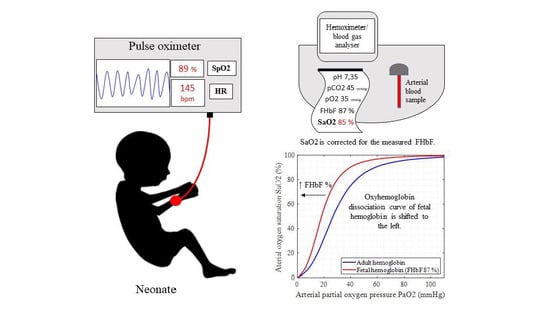
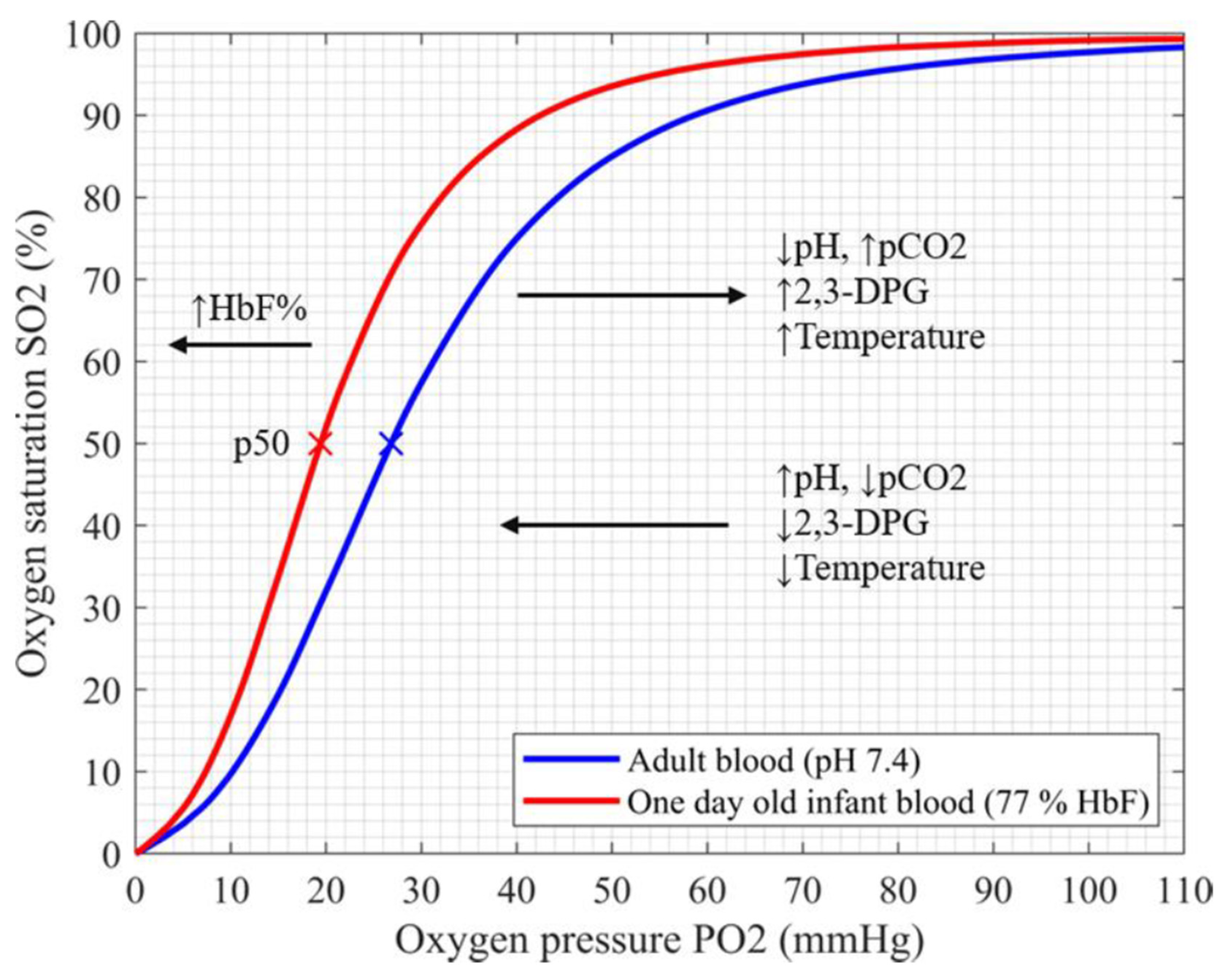
Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Madar, J.; Roehr, C.C.; Ainsworth, S.; Ersdal, H.; Morley, C.; Rüdiger, M.; Skåre, C.; Szczapa, T.; Te Pas, A.; Trevisanuto, D.; et al. European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 2021, 161, 291–326. [Google Scholar] [CrossRef]
- Aziz, K.; Lee, H.C.; Escobedo, M.B.; Hoover, A.V.; Kamath-Rayne, B.D.; Kapadia, V.S.; Magid, D.J.; Niermeyer, S.; Schmölzer, G.M.; Szyld, E.; et al. Part 5: Neonatal Resuscitation 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2021, 147 (Suppl. 1), e2020038505E. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.A.; Kamlin, C.O.F.; Vento, M.; Wong, C.; Cole, T.J.; Donath, S.M.; Davis, P.G.; Morley, C.J. Defining the reference range for oxygen saturation for infants after birth. Pediatrics 2010, 125, e1340–e1347. [Google Scholar] [CrossRef] [PubMed]
- Jennis, M.S.; Peabody, J.L. Pulse oximetry: An alternative method for the assessment of oxygenation in newborn infants. J. Pediatr. 1987, 79, 524–528. [Google Scholar]
- Praud, J.P.; Gaultier, C.L.; Carofilis, A.; Lacaille, F.; Dehan, M.; Bridey, F. Accuracy of two wavelength pulse oximetry in neonates and infants. Pediatr. Pulmonol. 1989, 6, 180–182. [Google Scholar] [CrossRef]
- Rajadurai, V.S.; Walker, A.M.; Yu, V.Y.H.; Oates, A. Effect of fetal hemoglobin on the accuracy of pulse oximetry in preterm infants. J. Paediatr. Child Health 1992, 28, 43–46. [Google Scholar] [CrossRef]
- Ramanathan, R.; Durand, M.; Larrazabal, C. Pulse oximetry in very low birth weight infants with acute and chronic lung injury. Pediatrics 1987, 79, 612–617. [Google Scholar]
- Wukitsch, M.W.; Petterson, M.T.; Tobler, D.R.; Pologe, J.A. Pulse oximetry: Analysis of theory, technology, and practice. J. Clin. Monit. 1988, 4, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices 2014, 7, 231–239. [Google Scholar] [CrossRef]
- Louw, A.; Cracco, C.; Cerf, C.; Harf, A.; Duvaldestin, P.; Lemaire, F.; Brochard, L. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001, 27, 1606–1613. [Google Scholar] [CrossRef]
- Perkins, G.D.; McAuley, D.F.; Giles, S.; Routledge, H.; Gao, F. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit. Care 2003, 7, R67. [Google Scholar] [CrossRef] [PubMed]
- Jubran, A.; Tobin, M.J. Reliability of pulse oximetry in titrating supplemental oxygen therapy in ventilator-dependent patients. Chest 1990, 97, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Newth, C.; Khemani, R. Accuracy of pulse oximetry in children. Pediatrics 2014, 133, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wackernagel, D.; Blennow, M.; Hellström, A. Accuracy of pulse oximetry in preterm and term infants is insufficient to de-termine arterial oxygen saturation and tension. Acta Paediatr. 2020, 109, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Oski, F.A.; Delivoria-Papadopoulos, M. The shift to the left. Pediatrics 1971, 48, 853–856. [Google Scholar] [PubMed]
- Sankaran, V.G.; Orkin, S.H. The Switch From Fetal to Adult Hemoglobin. Cold Spring Harb. Perspect. Med. 2013, 3, a011643. [Google Scholar] [CrossRef]
- Bunn, H.F.; Briehl, R.W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J. Clin. Investig. 1970, 49, 1088–1095. [Google Scholar] [CrossRef]
- Orzalesi, M.M.; Hay, W.W. The regulation of oxygen affinity of fetal blood. I. In vitro experiments and results in normal infants. Pediatrics 1971, 48, 857–864. [Google Scholar]
- Bard, H. Postnatal fetal and adult hemoglobin synthesis in early preterm newborn infants. J. Clin. Investig. 1973, 52, 1789–1795. [Google Scholar] [CrossRef]
- Wilson, K.; Hawken, S.; Murphy, M.S.; Atkinson, K.M.; Potter, B.K.; Sprague, A.; Walker, M.; Chakraborty, P.; Little, J. Postnatal prediction of gestational age using newborn fetal hemoglobin levels. EBioMedicine 2017. [Google Scholar] [CrossRef]
- Cochran-Black, D.L.; Cowan, L.D.; Neas, B.R. The relation between newborn hemoglobin F fractions and risk factors for sudden infant death syndrome. Arch. Pathol. Lab. Med. 2001, 125, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Stutchfield, C.J.; Jain, A.; Odd, D.; Wiliams, C.; Markham, R. Foetal haemoglobin, blood transfusion, and retinopathy of prematurity in very preterm infants: A pilot prospective cohort study. Eye 2017, 31, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Takabe, F.; Maeno, Y.; Iwasa, M. Identification of fetal hemoglobin in blood stains by high performance liquid chro-matography. Z. Rechtsmed. 1989, 102, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Walsh, B.K.; Sittig, S.E.; Restrepo, R.D. AARC Clinical practice guideline: Blood gas analysis and hemoximetry: 2013. Respir. Care 2013, 58, 1694–1703. [Google Scholar] [CrossRef]
- ABL800 FLEX Reference Manual from Software Version 6.00; Code number: 989-963; Radiometer: Copenhagen, Denmark, 2008.
- Zijlstra, W.G.; Buursma, A.; Meeuwsen-van der Roest, W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991, 37, 1633–1638. [Google Scholar] [CrossRef]
- Krzeminski, A. How Is Fetal Hemoglobin Determined and Corrected for in the OSM3, the ABL 510, and the ABL 520? Radiometer: Copenhagen, Denmark, 1992; pp. 1–4. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis-protocols (Prisma P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Shiao, S.Y.P.K. Effects of fetal hemoglobin on accurate measurements of oxygen saturation in neonates. J. Perinat. Neonatal Nurs. 2005, 19, 348–361. [Google Scholar] [CrossRef]
- Shiao, S.Y.P.K.; Ou, C.N.; Pierantoni, H. The measurement of accurate fetal hemoglobin and related oxygen saturation by the hemoximeter. Clin. Chim. Acta 2006, 374, 75–80. [Google Scholar] [CrossRef]
- Shiao, S.Y.; Ou, C.N. Validation of oxygen saturation monitoring in neonates. Am. J. Crit. Care 2007, 16, 168–178. [Google Scholar] [CrossRef]
- Durand, M.; Ramanathan, R. Pulse oximetry for continuous oxygen monitoring in sick newborn infants. J. Pediatr. 1986, 109, 1052–1056. [Google Scholar] [CrossRef]
- Wimberley, P.D.; Helledie, N.R.; Friis-Hansen, B.; Fogh-Andersen, N.; Olesen, H. Pulse oximetry versus transcutaneous pO2 in sick newborn infants. Scand. J. Clin. Lab. Investig. 1987, 188, 19–25. [Google Scholar] [CrossRef]
- Nitzan, I.; Hammerman, C.; Mimouni, F.B.; Bin-Nun, A. Packed red blood cells transfusions in neonates: Effect on FiO2 and PaO2/SaO2 ratio and implications for neonatal saturation targeting. J. Perinatol. 2018, 38, 693–695. [Google Scholar] [CrossRef]
- Cornelissen, P.J.H.; van Woensel, C.L.M.; van Oel, W.C.; de Jong, P.A. Correction factors for hemoglobin derivatives in fetal blood, as measured with the IL 282 Co-oximeter. Clin. Chem. 1983, 29, 1555–1556. [Google Scholar] [CrossRef]
- Émond, D.; Lachance, C.; Gagnon, J.; Bard, H. Arterial partial pressure of oxygen required to achieve 90% saturation of hae-moglobin in very low birth weight newborns. Pediatrics 1993, 91, 602–604. [Google Scholar]
- Bohnhorst, B.; Peter, C.S.; Poets, C.F. Detection of hyperoxaemia in neonates: Data from three new pulse oximeters. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F217–F219. [Google Scholar] [CrossRef] [PubMed]
- Support Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network; Carlo, W.A.; Finer, N.N.; Walsh, M.C.; Rich, W.; Gantz, M.G.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; et al. Target ranges of oxygen saturation in extremely pre-term infants. N. Engl. J. Med. 2010, 362, 1959–1969. [Google Scholar]
- Vaucher, Y.E.; Peralta-Carcelen, M.; Finer, N.N.; Carlo, W.A.; Gantz, M.G.; Walsh, M.C.; Laptook, A.R.; Yoder, B.A.; Faix, R.G.; Das, A.; et al. Neurodevelopmental outcomes in the early CPAP and pulse oximetry trial. N. Engl. J. Med. 2012, 367, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Whyte, R.K.; Asztalos, E.V.; Moddemann, D.; Poets, C.; Rabi, Y.; Solimano, A.; Roberts, R.S.; the Canadian Oxygen Trial (COT) Group. Effects of targeting higher vs lower arterial oxygen saturations on death or disability in extremely preterm infants: A randomized clinical trial. JAMA 2013, 309, 2111–2120. [Google Scholar] [CrossRef]
- Group BIUKC; Group BIAC; Group BINZC; Stenson, B.J.; Tarnow-Mordi, W.O.; Darlow, B.A.; Simes, J.; Juszczak, E.; Askie, L.; Battin, M.; et al. Oxygen saturation and outcomes in preterm infants. N. Engl. J. Med. 2013, 368, 2094–2104. [Google Scholar]
- Darlow, B.A.; Marschner, S.L.; Donoghoe, M.; Battin, M.R.; Broadbent, R.S.; Elder, M.J.; Hewson, M.P.; Meyer, M.P.; Ghadge, A.; Graham, P.; et al. Randomized controlled trial of oxygen saturation targets in very pre-term infants: Two year outcomes. J. Pediatr. 2014, 165, 30–35. [Google Scholar] [CrossRef] [PubMed]
- The BOOST-II Australia and United Kingdom Collaborative Groups; Tarnow-Mordi, W.O.; Stenson, B.J.; Kirby, A.; Juszczak, E.; Donoghoe, M.; Deshpande, S.; Morley, C.; King, A.; Doyle, L.W.; et al. Outcomes of two trials of oxygen-saturation targets in preterm infants. N. Engl. J. Med. 2016, 374, 749–760. [Google Scholar] [PubMed]
- Khadawardi, E.; Al Hazzani, F. Oxygen saturation and outcomes in preterm infants: The BOOST II United Kingdom, Australia, and New Zealand Collaborative Groups. J. Clin. Neonatol. 2013, 2, 73–75. [Google Scholar] [CrossRef]
- Lakshminrusimha, S.; Manja, V.; Mathew, B.; Suresh, G.K. Oxygen targeting in preterm infants: A physiologic interpretation. J. Perinatol. 2015, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Poets, C.F. Noninvasive monitoring and assessment of oxygenation in infants. Clin. Perinatol. 2019, 46, 417–433. [Google Scholar] [CrossRef] [PubMed]

| Ref | 1st Author, Year | Number of Patients/ HbF Blood Samples | Blood Sample Type | HbF Measurement Method | Gestation Distribution (Weeks) | Time of Sample Collection and Non-Invasive Monitoring | Blood Oxygenation Parameters | Blood Gas Analyzer /Hemoximeter | Pulse Oximeter (Company Name) | Additional Bedside Oxygenation Monitoring Device (Company Name) | Relevant Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [32]. | Durand, 1986 | 75/140 | Arterial | Alkali denaturation method | 24–42 | 1–14 days + 30–153 days after birth | paO2, SaO2 | Radiometer BMS3 Mark II / Co-oximeter IL 282 | Nellcor N-100 (Hayward, CA, USA) | tc-pO2 Oxygen electrode (Novametrix, Wallingford, CT, USA) | HbF values of 4.3% to 95% did not influence the accuracy of pulse oximeter readings. |
| [7]. | Ramanathan, 1987 | 68/132 | Arterial | Alkali denaturation method | 25–31 | 1–6 days + 20–80 days after birth | paO2, SaO2 | Radiometer BMS3 Mark II / Co-oximeter IL 282 | Nellcor N-100 (Hayward, CA, USA) | tc-pO2 Oxygen electrode (Novametrix, Wallingford, CT, USA) | HbF values of 4.3% to 92.2% did not influence the accuracy of pulse oximeter readings. |
| [33]. | Wimberley, 1987 | 18/18 | Arterial | Alkali denaturation method | 25–34 | Within 5 days after birth | paO2, SaO2 | ABL300/ Hemoximeter OSM3 | Ohmeda Biox 3700 | tc-pO2 Radiometer TCM3 | FHbF ranged from 44–97%. The variations in the levels of HbF, pH, pCO2 and 2,3-DPG resulted in a variable paO2-SaO2 relation. |
| [4]. | Jennis, 1987 | 26/49 | Arterial | Electrophoresis | 24–40 | 1–49 days after birth | SaO2 | Co-oximeter IL-282 | Nellcor N-100 (Hayward, CA, USA) | NA | FHbF > 50% generated a 2.8% to 3.6% error (underestimation) in SpO2 reading. |
| [5]. | Praud, 1989 | 71/52 | Arterial | Electrophoresis and alkali denaturation method | 25–40 | 1–14 days after birth + 4.5–38 weeks after birth | SaO2 | Hemoximeter OSM2 | Nellcor N-100 (Hayward, CA, USA) | NA | For FHbF < 50% and SaO2 ≤ 95%, SpO2 was overestimated. |
| Ref | 1st Author, Year | Number of Patients/ HbF Blood Samples | Blood Sample Type | HbF Measurement Method | Gestation Distribution (Weeks) | Time of Sample Collection and Non-Invasive Monitoring | Blood Oxygenation Parameters | Blood Gas Analyzer/Hemoximeter | Pulse Oximeter (Company Name) | Additional Bedside Oxygenation Monitoring Device (Company Name) | Relevant Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [6]. | Rajadurai, 1992 | 22/64 | Arterial | Visible absorption spectroscopy (hemoximeter) | 25–36 | 1 h–73 days after birth | Functional SaO2 * | ABL30 Analyzer/ Hemoximeter OSM3 | Nellcor N-100 (Hayward, CA, USA) | NA | Pulse oximeter saturations were unaffected by FHbF values which ranged from 0 to 100%. |
| [29]. | Shiao, 2005 | 20/210 | Arterial and venous | Visible absorption spectroscopy (hemoximeter) | 24–34 | First 5 days after birth | paO2, SaO2, SvO2, HbO2 | Hemoximeter OSM3 | Nellcor NPB 290 (Pleasanton, CA, USA) | NA | Bias of SpO2 vs HbO2 was +1.6% (2SD 5.6) and SpO2 vs SaO2 −0.6% (2SD 5.9). There was no statistical analysis of HbF contribution to the bias. |
| [30]. | Shiao, 2006 | 39/188 | Arterial and venous | Visible absorption spectroscopy (hemoximeter) + HPLC | 25–38 | First 5 days after birth | paO2, SaO2, SvO2, HbO2 | Hemoximeter OSM3 | Nellcor NPB 290 (Tyco Healthcare, Mansfield, MA, USA) | NA | Lower HbF levels after the transfusion resulted in lower SpO2 for the same paO2 range of 50–75 mmHg. There was no statistical analysis of HbF contribution to the SpO2-SaO2 bias. |
| [31]. | Shiao, 2007 | 78/771 | Arterial and venous | Visible absorption spectroscopy (hemoximeter) | 25–38 | First 5 days after birth (every 6–8 h) | paO2, SaO2, HbO2 | Hemoximeter OSM3 | Nellcor (NPB 290, Pleasanton, CA, USA) | SaO2m, SvO2m *** Oximetric 3-wavelength monitors (Abbott, Chicago, IL, USA) | Bias of SpO2 vs HbO2 in arterial blood samples was 2.5% (SD 3.1). There was no statistical analysis of HbF contribution to the SpO2-SaO2 bias. |
| [34]. | Nitzan, 2018 | 14/28 | Arterial | Visible absorption spectroscopy (hemoximeter) | 24–33 | Within 12 h before and after the blood transfusion (first 5 days after birth) | paO2, SaO2 | ABL 90 FLEX | Nellcor (Covidien-Medtronic, Mansfield, MA, USA) | NA | HbF declined significantly after transfusion and FiO2 increased by > 12% to keep SpO2 within the same range. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pritišanac, E.; Urlesberger, B.; Schwaberger, B.; Pichler, G. Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review. Children 2021, 8, 361. https://doi.org/10.3390/children8050361
Pritišanac E, Urlesberger B, Schwaberger B, Pichler G. Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review. Children. 2021; 8(5):361. https://doi.org/10.3390/children8050361
Chicago/Turabian StylePritišanac, Ena, Berndt Urlesberger, Bernhard Schwaberger, and Gerhard Pichler. 2021. "Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review" Children 8, no. 5: 361. https://doi.org/10.3390/children8050361
APA StylePritišanac, E., Urlesberger, B., Schwaberger, B., & Pichler, G. (2021). Accuracy of Pulse Oximetry in the Presence of Fetal Hemoglobin—A Systematic Review. Children, 8(5), 361. https://doi.org/10.3390/children8050361