Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- A.
- Gestational age ≥ 36+0 weeks, ≤6 h of life (HOL) AND
- B.
- Cord/arterial pH ≤ 7.0 OR base excess ≤ −16 in the first sixty minutes of life OR APGAR-Score ≤ 5 AND/OR continued need for resuscitation at 10 min of life (criteria of perinatal asphyxia) AND
- C.
- Evidence of moderate-to-severe encephalopathy [25] OR
- D.
- Abnormalities on amplitude-integrated electroencephalography (aEEG) for at least 20 min or clinical and/or aEEG-defined seizures [26]
2.2. Outcome Definition
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Laptook, A.R.; Pappas, A.; McDonald, S.A.; Das, A.; Tyson, J.E.; Poindexter, B.B.; Schibler, K.; Bell, E.F.; Heyne, R.J.; et al. Effect of Depth and Duration of Cooling on Death or Disability at Age 18 Months Among Neonates With Hypoxic-Ischemic Encephalopathy: A Randomized Clinical Trial. JAMA 2017, 318, 57–67. [Google Scholar] [CrossRef]
- Tan, W.K.M.; Williams, C.E.; During, M.J.; Mallard, C.E.; Gunning, M.I.; Gunn, A.; Gluckman, P.D. Accumulation of Cytotoxins During the Development of Seizures and Edema after Hypoxic-Ischemic Injury in Late Gestation Fetal Sheep. Pediatr. Res. 1996, 39, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.E.; Gunn, A.; Gluckman, P.D. Time course of intracellular edema and epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 1991, 22, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.J.; Gunn, T.R.; De Haan, H.H.; Williams, C.E.; Gluckman, P.D. Dramatic neuronal rescue with prolonged selective head cooling after ischemia in fetal lambs. J. Clin. Investig. 1997, 99, 248–256. [Google Scholar] [CrossRef]
- Jensen, E.C.; Bennet, L.; Hunter, C.J.; Power, G.C.; Gunn, A.J. Post-hypoxic hypoperfusion is associated with suppression of cerebral metabolism and increased tissue oxygenation in near-term fetal sheep. J. Physiol. 2006, 572, 131–139. [Google Scholar] [CrossRef]
- Roth, S.C.; Edwards, A.D.; Cady, E.B.; Delpy, D.T.; Wyatt, J.S.; Azzopardi, D.; Baudin, J.; Townsend, J.; Stewart, A.L.; Reynolds, E.O.R. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev. Med. Child Neurol. 2008, 34, 285–295. [Google Scholar] [CrossRef]
- Roth, S.C.; Baudin, J.; Cady, E.; Johal, K.; Townsend, J.P.; Wyatt, J.S.; Reynolds, E.O.R.; Stewart, A.L. Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome and head circumference at 4 years. Dev. Med. Child Neurol. 2008, 39, 718–725. [Google Scholar] [CrossRef]
- Lorek, A.; Takei, Y.; Cady, E.B.; Wyatt, J.S.; Penrice, J.; Edwards, A.D.; Peebles, D.; Wylezinska, M.; Owen-Reece, H.; Kirkbride, V.; et al. Delayed (“Secondary”) Cerebral Energy Failure after Acute Hypoxia-Ischemia in the Newborn Piglet: Continuous 48-Hour Studies by Phosphorus Magnetic Resonance Spectroscopy. Pediatr. Res. 1994, 36, 699–706. [Google Scholar] [CrossRef]
- Yenari, M.A.; Han, H.S. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat. Rev. Neurosci. 2012, 13, 267–278. [Google Scholar] [CrossRef]
- Wood, T.; Thoresen, M. Physiological responses to hypothermia. Semin. Fetal Neonatal Med. 2015, 20, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Rainaldi, M.A.; Perlman, J.M. Pathophysiology of Birth Asphyxia. Clin. Perinatol. 2016, 43, 409–422. [Google Scholar] [CrossRef]
- Morton, S.U.; Brodsky, D. Fetal Physiology and the Transition to Extrauterine Life. Clin. Perinatol. 2016, 43, 395–407. [Google Scholar] [CrossRef]
- Lapointe, A.; Barrington, K.J. Pulmonary hypertension and the asphyxiated newborn. J. Pediatr. 2011, 158 (Suppl. S2), e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Laporte, M.A.L.; Wang, H.; Sanon, P.-N.; Vargas, S.B.; Maluorni, J.; Rampakakis, E.; Wintermark, P. Association between hypocapnia and ventilation during the first days of life and brain injury in asphyxiated newborns treated with hypothermia. J. Matern. Neonatal Med. 2017, 32, 1312–1320. [Google Scholar] [CrossRef]
- Nadeem, M.; Murray, D.; Boylan, G.; Dempsey, E.M.; Ryan, C.A. Blood Carbon Dioxide Levels and Adverse Outcome in Neonatal Hypoxic-Ischemic Encephalopathy. Am. J. Perinatol. 2009, 27, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Klinger, G.; Beyene, J.; Shah, P.; Perlman, M. Do hyperoxaemia and hypocapnia add to the risk of brain injury after intrapartum asphyxia? Arch. Dis. Child. Fetal Neonatal Ed. 2005, 90, F49–F52. [Google Scholar] [CrossRef]
- Pappas, A.; Shankaran, S.; Laptook, A.R.; Langer, J.C.; Bara, R.; Ehrenkranz, R.A.; Goldberg, R.N.; Das, A.; Higgins, R.D.; Tyson, J.E.; et al. Hypocarbia and Adverse Outcome in Neonatal Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2011, 158, 752–758.e1. [Google Scholar] [CrossRef]
- Lingappan, K.; Kaiser, J.R.; Srinivasan, C.; Gunn, A.; on behalf of the CoolCap Study Group. Relationship between PCO2 and unfavorable outcome in infants with moderate-to-severe hypoxic ischemic encephalopathy. Pediatr. Res. 2016, 80, 204–208. [Google Scholar] [CrossRef]
- Laffey, J.G.; Kavanagh, B.P. Hypocapnia. N. Engl. J. Med. 2002, 347, 43–53. [Google Scholar] [CrossRef]
- Victor, S.; Appleton, R.E.; Beirne, M.; Marson, A.G.; Weindling, A.M. Effect of carbon dioxide on background cerebral electrical activity and fractional oxygen extraction in very low birth weight infants just after birth. Pediatr. Res. 2005, 58, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Pirot, A.L.; Fritz, K.I.; Ashraf, Q.M.; Mishra, O.P.; Delivoria-Papadopoulos, M. Effects of Severe Hypocapnia on Expression of Bax and Bcl-2 Proteins, DNA Fragmentation, and Membrane Peroxidation Products in Cerebral Cortical Mitochondria of Newborn Piglets. Neonatology 2007, 91, 20–27. [Google Scholar] [CrossRef]
- Koch, J.D.; Miles, D.K.; Gilley, J.A.; Yang, C.-P.; Kernie, S.G. Brief Exposure to Hyperoxia Depletes the Glial Progenitor Pool and Impairs Functional Recovery after Hypoxic-Ischemic Brain Injury. Br. J. Pharmacol. 2008, 28, 1294–1306. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.G.; Tan, A.; O’Donnell, C.P.; Schulze, A. Resuscitation of newborn infants with 100% oxygen or air: A systematic review and meta-analysis. Lancet 2004, 364, 1329–1333. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Sarnat, M.S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Al Naqeeb, N.; Edwards, A.D.; Cowan, F.M.; Azzopardi, D. Assessment of Neonatal Encephalopathy by Amplitude-integrated Electroencephalography. Pediatrics 1999, 103, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Hajnal, B.L.; Vigneron, D.; Sola, A.; Partridge, J.C.; Allen, F.; Ferriero, D.M. Prediction of neuromotor outcome in perinatal asphyxia: Evaluation of MR scoring systems. Am. J. Neuroradiol. 1998, 19, 143–149. [Google Scholar] [PubMed]
- Al Amrani, F.; Marcovitz, J.; Sanon, P.-N.; Khairy, M.; Saint-Martin, C.; Shevell, M.; Wintermark, P. Prediction of outcome in asphyxiated newborns treated with hypothermia: Is a MRI scoring system described before the cooling era still useful? Eur. J. Paediatr. Neurol. 2018, 22, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, M.; Hellström-Westas, L.; Liu, X.; De Vries, L.S. Effect of Hypothermia on Amplitude-Integrated Electroencephalogram in Infants With Asphyxia. Pediatrics 2010, 126, e131–e139. [Google Scholar] [CrossRef]
- Sarkar, S.; Barks, J.D.; Donn, S.M. Should amplitude-integrated electroencephalography be used to identify infants suitable for hypothermic neuroprotection? J. Perinatol. 2008, 28, 117–122. [Google Scholar] [CrossRef]
- Ruhfus, M.; Giannakis, S.; Markus, M.; Stein, A.; Hoehn, T.; Felderhoff-Mueser, U.; Sabir, H. Association of Routinely Measured Proinflammatory Biomarkers With Abnormal MRI Findings in Asphyxiated Neonates Undergoing Therapeutic Hypothermia. Front. Pediatr. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Toet, M.C.; Hellström-Westas, L.; Groenendaal, F.; Eken, P.; De Vries, L.S. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F19–F23. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, F.; Soeder, H.; Falk, G. DTV-Atlas zur Mathematik: Taf. u. Texte. Orig.-Ausg. Ed; Deutscher Taschenbuch-Verlag: München, Germany, 1974. [Google Scholar]
- Sabir, H.; Jary, S.; Tooley, J.; Liu, X.; Thoresen, M. Increased Inspired Oxygen in the First Hours of Life is Associated with Adverse Outcome in Newborns Treated for Perinatal Asphyxia with Therapeutic Hypothermia. J. Pediatr. 2012, 161, 409–416. [Google Scholar] [CrossRef]
- Greisen, G. Autoregulation of cerebral blood flow in newborn babies. Early Hum. Dev. 2005, 81, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Filippi, L.; Cristofori, G.; Colnaghi, M.; Ramenghi, L.; Agazzani, E.; Ronchi, A.; Florini, P.; Mosca, F. Does pulmonary function change during whole-body deep hypothermia? Arch. Dis. Child. Fetal Neonatal Ed. 2011, 96, F374–F377. [Google Scholar] [CrossRef] [PubMed]
- Dassios, T.; Austin, T. Respiratory function parameters in ventilated newborn infants undergoing whole body hypothermia. Acta Paediatr. 2014, 103, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Thoresen, M. Supportive care during neuroprotective hypothermia in the term newborn: Adverse effects and their prevention. Clin. Perinatol. 2008, 35, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Towfighi, J.; Heitjan, D.F.; Brucklacher, R.M. Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: An experimental study in the immature rat. Pediatrics 1995, 95, 868–874. [Google Scholar] [PubMed]
- Vannucci, R.C.; Brucklacher, R.M.; Vannucci, S.J. Effect of carbon dioxide on cerebral metabolism during hypoxia-ischemia in the immature rat. Pediatr. Res. 1997, 42, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, R.C.; Towfighi, J.; Brucklacher, R.M.; Vannucci, S.J. Effect of Extreme Hypercapnia on Hypoxic-Ischemic Brain Damage in the Immature Rat. Pediatr. Res. 2001, 49, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Bracci, R.; Perrone, S.; Buonocore, G. Red blood cell involvement in fetal/neonatal hypoxia. Biol. Neonate 2001, 79, 210–212. [Google Scholar] [PubMed]
- Saugstad, O.D.; Aasen, A.O. Plasma hypoxanthine concentrations in pigs. A prognostic aid in hypoxia. Eur. Surg. Res. 1980, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Núnez, A.; Benavente, I.; Blanco, D.; Boix, H.; Cabañas, F.; Chaffanel, M.; Fernández-Colomer, B.; Fernández-Lorenzo, J.R.; Loureiro, B.; Moral, M.T.; et al. Oxidative stress in perinatal asphyxia and hypoxic-ischaemic encephalopathy. An. Pediatría 2018, 88, 228.e1–228.e9. [Google Scholar] [CrossRef]
- Markus, T.; Hansson, S.; Amer-Wåhlin, I.; Hellström-Westas, L.; Saugstad, O.D.; Ley, D. Cerebral Inflammatory Response After Fetal Asphyxia and Hyperoxic Resuscitation in Newborn Sheep. Pediatr. Res. 2007, 62, 71–77. [Google Scholar] [CrossRef]
- Munkeby, B.H.; Børke, W.B.; Bjørnland, K.; Sikkeland, L.I.B.; Borge, G.I.A.; Halvorsen, B.; Saugstad, O.D.; Oslash, B.W.B. Resuscitation with 100% O2 increases cerebral injury in hypoxemic piglets. Pediatr. Res. 2004, 56, 783–790. [Google Scholar] [CrossRef]
- Saugstad, O.D.; Ramji, S.; Soll, R.F.; Vento, M. Resuscitation of Newborn Infants with 21% or 100% Oxygen: An Updated Systematic Review and Meta-Analysis. Neonatology 2008, 94, 176–182. [Google Scholar] [CrossRef]
- Dalen, M.L.; Liu, X.; Elstad, M.; Løberg, E.M.; Saugstad, O.D.; Rootwelt, T.; Thoresen, M. Resuscitation with 100% oxygen increases injury and counteracts the neuroprotective effect of therapeutic hypothermia in the neonatal rat. Pediatr. Res. 2012, 71, 247–252. [Google Scholar] [CrossRef][Green Version]
- Szakmar, E.; Jermendy, A.; El-Dib, M. Respiratory management during therapeutic hypothermia for hypoxic-ischemic encephalopathy. J. Perinatol. 2019, 39, 763–773. [Google Scholar] [CrossRef]
- Giannakis, S.; Ruhfus, M.; Rüdiger, M.; Sabir, H.; Network, T.G.N.H.; Network, G.N.H. Hospital survey showed wide variations in therapeutic hypothermia for neonates in Germany. Acta Paediatr. 2019, 109, 200–201. [Google Scholar] [CrossRef]


| Clinical Characteristics | Intubated (n = 53) | Non-Intubated (n = 18) | p-Value |
|---|---|---|---|
| Birth weight (g), median (IQR) | 3265 (2845–3840) | 3070 (2722.5–3545) | 0.09 |
| Male gender, n (%) | 30 (56.6%) | 4 (22.2%) | <0.01 |
| Gestational age in weeks, median (IQR) | 39+6 (37+5–40+4) | 38+6 (37+1–39+6) | 0.12 |
| APGAR score | |||
| 5 min, median (min, max) | 4 (0–10) | 5 (2–9) | 0.03 |
| 10 min, median (min, max) | 6 (0–10) | 7 (4–10) | <0.01 |
| First pH, median (IQR) | 6.81 (6.68–6.93) | 6.93 (6.85–6.98) | <0.01 |
| First base excess (mmol/L), median (IQR) | 22.15 (16.6–27) | 18 (14.2–22) | <0.01 |
| First lactate level (mmol/L), median (IQR) | 12.7 (8.7–17) | 10.95 (8.52–13.08) | 0.11 |
| HIE grade before cooling (n = mild, n = moderate, n = severe) | 7, 22, 21 | 11, 5, 0 | <0.01 |
| Inborn, n (%) | 29 (54.7%) | 14 (77.8%) | 0.03 |
| Resuscitation at birth, n (%) | 33 (62.3%) | 2 (11.1%) | <0.01 |
| Short-term adverse outcome | 17 (32.1%) | 1 (5.6%) | <0.01 |
| Death, n (%) | 9 (17.0%) | 0 (0%) | <0.01 |
| Initial temperature (℃) before start of TH, median (IQR) | 35.5 (34.2–36.9) | 36.1 (35.8–36.4) | 0.01 |
| Time (minutes) until start ofTH, median (IQR) | 37.5 (10–73.7) | 30 (10–105) | 0.50 |
| Time (minutes) to target temperature, median (IQR) | 120 (60–170) | 120 (60–127.5) | 0.37 |
| EEG time (minutes) to normal trace, median (IQR) | 13 (1–57) | 1 (1–4.5) | <0.01 |
| Lowest blood glucose levels (mg/dL) | |||
| first 6 HOL, median (IQR) | 81 (60–127) | 68 (62.75–77.5) | 0.04 |
| first 72 HOL, median (IQR) | 63 (48–77.5) | 60 (48.5–68.5) | 0.08 |
| Morphine | |||
| duration (hours), median (IQR) | 72 (65–92) | 72 (64–76) | 0.09 |
| cumulative dose (µg/kg/d), median (IQR) | 0.6 (0.3–1.15) | 0.25 (0.18–0.42) | <0.01 |
| Inotropic support, n (%) | 33 (62.3%) | 4 (22.2%) | <0.01 |
| Oxygen supplementation | |||
| duration (minutes), median (IQR) | 24 (8–102) | 25 (0–3.25) | <0.01 |
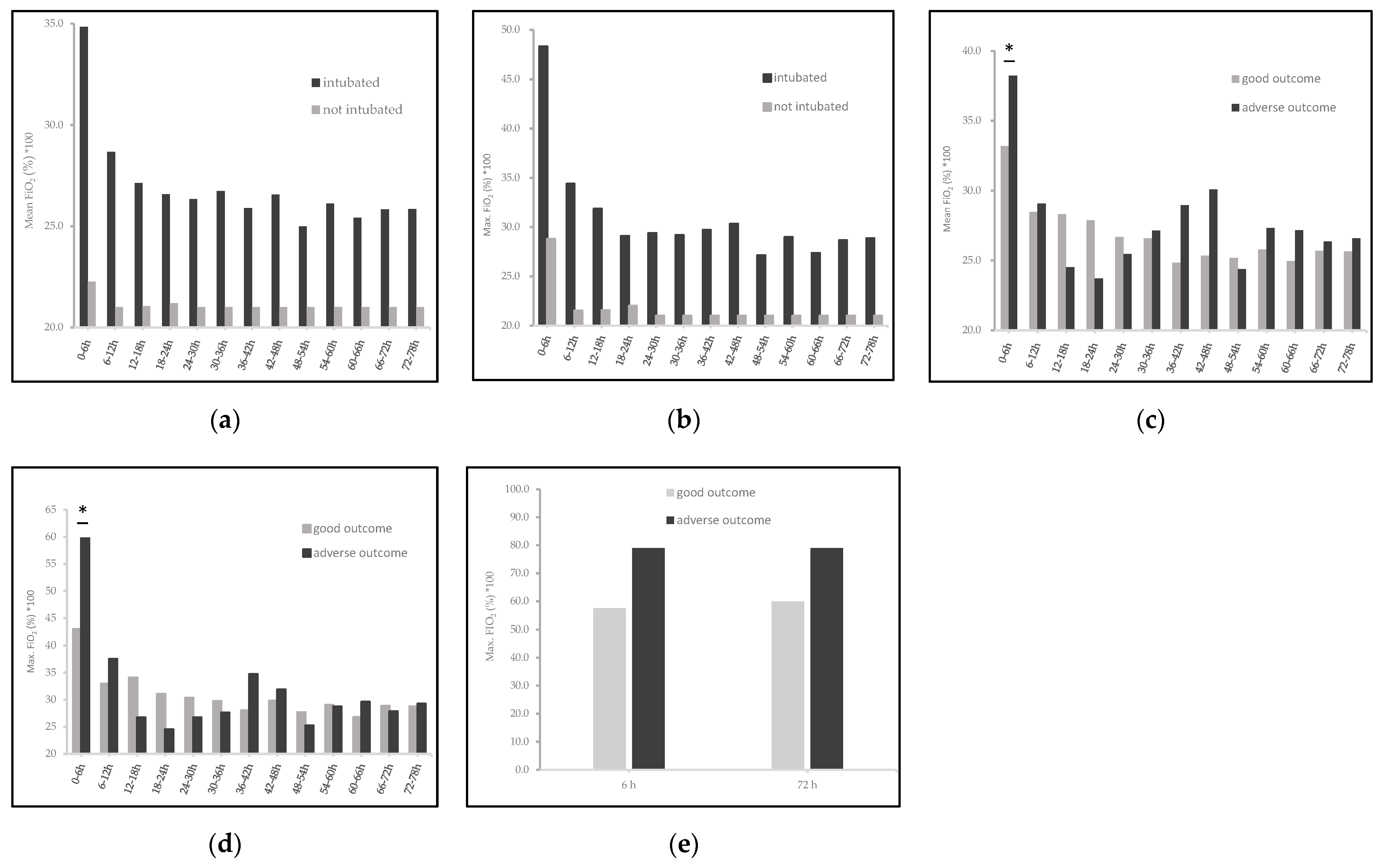
| highest FiO2(%) × 100 | |||
| first 6 HOL, median (IQR) | 80 (40–100) | 21 (21–58) | 0.01 |
| first 72 HOL, median (IQR) | 80 (48–100) | 21 (21–58) | <0.01 |
| Area under the curve (AUC) FiO2(%) × 100 over 78 h | |||
| maximum FiO2, median (IQR) | 23.6 (21.3–29.6) | 21 (21–21.5) | <0.01 |
| mean FiO2, median (IQR) | 21.6 (21–24) | 21 (21–21) | 0.06 |
| AUC pCO2 over 78 h | |||
| maximum pCO2, median (IQR) | 42.6 (38.8–45.5) | 45.7 (41.4–50.6) | 0.03 |
| minimum pCO2, median (IQR) | 46.7 (44.1–53.5) | 47.5 (41.7–54.9) | 0.29 |
| ΔpCO2 in mmHg | |||
| first 6 HOL, median (IQR) | 44.0 (15–74.3) | 37.4 (27.7–58.75) | 0.14 |
| first 72 HOL, median (IQR) | 55.1 (30.3–79.4) | 43.7 (31.8–57.8) | 0.03 |
| Clinical Characteristics | Good Short-Term Outcomes (n = 36) | Adverse Short-Term Outcomes * (n = 17) | p-Value |
|---|---|---|---|
| Birth weight (g), median (IQR) | 3212.5 (2827.5–3855) | 3300 (2775–3827.5) | 0.40 |
| Male gender, n (%) | 22 (61.1%) | 8 (47.1%) | 0.18 |
| Gestational age (weeks), median (IQR) | 38+6 (37 + 1–40 + 4) | 40+2 (38 + 5–40 + 6) | 0.07 |
| APGAR score | |||
| 5 min, median (min, max) | 5 (0–10) | 2 (0–6) | <0.01 |
| 10 min, median (min, max | 7 (1–10) | 4 (0–7) | <0.01 |
| First pH, median (IQR) | 6.85 (6.78–6.95) | 6.8 (6.6–6.92) | 0.03 |
| First base excess (mmol/L), median (IQR) | 21.8 (16.6–25.1) | 23 (16.35–29.6) | 0.27 |
| First lactate level (mmol/L), median (IQR) | 12.2 (7.65–17) | 13.5 (11.2–19) | 0.11 |
| HIE grade before cooling (n = mild, n = moderate, n = severe) | 7, 18, 9 | 0, 4, 12 | <0.01 |
| Inborn, n (%) | 18 (50.0%) | 11 (64.7%) | 0.16 |
| Resuscitation at birth, n (%) | 20 (55.6%) | 13 (76.5%) | 0.08 |
| Meconium aspiration, n (%) | 8 (22.2%) | 8 (47.1%) | 0.15 |
| Seizures, n (%) | 15 (41.7%) | 14 (82.4) | <0.01 |
| Initial temperature (℃) before Start TH, median (IQR) | 35.65 (34.1–37) | 35.15 (34.3–36.5) | 0.46 |
| Time (minutes) until Start TH, median (IQR) | 45 (10–77.5) | 20 (10–71.3) | 0.14 |
| Time (minutes) to target temperature, median (IQR) | 105 (52.5–150) | 135 (60–183.5) | 0.24 |
| EEG time (minutes) to normal trace, median (IQR) | 12 (1–23) | 73 (2–300) | <0.01 |
| Minimum blood glucose levels (mg/dL) | |||
| first 6 HOL median (IQR) | 77 (50–113) | 86 (72–155) | 0.25 |
| first 72 HOL, median (IQR) | 60 (45–79) | 66 (50.3–74.8) | 0.18 |
| Morphine | |||
| duration (hours), median (IQR) | 79.5 (72–96) | 67 (35–72) | <0.01 |
| Cumulative dose (µg/kg/d), median (IQR) | 0.61 (0.34–1.47) | 0.42 (0.25–0.8) | 0.43 |
| Inotropic support, n (%) | 22 (61.1%) | 11 (64.7%) | 0.39 |
| Oxygen supplementation | |||
| duration (minutes), median (IQR) | 31.5 (3.75–106.5) | 24 (11–88) | 0.26 |
| highest FiO2(%) × 100 | |||
| first 6 HOL, median (IQR) | 60 (30–100) | 80 (30–100) | 0.01 |
| first 72 HOL, median (IQR) | 95 (68–100) | 90 (67–100) | 0.05 |
| Area under the curve (AUC) FiO2(%) × 100 over 78 h | |||
| maximum FiO2, median (IQR) | 22.6 (21.2–28.3) | 26.2 (23.6–37.2) | 0.46 |
| mean FiO2, median (IQR) | 21.8 (21–23.9) | 24.3 (21.6–32.8) | 0.37 |
| AUC pCO2 (mmHg) over 78 h | |||
| maximum pCO2, median (IQR) | 46.1 (43.6–50) | 50 (45.5–55.9) | 0.049 |
| minimum pCO2, median (IQR) | 42.6 (39.6–45.5) | 42.4 (37.1–46.0) | 0.20 |
| ΔpCO2 in mmHg | |||
| first 6 HOL, median (IQR) | 25.9 (14.4–63.9) | 70.2 (40.9–101.3) | <0.01 |
| first 72 HOL, median (IQR) | 41.0 (29.2–76.8) | 66.3 (55.1–98.7) | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakis, S.; Ruhfus, M.; Markus, M.; Stein, A.; Hoehn, T.; Felderhoff-Mueser, U.; Sabir, H. Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns. Children 2021, 8, 430. https://doi.org/10.3390/children8060430
Giannakis S, Ruhfus M, Markus M, Stein A, Hoehn T, Felderhoff-Mueser U, Sabir H. Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns. Children. 2021; 8(6):430. https://doi.org/10.3390/children8060430
Chicago/Turabian StyleGiannakis, Stamatios, Maria Ruhfus, Mona Markus, Anja Stein, Thomas Hoehn, Ursula Felderhoff-Mueser, and Hemmen Sabir. 2021. "Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns" Children 8, no. 6: 430. https://doi.org/10.3390/children8060430
APA StyleGiannakis, S., Ruhfus, M., Markus, M., Stein, A., Hoehn, T., Felderhoff-Mueser, U., & Sabir, H. (2021). Mechanical Ventilation, Partial Pressure of Carbon Dioxide, Increased Fraction of Inspired Oxygen and the Increased Risk for Adverse Short-Term Outcomes in Cooled Asphyxiated Newborns. Children, 8(6), 430. https://doi.org/10.3390/children8060430







