1. Introduction
The Ewing sarcoma family of tumors (ESFT) is a collection of small, rounded tumor cells that have similar neural histological and genetic characteristics [
1,
2,
3,
4]. ESFT is categorized into four types based on the origin of the tumor: Ewing sarcoma of the bone, peripheral primitive neuroectodermal tumor (pPNET), Askin tumor, which originates from the chest wall, and, finally, the extraosseous or extraskeletal Ewing sarcoma (EES). EES, which occurs in around 20% of ES cases, typically originates from the soft tissues of the trunk and extremities [
5], and the majority of these cases are reported among patients who are 10–30 years of age [
6].
Based on a previous report, the incidence of EES is 0.4 per million individuals, which is lower than that of ES of the bone by 10-fold [
7]. Although uncommon, the occurrence of EES seems to have a bimodal distribution, where there is a peak in the occurrence rate among children (<5 years) and adults (>35 years) [
8], with an increased likelihood of presenting among older populations compared to ES of the bone. Unlike Ewing earcoma of the bone, no evidence supports a link between the tumor and race or biological sex [
8,
9,
10].
The management of EES includes surgery [
11] and chemotherapy [
10,
12,
13] in resectable tumors. Under unresectable conditions, radiotherapy is usually considered [
14]. According to the National Comprehensive Cancer Network (NCCN), the optimum management of EES remains not clearly defined [
15,
16], although some studies have highlighted an added value of surgery among EES cases compared to Ewing sarcoma of the bone in terms of better survival rates [
17,
18]. In general, the prognosis of EES is more favorable than that of the bone [
9,
10].
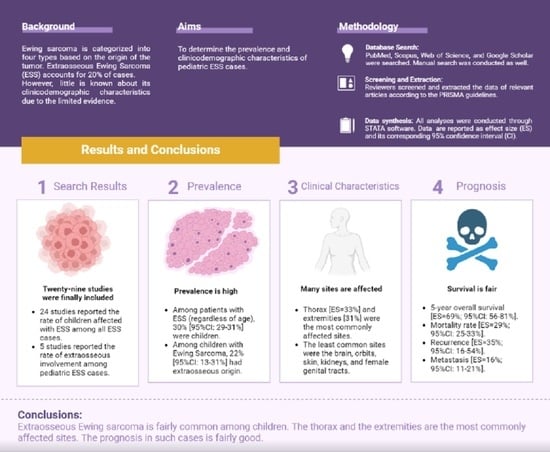
To date, there is no clear picture regarding the occurrence rate of EES among children and adolescents (<21 years), as well as their demographic characteristics, tumor characteristics (i.e., location), treatment modalities, and clinical outcomes (i.e., survival, mortality, recurrence). Therefore, we conducted this systematic review and meta-analysis to provide collective evidence regarding the clinical characteristics and outcomes in this patient population.
2. Materials and Methods
2.1. Study Design and Search Strategy
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [
19]. A protocol was not registered, since it is not mandatory, as per several recommendations [
20,
21]. On 26 July 2022, PubMed, Scopus, Web of Science, and Google Scholar were searched for articles that report the presentation of EES in the pediatric population (children and young adolescents <21 years of age). Of note, only the first 200 records from Google Scholar were retrieved and screened according to recently published guidelines [
22]. We updated the database search on 24 August 2022 to ensure that no additional relevant reports had been published prior to the qualitative and quantitative analyses [
23].
We used a combination of keywords and terms in our search, which included the following: (“Ewing Sarcoma” OR “Ewing’s Sarcoma”) AND (adolescen* OR Child* OR Pediatric* OR “young adult”) AND (“soft tissue” OR extraskeletal OR extraosseous) AND (clinicopathologic* OR “clinical feautre” OR “clinical characteristic*” OR “clinical outcome*”). The terms of the Medical Subject Headings (MeSH) were also added (particularly in PubMed) to retrieve all the possibly relevant articles. The detailed search criteria used for each database are described in
Supplementary Table S1.
Moreover, we conducted a manual search to find any relevant articles that may have potentially been excluded during the screening phase or were not found during the database search [
24,
25]. This strategy was conducted through three different approaches: (1) screening the titles of the reference list of the final included papers, (2) reading the titles and abstracts of articles similar to final included studies through the “similar articles” function on PubMed, and (3) conducting a random search on Google using keywords similar to those of the original database search, such as: “Ewing sarcoma” + “child”. It is noteworthy that no filters were used during the database search regarding the language of the research paper, year in which the paper was published, or the country of the first author.
2.2. Eligibility Criteria
The methodology and design of this review were conducted as per the PICO framework [
26,
27], including the population (pediatric cases of EES), intervention (none), comparison (none), and outcome (primary outcome: prevalence rate of EES in children and adolescents; secondary outcomes: clinicodemographic characteristics, tumor characteristics, and clinical outcomes in pediatric cases of EES).
For articles to be included, a study had to: (1) report original data, (2) include cases of EES, (3) report cases aged <21 years. On the other hand, studies were excluded if they were compliant with one of the following criteria: (1) non-original research (i.e., review articles, editorials without human data, commentaries, theses, conference abstracts/posters, and books), (2) animal, in vivo, and in vitro studies, (3) case reports and case series of <5 cases, (4) studies reporting EES cases of mixed ages (children, adolescents, adults, and elderly) without stratifying the cases according to their age, (5) studies reporting Ewing sarcomas of mixed origin (extraosseous and skeletal) in children without stratifying the cases according to their origin, and (6) duplicated records.
2.3. Screening and Study Selection
Following the retrieval of records through the database search [
28], the references were imported to EndNote (Version 8) for duplicate removal and to organize the screening sheet [
29]. The screening sheet included the following: article ID, list of authors’ names, year of publication (YOP), research paper’s title, DOI, journal name, and abstract. The screening was carried out in three separate stages: title, abstract, and full-text screening. All of these steps were performed by two sets of two reviewers each. Any differences between the reviewers were reviewed and resolved by the senior author [
30].
Significantly, upon reviewing the literature, two categories of articles were found to be consistent with our eligibility criteria. The first group of articles included patients with EES, among whom pediatric cases were counted, and the second group of articles included pediatric cases, of whom the origin of Ewing sarcoma was determined (extraosseous or skeletal). Both of these categories were included, extracted, and presented separately in our review.
2.4. Extraction and Quality Assessment
The data extraction process was carried out in a similar manner as the screening stage [
31]. The senior author designed a pilot data extraction sheet through the Excel software (version 2021) that was consistent with the study objectives. The sheet included 5 domains. The first domain highlighted the baseline characteristics of the included studies (authors’ names, year of publication, country, study design, sample size, and follow-up duration). The second domain included the demographic characteristics of the included participants, such as age and biological sex. The third domain included the location of the EES among the pediatric cases (i.e., cranium, female genital tract, orbit, head and neck, pelvis, extremities, thorax, abdomen). The fourth domain included the tumor’s characteristics (i.e., management modalities (i.e., surgery alone, surgery combined with radiotherapy, surgery combined with chemotherapy, etc.). The final domain included the patients’ clinical outcomes in terms of the overall survival (OS), progression-free survival (PFS), disease-specific survival (DSS), secondary metastasis, no evidence of disease (NED), mortality, and recurrence. Two reviewers extracted the data from the included studies for further qualitative and quantitative synthesis, as per the recommended guidelines [
32,
33].
2.5. Data Synthesis
All quantitative analyses were conducted using the STATA software (version 17) with the metaprop command [
34]. The exact cimethod [
34] was used to pool the effect size (ES)—occurrence rate of EES in the pediatric cases—along with its 95% confidence interval (CI). Importantly, for the purposes of discussing the findings of our review, the term ES will refer to the effect size and not Ewing sarcoma (which will not be abbreviated in this manuscript). The random-effects and fixed-effects models were used according to the presence or absence of heterogeneity, respectively [
35,
36]. Heterogeneity was measured using the
I2 statistic, where a value of >50% or a
p-value of <0.05 indicates significant heterogeneity.
4. Discussion
There is limited evidence regarding the occurrence rate and clinical characteristics of EES in children. Our systematic review is the first to comprehensively discuss the prevalence, clinical features, and outcomes of EES patients of pediatric age (less than 21 years). A summary of our key findings can be found in
Table 6. Overall, a total of 29 studies reporting on 5752 patients were analyzed. In our study, we found that the rate of affected children and adolescents with EES in a population with EES (mixed age) varied substantially between the studies, ranging from 5.63% [
60] to as high as 100% [
55]. This discrepancy could be related to the design and methodology of the included studies, since some studies included patients with EES regardless of the age group at baseline, while a few studies included pediatric cases of EES at baseline [
55,
56]. Overall, our meta-analysis revealed that 30% of the EES cases occurred among children and adolescents. Consistent with previous observations [
8,
9], no link was noted between EES presentation in children and biological sex. The pooled rate of male pediatric patients with EES was 52.45%, which is relatively similar to that of female cases (47.55%).
In addition, five studies included children affected with ES at baseline, and then the origin of the tumor was analyzed in these cases. The rate of EES out of all the ES types ranged from 10% to 55.55% among the individual studies. Again, the difference in reported rates could be related to the design and methodology implemented in each study. That being said, in our meta-analysis, the rate of EES occurrence among the pediatric ES cases was 22%, of whom 54.70% were males.
Data on the location of EES among pediatric cases is scarce, since the majority of the available studies in the literature include patients with mixed ages and tend to stratify the outcomes (i.e., survival) based on age (children vs. adults or the elderly), without stratifying the clinical characteristics or tumor characteristics based on the age of the examined patients. Therefore, the data reported in our review regarding the EES location in the pediatric cases rely mainly on case series with a case-by-case description of the tumor characteristics. Thirteen studies reported relevant data on the location of EES, and our pooled meta-analysis revealed that the thorax is the most predominant origin for EES in children and adolescents, followed by the extremities, the head and neck, the pelvis, the abdomen, the spine, and the intracranial space, respectively. In certain cases, the EES originated in the orbit among the pediatric cases; however, the occurrence rate did not surpass the rare event assumption (>5%). Additionally, other sites, such as the great toe [
63], the mesocolon [
64], the frontal sinus [
65], and the penis [
66], have been described as rare cases. Moreover, the kidneys [
41,
53], the skin [
37], and the female genital tract [
49] have been reported as sites of origin of EES in pediatric cases in several case series; however, not enough data were present to perform a meta-analysis of the prevalence in this case.
There is a debate on the best management approach for EES cases occurring in children, and this uncertainty is related to the rarity of EES, the discrepancy in its clinical presentation, and the differences in the patients’ characteristics [
67]. In addition, this patient population is underrepresented in clinical trials directed towards the investigation of the efficacy and safety of various treatment modalities among pediatric cases of EES. In our review, only ten studies reported the treatment modalities according to different age groups, and the majority of the data were pooled from case series. Overall, the majority of cases were treated with concurrent chemotherapy and radiotherapy (57% of cases), followed by surgery and radiotherapy (55%), surgery alone (53%), surgery and chemotherapy (29%), and radiotherapy alone in cases of unresectable tumors (16%). It is important to mention that the confidence interval of these reported rates is wide, reflecting the imprecision of the reported effect estimates. Therefore, these data should be interpreted with caution and should not be perceived as representative of the EES pediatric population. More data from properly designed research studies are still needed to confirm this observation. Additionally, the available data did not present survival outcomes stratified by these treatment modalities in the pediatric cases separately. Therefore, future studies should carefully consider stratifying data (clinical characteristics and outcomes) based on the origin of the tumor (skeletal vs. extraskeletal) and age of the included patients (children vs. adults vs. the elderly).
In our study, we found that a great proportion of pediatric EES patients have a preferable prognosis in terms of their 5-year overall survival (with an overall rate of 69%), which is consistent with that of cases with no evidence of disease following treatment (an overall rate of 69%). However, mortality was documented in almost one-third of the pediatric EES population (120 deaths out of 404 cases, an overall rate of 29%). Additionally, recurrence was reported in 35% of cases, while secondary metastasis was reported in 16%. That being said, these rates should be based on the available data of 11 studies out of the 29 studies included in our review. Therefore, the presented data are not generalizable to the whole EES pediatric population.
Meanwhile, our review has several limitations. The most important is the fact that our estimates regarding the prevalence of childhood EES among EES cases (of all ages) or the prevalence of cases of extraosseous origin among the pediatric Ewing sarcoma cases could be overestimated, since the majority of the included studies investigated EES cases and not the Ewing sarcoma population as a whole. In addition, most of these studies are based on retrospective analyses and not cross-sectional in design, which further limits the generalizability of our findings.