019, 2019, 012530. [Google Scholar] [CrossRef] [PubMed]
- Buckley, B.S.; Sanders, C.D.; Spineli, L.; Deng, Q.; Kwong, J.S. Conservative interventions for treating functional daytime urinary incontinence in children. Cochrane Database Syst. Rev. 2019, 2019, CD012367. [Google Scholar] [CrossRef]
- Sureshkumar, P.; Bower, W.; Craig, J.C.; Knight, J.F. Treatment of daytime urinary incontinence in children: A sys-tematic review of randomized controlled trials. J. Urol. 2003, 170, 196–200. [Google Scholar] [CrossRef]
- Ernst, E. Serious adverse effects of unconventional therapies for children and adolescents: A systematic review of recent evidence. Eur. J. Nucl. Med. Mol. Imaging 2003, 162, 72–80. [Google Scholar] [CrossRef]
- Rutten, J.M.T.M.; Korterink, J.J.; Venmans, L.M.A.J.; Benninga, M.A.; Tabbers, M.M. Nonpharmacologic Treatment of Functional Abdominal Pain Disorders: A Systematic Review. Pediatrics 2015, 135, 522–535. [Google Scholar] [CrossRef] [Green Version]
- Fisher, B.E.; Heathcote, B.L.; Palermo, T.M.; de Williams, A.C.C.; Lau, B.J.; Eccleston, B.C. Systematic Review and Meta-Analysis of Psychological Therapies for Children with Chronic Pain. J. Pediatr. Psychol. 2014, 39, 763–782. [Google Scholar] [CrossRef] [Green Version]
- Vohra, S.; Johnston, B.C.; Cramer, K.; Humphreys, K. Adverse Events Associated with Pediatric Spinal Manipulation: A Systematic Review. Pediatrics 2007, 119, e275–e283. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Cranswick, N.; South, M. Adverse events associated with the use of complementary and alternative medicine in children. Arch. Dis. Child. 2010, 96, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Astin, J.A.; Shapiro, S.L.; Eisenberg, D.M.; Forys, K.L. Mind-Body Medicine: State of the Science, Implications for Practice. J. Am. Board Fam. Med. 2003, 16, 131–147. [Google Scholar] [CrossRef]
- Tsang, R.; Colley, L.; Lynd, L.D. Inadequate statistical power to detect clinically significant differences in adverse event rates in randomized controlled trials. J. Clin. Epidemiol. 2009, 62, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Biegel, G.M.; Brown, K.W.; Shapiro, S.L.; Schubert, C.M. Mindfulness-based stress reduction for the treatment of adolescent psychiatric outpatients: A randomized clinical trial. J. Consult. Clin. Psychol. 2009, 77, 855–866. [Google Scholar] [CrossRef] [Green Version]
- Joyce, A.; Etty-Leal, J.; Zazryn, T.; Hamilton, A. Exploring a Mindfulness Meditation Program on the Mental Health of Upper Primary Children: A Pilot Study. Adv. Sch. Ment. Health Promot. 2010, 3, 17–25. [Google Scholar] [CrossRef]
- Napoli, M.; Krech, P.R.; Holley, L.C. Mindfulness Training for Elementary School Students. J. Appl. Sch. Psychol. 2005, 21, 99–125. [Google Scholar] [CrossRef]
- Zenner, C.; Herrnleben-Kurz, S.; Walach, H. Mindfulness-based interventions in schools—a systematic review and meta-analysis. Front. Psychol. 2014, 5, 603. [Google Scholar] [CrossRef] [Green Version]
- Shekelle, P.G.; Morton, S.C.; Suttorp, M.J.; Buscemi, N.; Friesen, C. Challenges in Systematic Reviews of Complementary and Alternative Medicine Topics. Ann. Intern. Med. 2005, 142, 1042–1047. [Google Scholar] [CrossRef]
- Busse, J.W.; Bruno, P.; Malik, K.; Connell, G.; Torrance, D.; Ngo, T.; Kirmayr, K.; Avrahami, D.; Riva, J.J.; Ebrahim, S.; et al. An efficient strategy allowed English-speaking reviewers to identify foreign-language articles eligible for a systematic review. J. Clin. Epidemiol. 2014, 67, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Zorzela, L.; Loke, Y.K.; Ioannidis, J.P.; Golder, S.; Santaguida, P.; Altman, D.G.; Moher, D.; Vohra, S.; PRISMA Harms Group. PRISMA harms checklist: Improving harms reporting in systematic reviews. BMJ 2016, 352, i157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ioannidis, J.P.A.; Lau, J. Completeness of Safety Reporting in Randomized Trials. JAMA 2001, 285, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.K.; Derry, S. Reporting of adverse drug reactions in randomised controlled trials—a systematic survey. BMC Clin. Pharmacol. 2001, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loke, Y.K.; Price, D.; Herxheimer, A. Cochrane Adverse Effects Methods Group. Systematic reviews of adverse ef-fects: Framework for a structured approach. BMC Med. Res. Methodol. 2007, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Basch, E.; Reeve, B.B.; Mitchell, S.A.; Clauser, S.B.; Minasian, L.M.; Dueck, A.C.; Mendoza, T.R.; Hay, J.; Atkinson, T.M.; Abernethy, A.P.; et al. Development of the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). J. Natl. Cancer Inst. 2014, 106, dju244. [Google Scholar] [CrossRef]
- Mason, K.P.; Green, S.M.; Piacevoli, Q. Adverse event reporting tool to standardize the reporting and tracking of adverse events during procedural sedation: A consensus document from the World SIVA International Sedation Task Force. Br. J. Anaesth. 2012, 108, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Sparks, E.; Zorzela, L.; Necyk, C.; Khamba, B.; Urichuk, L.; Barnes, J.; Vohra, S. Study of Natural products Adverse Reactions (SONAR) in children seen in mental health clinics: A cross-sectional study. BMJ Paediatr. Open 2020, 4, e000674. [Google Scholar] [CrossRef]
- Zorzela, L.; Boon, H.; Mior, S.; Yager, J.; Gross, A.; Vohra, S. Serious adverse events associated with pediatric complementary and alternative medicine. Eur. J. Integr. Med. 2014, 6, 467–472. [Google Scholar] [CrossRef]
- Zorzela, L.; Mior, S.; Boon, H.; Gross, A.; Yager, J.; Carter, R.; Vohra, S. Tool to assess causality of direct and indirect adverse events associated with therapeutic interventions. Curr. Med. Res. Opin. 2017, 34, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Gluud, L.L. Bias in Clinical Intervention Research. Am. J. Epidemiol. 2006, 163, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| MB Intervention | First Author, Year, Country | Study Design | # of Participants | Age Mean (SD), Range | Sex (% Male) | Reason for Seeking Treatment | MB Provider | Frequency and Length of MB Therapy | Limitation(s) |
|---|---|---|---|---|---|---|---|---|---|
| Biofeedback | |||||||||
| Bolek (2006), USA [19] | Retrospective cohort | 16 | 8.31 (5.15), 4–18 | NR | To improve motor control (e.g., standing, sitting, head control, etc.) | Therapist | Patient-specific planning | No concurrent control group | |
| Dahl (1988), USA [20] | SAE | 3 | 14 (1.7), 12–15 | 66% | To treat frequent refractory epileptic seizures | Psychologist | Patient-specific treatment | No concurrent control group, poor representation of the population | |
| Hypnosis | |||||||||
| Anbar (2005), UK [21] | Case report | 1 | 15 | 100% | To improve adherence to cystic fibrosis therapy | Physician | On a daily basis for 7 years | Individual anecdotal report of AE | |
| Haber (1979), USA [22] | SAE | 8 | 15.44 (1.55), 13–17 | 50% | To treat resistant obesity (to decrease food consumption) | NR | NR | No concurrent control group, poor representation of the population | |
| Kellerman (1983), US [23] | SAE | 16 | 14.0 (1.6) | 44% | To ameliorate discomfort and anxiety in adolescents with cancer | Pediatricians and psychologists | Training session and during procedure | No concurrent control group | |
| LeBaron (1985), US [24] | Case report | 1 | 18 | 100% | To reduce pain, codeine usage, and bleeding associated with hemophilia | NR | 5 months | Individual report of AE | |
| Page (1990), US [25] | Case series | 2 | 18 (0) | 50% | Nonclinical study volunteers | NR | NR | Individual reports of AE | |
| Smith (1984), US [26] | Case report | 1 | 13 | 0% | To reduce procedural anxiety, muscle contraction, and headaches | Therapist | Utilized twice daily 4 days prior to hospitalization | Individual report of AE | |
| Zeltzer (1983), US [27] | SAE | 9 | 14.2 (3.3), 10–20 | 58% | To reduce chemotherapy side effects (e.g., vomiting) in cancer patients | Psychologist | 1–3 sessions prior to and during chemotherapy | No concurrent control group, different level of acceptance of hypnosis amongst participants | |
| Imagery | |||||||||
| Huth (2004), Netherlands [28] | RCT | 36 (treatment) | 9.42 (1.74), 6–12 | 44% | To reduce pain in tonsillectomy/adenoidectomy | Investigator | 2–22 days prior to surgery and post operatively | Potential for children to over-report to please investigator, inability to provide sham treatment, inability to control pre-test pain equivalency | |
| Meditation | |||||||||
| St Louis (2006), UK [29] | Case report | 1 | 18 | 0% | Practicing transcendental meditation since childhood | NR | Not clear but practicing since childhood | Individual report of AE | |
| Relaxation | |||||||||
| McNally (2018), USA [30] | SAE | 26 (completers) | 15.9 (2) | 32% | To treat persistent post-concussive symptoms | Psychologist | 2–5 sessions (45–60 min duration each) | No concurrent control group, findings may not be generalizable to other clinical concussion populations | |
| Zarkowska (1989), UK [31] | Case report | 1 | 13 | 0% | To treat Tourette Syndrome in a cognitively delayed child | NR | Individual-specific schedule | Individual report of AE | |
| Yoga | |||||||||
| Benavides (2009), UK [32] | SAE | 14 | 11.7 (1.5), 8.8–14.7 | 21% | Weight management and to improve self-concept/psychiatric symptoms | Yoga instructor | 3 days/week for 12 weeks, 75 min sessions | Small sample size, lack of control, unable to fully evaluate long-term outcomes | |
| Bianchi (2004), Italy [33] | Case report | 1 | 14 | 0% | Yoga in physical education class | Therapist | Once | Individual report of AE | |
| Moody (2017), USA [34] | RCT | 35 (treatment) | NR, 6–20 | 40% | Sickle cell disease vaso-occlusive crises | Yoga instructor | Daily 30 min sessions, average 2.5 (1.6) sessions total | Randomization not blinded, small sample size, limited number of yoga sessions, single institution | |
| Thygeson (2010), US [35] | SAE | 16 | 8.5 (1.75), 7–12; 15.4 (1.82), 13–18 | 63% | To reduce distress associated with diagnoses on hematology/oncology unit | Registered yoga teacher | Single yoga session | Recruitment issues (selection bias) due to lack of yoga experience among participants and parents | |
| Multiple | |||||||||
| Biofeedback and progre-ssive muscle relaxation | Armstrong (1976), USA [36] | Case report | 1 | 17 | 100% | Tension headaches | Therapist | NR | Individual report of AE |
| Biofeedback and Relaxation/Imagery | Smith (1989), US [37] | RCT | 20 (treatment) | NR, 9–18 | NR | To ameliorate symptoms of mitral valve prolapse (e.g., chest pain, fatigue, etc.) | NR | 8 sessions (40 minutes) + twice daily practice (15 minutes) | Small sample size, inadequate duration of treatment, lack of compliance in home practice |
| Self-management, progressive relaxation | Vazquez (1993), UK [38] | CCT | 9 (treatment) | 10.81 (NR), 8–13 | 70% | To treat bronchial asthma | NR | 6 weekly one hour sessions | Small sample size, patient heterogeneity may confound relationship between intervention and outcome |
| Various MB therapies | Ding (2017), AUS [39] | Cross-Sectional Survey | 381 | NR, 0–18 | 52% | Various, aimed to determine 12 month prevalence/nature of alternative therapy use in pediatric patients | NR | NR | Observational study Minimal details of AEs |
| First Author (Year), Country | MB Practice | # of AE (s) | Age/Sex | # of Study Partici-Pants | AE Description | Timing of AE | Outcome of AE | Results/Conclusion by Authors |
|---|---|---|---|---|---|---|---|---|
| Severity Grade 3 | ||||||||
| Bianchi (2004), Italy [33] | Yoga | 1 | 14F | 1 | Fracture of distal tibia | While attempting to assume “lotus” yoga position | Resolved with standard leg immobilization, casting, and rehabilitation | Yoga can result in severe damage in adolescents due to age and open growth plates |
| LeBaron (1985), US [24] | Hypnosis | 1 | 18M | 1 | Spontaneous intra-abdominal bleed | A few hours after administration of hypnotic scale | Resolved by hematologist treatment | Physiological effects of hypnosis in hemophilia population is unknown and potential risk may exist |
| Smith (1984), US [26] | Self-hypnosis | 1 | 13F | 1 | Self-hypnosis misinterpreted as CNS deterioration in ALL case | Four days after learning self-hypnosis | Resolved with therapist’s help, returned to stable/alert state | Self-hypnosis needs a conscientious practice of the technique and appropriate communication with others |
| Severity Grade 2 | ||||||||
| Armstrong (1976), US [36] | Biofeedback and prog-ressive muscle relaxation | 1 | 17M | 1 | Depression and unavailability from therapeutic engagement | Post-intervention | NR | Removal of patient’s somatic complaint eliminated the only channel open to therapeutic engagement |
| Page (1990), US [25] | Hypnosis | 1 | 18F | 1 | Apparent epileptic seizure | While in the hypnotic state | Resolved, normal EEG post event | Pre-induction precautions, omitting references to after effects, and careful observation during hypnosis suggested |
| St. Louis (2006), UK [29] | Transcendental Meditation | 1 | 18F | 1 | Temporal lobe epilepsy (4 “spells” in a year and 3 generalized tonic-colonic seizures) | Following sleep deprivation and missed medication doses | Became seizure free for 6 months with medication and continued meditation practice | Further retrospective and prospective studies needed to determine whether meditation can precipitate epilepsy |
| Severity Grade 1 | ||||||||
| Anbar (2005), UK [21] | Self-hypnosis | 1 | 15M | 1 | Blue-tinted vision and concurrent penile erection | Half of the times therapy utilized | Continued to occur with self-hypnosis | Controlled studies with biological measurement of retinal blood flow after self-hypnosis may determine cause of blue-tinted vision |
| Bolek (2006), US [19] | Biofeedback | 2 | 13F, 13M | 16 | Anxiety (n = 1) and foot pain (n = 1) due to weight issues on standing | During therapy | Anxiety improved with distraction by program’s video; discontinued therapy | Surface electromyography helps improve motor performance in treatment resistant children |
| Dahl (1988), US [20] | Biofeedback | 2 | NR/NR | 3 | Anxiety when aware of early seizure signals | During therapy | NR | Biofeedback reduced refractory seizure behaviour and paroxysmal EEG activity |
| Haber (1979), US [22] | Hypnosis | 3 | 14M, 14M, 17M | 8 | Dissociated state (n = 1), depersonalization and anxiety (n = 1), increased anxiety (n = 1) | During therapy and post-hypnosis | Resolved with discontinuation and counseling | Hypnosis may have associated adverse events and did not appear to have any advantages over other therapeutic options |
| Huth (2004), Netherlands [28] | Imagery | 2 | NR/2M | 36 | Distress (n = 1), physical shaking (n = 1) | In anticipation of therapy; during therapy | Withdrew from the study | Imagery is associated with a reduction in post-operative pain and anxiety |
| Kellerman (1983), US [23] | Hypnosis | 1 | NR/1M | 16 | Feeling uncomfortable while practicing hypnosis | During therapy | Declined further treatment | Hypnosis has value in reducing procedural associated anxiety and discomfort in adolescent cancer patients |
| Page (1990), US [25] | Hypnosis | 1 | 18M | 1 | Retroactive amnesia; unable to recall phone numbers | ~100 minutes following hypnosis | Resolved by looking at numbers again, no further retroactive amnesia experienced | Suggest that therapists employ careful observation during their routine |
| Smith (1989), US [37] | Biofeedback, imagery, relaxation | 1 | NR/NR | 20 | Increased chest pain | Post-therapy | NR | Chest pain decreased at 6 months in mitral valve prolapse with biofeedback and relaxation/imagery treatment |
| Thygeson (2010), US [35] | Yoga | 1 | NR/NR | 16 | Dizziness | During yoga | Withdrew from study | Yoga is a feasible intervention and beneficial to adolescent patients and parents |
| Vazquez (1993), UK [38] | Progressive muscle relaxation | 4 | NR/NR | 9 | Increased drug consumption in emotionally-triggered asthma | During therapy | NR | Relaxation was found to be effective in emotionally-triggered asthma |
| Zarkowska (1989), UK [31] | Cue-controlled relaxation training | 1 | 13F | 1 | Increased tic frequency from baseline | Post-intervention | Resolved with a trial of medication | Relaxation failed to reduce tic frequency |
| Zeltzer (1983), US [27] | Hypnosis | 1 | 13M | 9 | Physical discomfort | During therapy | Discontinued therapy | The results support the efficacy of hypnosis as a means of reducing emesis |
| Unclassified | ||||||||
| Benavides (2009), UK [32] | Ashtanga yoga | 4 | NR/NR | 14 | Lower self-esteem (n = 2), Increased depression symptoms (n = 2) | Post-intervention | NR | Yoga may represent an alternative for weight loss and provide mental health benefits |
| Ding (2017), AUS [39] | Yoga (n = 2), massage (n = 1), hypno-therapy (n = 1) | 4 | NR/NR | 381 | Hypnotherapy: increased anxiety; NR for other AE | NR | NR | Alternative therapy use is common among pediatric ER patients. Parents who arrange alternative therapy have differing perceptions of its usefulness/safety from those who do not |
| McNally (2018), USA [30] | Relaxation | 1 | NR/NR | 26 | Worsened concussion symptoms | NR | NR | Brief cognitive behavioural intervention a promising treatment for children and adolescents experiencing persistent post-concussive symptoms |
| Moody (2017), USA [34] | Yoga | 2 | NR/NR | 35 | Avascular necrosis (n = 1) Acute splenic sequestration (n = 1) | NR | NR | Yoga is an acceptable, feasible and helpful intervention for hospitalized children with vaso-occlusive crisis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyszczyk, M.; Karkhaneh, M.; Gladwin, K.K.; Funabashi, M.; Zorzela, L.; Vohra, S. Adverse Events of Mind-Body Interventions in Children: A Systematic Review. Children 2021, 8, 358. https://doi.org/10.3390/children8050358
Lyszczyk M, Karkhaneh M, Gladwin KK, Funabashi M, Zorzela L, Vohra S. Adverse Events of Mind-Body Interventions in Children: A Systematic Review. Children. 2021; 8(5):358. https://doi.org/10.3390/children8050358
Chicago/Turabian StyleLyszczyk, Meagan, Mohammad Karkhaneh, Kerri Kaiser Gladwin, Martha Funabashi, Liliane Zorzela, and Sunita Vohra. 2021. "Adverse Events of Mind-Body Interventions in Children: A Systematic Review" Children 8, no. 5: 358. https://doi.org/10.3390/children8050358





